Telomere length control and transcriptional regulation of subtelomeric adhesins in Candida glabrata
Summary
The pathogenic yeast Candida glabrata is able to bind in vitro to human epithelial cells. This interaction depends on expression of the adhesin Epa1p. The genome contains a number of EPA1 paralogues which localize to the subtelomeric regions of the C. glabrata. We have identified three hyperadherent mutants of C. glabrata. The first has an insertion adjacent to EPA7, an EPA1-related adhesin. The others disrupt the SIR3 and RIF1 genes of C. glabrata. We show that SIR3 and RIF1 are required for subtelomeric silencing in C. glabrata and that RIF1 regulates telomere length in C. glabrata. We show that the hyperadherent phenotype of the sir3Δ and rif1Δ deletion strains depends primarily on derepression of two novel members of the EPA gene family –EPA6 and EPA7. The sir3Δ and rif1Δ mutants show increased colonization of the kidney in a murine model of disseminated infection and this hypercolonization depends, at least in part, on derepression of EPA6 and EPA7. The analysis here is the first evidence that multiple EPA genes encode adhesins and demonstrates that transcription of at least two of these adhesins is regulated by subtelomeric silencing.
Introduction
The incidence of opportunistic fungal infections in the immunocompromised patient population has increased significantly over the last two decades (Schuman et al., 1998; Trick et al., 2002). Candida species account for the majority of fungal infections, and these are primarily caused by Candida albicans (Pfaller et al., 2002; Trick et al., 2002). However, there has been a notable increase in the relative proportion of infections caused by non-albicans species of Candida (Nguyen et al., 1996; Trick et al., 2002). In the USA, Candida glabrata is the most important non-albicans species and is now the second leading cause of both blood stream and mucosal candidiasis (Pfaller et al., 1998; Vazquez et al., 1999; Bodey et al., 2002; Trick et al., 2002).
Adherence of Candida species to host cells is proposed to be of prime importance in the virulence of these organisms, and a number of adhesins have been identified in Candida species. Perhaps the best studied adhesin in C. albicans is encoded by the HWP1 gene. Glutamine residues in the N-terminal domain of Hwp1 are cross-linked by host transglutaminase activity to unidentified host proteins and result in covalent attachment of the yeast to host epithelial cells. This interaction has been shown to be important for colonization in the oral cavity (Sundstrom et al., 2002). In C. albicans, the ALS family of proteins (Hoyer, 2001) also includes at least two proteins that are demonstrably adhesins: ALS1 and ALS5 (or ALA1). The host ligands for Als1 and Als5 likely include components of the extracellular matrix (Klotz et al., 2004 and references therein), but have not been definitively identified.
In C. glabrata, adherence to epithelial cells in vitro is mediated by a cell wall protein encoded by the EPA1 gene (Cormack et al., 1999). Deletion of this gene reduces in vitro adherence to host epithelial cells to background levels. EPA1 encodes a lectin that recognizes host N-acetyl lactosamine containing glycoconjugates. EPA1, like the ALS genes of C. albicans, actually forms part of a family of genes in C. glabrata, defined by significant homology within the N-terminal ligand-binding domain (De Las Peñas et al., 2003). EPA1 itself is part of a cluster of three EPA genes located within a few kilobases of a telomere. EPA4 and EPA5 are also encoded in a cluster located 5 kb from a telomere. These additional EPA genes are not transcribed during in vitro growth, in part because both subtelomeric regions are subject to chromatin-based transcriptional silencing (De Las Peñas et al., 2003).
In Saccharomyces cerevisiae, silencing at telomeres is thought to initiate with Rap1 binding to the telomeric repeats, which contain a Rap1 binding site on average every 40 base pairs. Rap1 recruits the Sir complex which spreads into adjacent subtelomeric domains through the action of Sir2, an NAD-dependent histone deacetylase which deacetylates the N-termini of histones H3 and H4 providing high affinity binding sites for Sir3 and Sir4 (reviewed in Rusche et al., 2003). Sir3 and Sir4 binding, which is coextensive with the region of silencing, is thought to form a repressive higher order chromatin structure.
Rap1 also interacts in S. cerevisiae with two additional proteins – Rif1 and Rif2 (Rap1-interacting factors 1 and 2) (Hardy et al., 1992; Wotton and Shore, 1997). These proteins have a primary role in regulating telomere length. In the absence of Rif1, and to a lesser extent Rif2, telomere length regulation is compromised and the telomeres which are normally limited in size to 250 bp increase in length to an average of 500 bp. S. cerevisiae rif1Δ mutants also show an alteration in silencing at the silent mating loci and in subtelomeric regions (Hardy et al., 1992). In the current model, the loss of RIF1 has a direct effect on telomere length regulation and an indirect effect on silencing. Because the telomere repeats contain Rap1 binding sites, the longer telomeres in a rif1Δ strain are thought to titrate Rap1 and the Sir proteins from other genomic loci, resulting in loss of silencing, notably at the silent mating loci HMR and HML. At telomeres themselves, the rif1Δ mutant actually shows a modest increase in silencing of marker genes placed immediately adjacent to the telomeric repeats, probably as a result of increased Rap1p-Sir3/Sir4 recruitment to the region (Wotton and Shore, 1997). In Chromatin immunoprecipitation (ChIP) experiments, Rif1 can be found along with the Sir proteins at distances far from the telomere repeats themselves and well into the subtelomeric regions subject to silencing (Smith et al., 2003). This finding suggests either a broader than expected spreading of Rif1 into subtelomeric regions or alternatively suggests the existence of higher order structure at the chromosome ends in which the telomeric repeats and the proteins bound there are in complex with chromatin several kilobases proximal to the telomeric repeats.
In this article, we describe the identification and characterization of three hyperadherent mutants of C. glabrata. One mutant had insertions downstream of a novel EPA gene, EPA7. We show that this insertion resulted in derepression of the EPA7 locus, which is able, like EPA1, to mediate adherence to epithelial cells. We also identified mutants with insertions in the SIR3 and RIF1 genes of C. glabrata. These mutants show increased adherence of C. glabrata to epithelial cells. rif1Δ strains, but not sir3Δ strains, showed a loss of telomere length regulation. In addition, several EPA-related genes were transcriptionally induced in the sir3Δ and rif1Δ strains relative to the wild type strain. Two of these, EPA6 and EPA7, were primarily responsible for the hyperadherent phenotype of the sir3Δ and rif1Δ strains. Strikingly, in a murine model of disseminated infection, the sir3Δ and rif1Δ strains show a significant increased colonization of kidney, and at least for SIR3, that increased colonization depends on overexpression of EPA genes. This work provides evidence that multiple EPA genes in C. glabrata are able to mediate adherence to epithelial cells and implicates subtelomeric silencing in the regulation of C. glabrata adherence and virulence.
Results
EPA1 mediates adherence of C. glabrata to epithelial cells. We previously showed that there are at least five additional EPA-related genes encoded in the genome (De Las Peñas et al., 2003). Inspection of the C. glabrata genome sequence showed the existence of additional EPA-related sequences some of which, we hypothesized, might correspond to adhesins (data not shown). To further analyse the adherence capability of C. glabrata, and in particular the role of the potential additional EPA family members in adherence, we undertook a genetic screen for mutants that restore adherence to cells lacking EPA1 function. Under certain growth conditions EPA1 is not significantly expressed and the cells are non-adherent. Specifically, EPA1 is transcribed weakly in stationary phase (see Fig. 2 and data not shown); as a result, C. glabrata stationary phase cells are largely non-adherent to a variety of epithelial cells [Lec2 cells (Table 1), as well as HeLa cells, A498 cells and T24 cells (data not shown)]. We used this fact to screen for mutants that increase adherence of stationary phase C. glabrata to epithelial cells. We had previously generated two different libraries of insertion mutants in strain BG14, a ura3 derivative of BG2 (Cormack and Falkow, 1999). The first library of insertion mutants was generated by non-homologous integration of the plasmid YIplac211 into the C. glabrata genome (Cormack and Falkow, 1999). The second library was generated by random Tn7 insertions of C. glabrata genomic clones, and then introduced into C. glabrata genome by homologous recombination (Castaño et al., 2003). Five thousand mutants of the first library, and 10 000 of the second library were screened for insertions that show a significant adherence of stationary phase cells to Lec2 cells, T24 cells or A498 cells. We identified two mutants from each library that were notably hyperadherent to all three cell lines (Table 1 and data not shown). To identify the disrupted locus in each case, we digested the mutant genomic DNA with a restriction enzyme not present in the plasmid or the Tn7 transposon, ligated this and transformed the resulting plasmid into Escherichia coli. The first two plasmids (pSP35-4 and pSP18-17) consist of the original YIplac211 plasmid flanked by the disrupted genomic locus. Sequence analysis showed that in both cases, the same locus had been disrupted. One insertion was in the promoter and one in the open reading frame of a gene homologous to the RIF1 gene of S. cerevisiae. The other two plasmids, pJA2 and pIC63 were also sequenced. pJA2 was found to be a Tn7 insertion in the promoter region of a gene homologous to SIR3 of S. cerevisiae while pIC63 contained a large insertion downstream of a novel EPA gene –EPA7. We will first describe our analysis of the SIR3 and RIF1 genes and then the analysis of the cis insertion at the EPA7 locus.
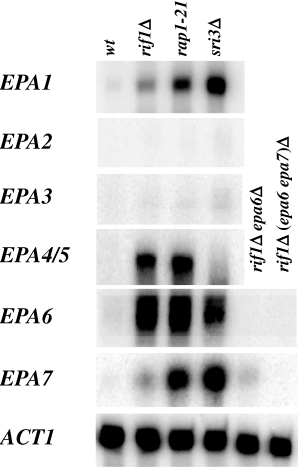
Transcript levels for EPA1–EPA7 in wild type and mutant backgrounds. RNA was isolated from four isogenic strains grown to stationary phase: wild-type (BG14), rif1Δ (BG509), sir3Δ (BG676) and rap1–21(BG592) and analysed by S1 nuclease protection. Seven probes were used corresponding to EPA1, EPA2, EPA3, EPA4/5, EPA6, EPA7 and ACT1 as positive control.
| Strain | Genotype | % adherence |
|---|---|---|
| BG14 | Wild-type | 4.78 ± 2.15 |
| BG509 | rif1Δ | 30.02 ± 5.11 |
| BG676 | sir3Δ | 33.42 ± 4.85 |
| BG733 | EPA7::Tn7 | 21.13 ± 2.34 |
| BG1016 | rif1Δ::RIF1 | 4.08 ± 0.09 |
| BG1031 | sir3Δ::SIR3 | 3.84 ± 0.34 |
- Yeast cells were grown overnight into stationary phase and used in quantitative adherence assays as described in Experimental procedures. Numbers represent the mean of 3 experiments each done in triplicate. Strains analysed (see Table S2) include strains deleted for RIF1 and SIR3, and derivatives of those strains in which RIF1 or SIR3 have been restored. Also analysed is strain BG733 which contains a large insertion between EPA7 and its telomere.
To verify that disruption of SIR3 and RIF1 were responsible for the hyperadherent phenotype of the two mutants in SIR3 and RIF1, we generated new strains isogenic with our parental lab strain, in which the SIR3 and RIF1 coding regions were deleted (see Experimental procedures). In both cases, the deletion strains, BG676 (sir3Δ) and BG509 (rif1Δ) showed hyperadherent phenotypes demonstrating that disruption of SIR3 and RIF1 was in fact responsible for the hyperadherent phenotype (Table 1). Restoration of SIR3 or RIF1 in strains BG676 and BG509 reverted the strains to a non-hyperadherent phenotype, again showing that the hyperadherent phenotype was resulting from SIR3 or RIF1 disruption. All further experiments in this article were carried out with strains BG676 (sir3Δ) and BG509 (rif1Δ) and their derivatives rather than the original Tn7 insertion mutants.
RIF1 controls telomere length in C. glabrata
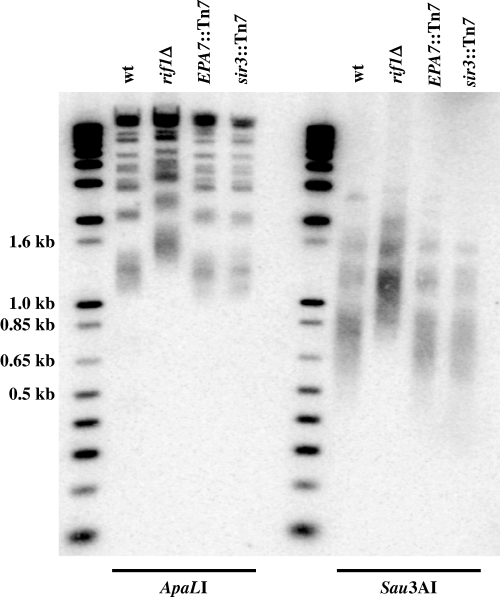
We sequenced the RIF1 gene (GenBank AY646924) and found that overall it was 24% identical and 41% similar to the S. cerevisiae RIF1 gene. Upstream of RIF1, we found an orthologue of PPS1 which is also located upstream of the same locus in S. cerevisiae. To determine if rif1Δ mutants in C. glabrata have defects in telomere length regulation, as expected based on the rif1Δ phenotype of S. cerevisiae, we carried out Southern analysis of genomic DNA from four strains: the wild-type strain, and three hyperadherent strains –BG509 (rif1Δ), BG542 (sir3::Tn7) and BG550, which carries the large insertion downstream of EPA7. We digested the DNA with either of two restriction enzymes and probed the blot with an oligonucleotide corresponding to the telomeric repeat. The resulting pattern shows a collection of fragments corresponding to the distal fragments of each chromosome (Fig. 1). Telomeres are not of uniform length in a population of yeast cells and so, in wild type cells the terminal telomeric fragments run as a smear, which is particularly obvious among the smaller bands on the gel. In the rif1Δ background, the average molecular weight of the fragments increases by 300–400 bp, corresponding to an increase in the number of telomere repeats and a concomitant increase in molecular weight of the telomeric fragments. This same effect is not seen in the genomic DNA from the other two hyperadherent mutants demonstrating that an increase in telomere length is not a common feature of hyperadherent mutants but is specific to the rif1Δ mutant. We conclude that in C. glabrata, as in S. cerevisiae, RIF1 is important for correct regulation of telomere length.
Average telomere length increases in a rif1Δ mutant. Genomic DNA from the wild-type strain (BG14), rif1Δ (BG509), a strain carrying a large insertion between EPA7 and its telomere (BG550) and a sir3::Tn7 strain (BG542), were digested with either ApaL I or Sau3A I and analysed by Southern blot using as probe a 32-mer containing two 16 bp -telomere repeat. The first lane of each set are labelled molecular weight markers.
Which EPA genes are responsible for the hyperadherent phenotypes of sir3Δ and rif1Δ strains?
Epa1 is able to mediate adherence to a variety of epithelial cells. We tested whether EPA1 overexpression in the sir3Δ or rif1Δ background might be responsible for the hyperadherent phenotype in those two backgrounds. rif1Δepa1Δ and sir3Δepa1Δ strains were still hyperadherent (see Table 3), suggesting that additional adhesins were induced in these two backgrounds. We also tested whether the four additional previously identified EPA genes (De Las Peñas et al., 2003) might be responsible for the sir3Δ or rif1Δ hyperadherent phenotypes by determining if they are even capable of mediating adherence to cultured epithelial cells. While EPA1 expressed in S. cerevisiae allows S. cerevisiae to bind to the Lec2 epithelial cell line (Table 2 and Cormack et al., 1999), we found that expression of EPA2, 3, 4 or 5 in S. cerevisiae did not result in adherence to Lec2 cells (Table 2). This could have been resulting from a failure of expression of EPA2−5 in S. cerevisiae. We do not have specific antibodies to any of the Epa proteins, and therefore could not directly monitor protein levels in these experiments. To partially address this issue, we made chimeric genes fusing the putative N-terminal ligand-binding domains of Epa1–5 with an epitope tagged C-terminal region of Epa1p. All five chimeric proteins were expressed at the S. cerevisiae cell surface as measured by labelling with antibody to the common epitope tag (data not shown), but only constructs containing the N-terminal ligand-binding domain of Epa1 were able to meditate adherence to epithelial cells (data not shown). This was consistent with the data in Table 2 and suggests that Epa2–5 do not mediate adherence to Lec2 cells.
| Strain | Genotype | % adherence |
|---|---|---|
| BG14 | wild-type | 4.2 ± 2.1 |
| BG509 | rif1Δ | 47.8 ± 4.6 |
| BG504 | rif1Δepa1Δ | 40.6 ± 6.2 |
| BG611 | rif1Δepa6Δ | 19.5 ± 5.6 |
| BG1098 | rif1Δepa7Δ | 32.9 ± 0.8 |
| BG999 | rif1Δ (epa1, epa6)Δ | 10.8 ± 5.0 |
| BG1099 | rif1Δ (epa1, epa7)Δ | 38.5 ± 12.4 |
| BG945 | rif1Δ (epa1, epa6, epa7)Δ | 5.4 ± 2.2 |
| BG1016 | rif1Δ::RIF1 | 4.1 ± 0.1 |
| BG676 | sir3Δ | 39.2 ± 10.7 |
| BG679 | sir3Δepa1Δ | 36.4 ± 8.4 |
| BG680 | sir3Δepa6Δ | 31.6 ± 9.5 |
| BG992 | sir3Δepa7Δ | 38.5 ± 8.6 |
| BG1007 | sir3Δ (epa1, epa6)Δ | 23.0 ± 6.0 |
| BG1012 | sir3Δ (epa1, epa7)Δ | 34.4 ± 3.6 |
| BG995 | sir3Δ (epa6, epa7)Δ | 26.3 ± 6.2 |
| BG966 | sir3Δ (epa1, epa6, epa7)Δ | 16.1 ± 5.8 |
| BG1031 | sir3Δ::SIR3 | 3.8 ± 0.34 |
| BY4742 | S. cerevisiae | 0.5 ± 0.15 |
- EPA genes important for the hyperadherent phenotypes of rif1Δ or sir3Δ strains. Quantitative adherence assays were performed as described in Experimental procedures. Numbers represent averages of 2 or 3 experiments each done in triplicate. Strains are described in Table S2. Note that adherence of the rif1Δ strain is mainly because of derepression of EPA1 and EPA6 while in a sir3Δbackground EPA1, EPA6 and EPA7 all contribute to adherence.
| Strain | Plasmid expressing | % adherence Lec2 |
|---|---|---|
| Sc96 | pVector | 0.24 ± 0.1 |
| Sc25 | pEPA1 | 62.43 ± 22.6 |
| Sc273 | pEPA2 | 0.47 ± 0.03 |
| Sc274 | pEPA3 | 0.44 ± 0.5 |
| Sc104 | pEPA4 | 1.14 ± 0.4 |
| Sc106 | pEPA5 | 0.81 ± 0.3 |
| Sc97 | pEPA6 | 31.36 ± 6.3 |
| Sc27 | pEPA7 | 53.39 ± 4.3 |
- EPA1, EPA6 and EPA7 mediate adherence when heterologously expressed in Saccharomyces cerevisiae. Yeast cells from S. cerevisiae strain BY4742 were transformed with the plasmids indicated that express EPA1, EPA2, EPA3, EPA4, EPA5, EPA6 and EPA7[plasmids pBC266, pBC273, pBC274, pMZ138, pMZ140, pIC109 and pIC100 (see TableS3)], or with the empty vector pBC169. Quantitative adherence assays were performed as described in Experimental procedures. Numbers represent averages of 2 or 3 experiments each done in triplicate.
These data suggested strongly that the hyperadherent phenotype of the sir3Δ and rif1Δ strains was because of misregulation of genes (possibly EPA genes) other than EPA1–5. As a first step in identifying the adhesins responsible for the hyperadherent phenotype of the sir3Δ and rif1Δ strains, we first characterized in more detail the complement of EPA genes present in BG2, our standard lab strain. We had previously characterized two loci containing a total of five EPA genes (De Las Peñas et al., 2003). Sequence of the C. glabrata genome (strain CBS138) revealed that there were a number of sequences with homology to the N-terminal ligand-binding domain of EPA1. Using PCR amplification of genomic DNA from strain BG2, our lab strain, we found that the majority of the EPA-related sequences from strain CBS138 were also present in BG2. We made of list of 16 distinct putative EPA-related sequences that were demonstrably present in strain BG2. For each gene, we designed specific oligonucleotides to assess transcript levels by RT-PCR or S1 nuclease protection (see Experimental procedures). We first assessed, using RT-PCR, whether EPA family gene expression was altered in a rif1Δ background. We found that expression for the majority of EPA genes was not significantly increased in the rif1Δ background. Expression of EPA2,3,4/5,6,7,12 and 15 were increased as measured by RT-PCR (TableS1), but only expression of EPA1 EPA4/5 and EPA6 was significantly affected in the rif1Δ background, as measured by S1 nuclease protection assay (Fig. 2, TableS1 and data not shown). EPA4 and EPA5 are present in the BG2 genome as an inverted repeat and are essentially identical (De Las Penas et al., 2003); because they cannot be distinguished in our S1 assay, we treat them as a single gene (EPA4/5) for the purposes of our analysis. In the sir3Δ mutant background there was a significant transcriptional induction of EPA1, EPA6 and EPA7 as determined by S1 nuclease protection assay (Fig. 2). Although the EPA6 and EPA7 genes are highly homologous at the nucleotide level (see below), the S1 probes used are specific for each gene, as shown by controls in Fig. 2, in which the EPA6 signal, but not the EPA7 signal is lost in an EPA6 null strain, while loss of the EPA7 signal occurs only with subsequent deletion of EPA7.
These experiments moved us to focus on EPA6 and EPA7 as potential adhesins responsible for the hyperadherent phenotypes in the sir3Δ and rif1Δ mutant strains.
EPA6 and EPA7 are two novel subtelomeric EPA genes encoding adhesins
The results above suggested that EPA6 and EPA7 were potentially responsible for the adherence phenotype of the rif1Δ and sir3Δ mutants. We identified three fosmids that contain EPA6 from a previously generated fosmid library of the C. glabrata genome (Castaño et al., 2003). Sequence analysis of one of the fosmids revealed that EPA6 is predicted to encode a GPI-anchored cell wall protein (GenBank AY646925). In the N-terminal ligand-binding domain, EPA6 is a close relative of EPA1, sharing about 72% amino acid identity. By contrast, the C-terminal region is quite different and shares little homology with any of EPA1–5. We expressed full length EPA6 as well as a chimeric protein in which the Epa6 N-terminal domain is fused to the epitope-tagged C-terminal domain of Epa1 in S. cerevisiae and tested how well the strains could adhere to epithelial cells. In both cases, the constructs conferred on S. cerevisiae the ability to adhere avidly to Lec2 epithelial cell (Table 2 and data not shown). Thus, EPA6, whose transcription is strongly induced in a rif1Δ background, can mediate adherence to human epithelial cells.
We next characterized the genomic region surrounding the EPA6 locus. Sequence of the EPA6 containing fosmid showed that upstream of EPA6, there was a long intergenic region of almost 10 kb before the next ORF (GenBank AY646925). The nearest upstream gene is predicted to encode an apparent homologue of the S. cerevisiae gene DAN1(Fig. 3). Like the EPA genes, DAN1 is predicted to be a GPI-anchored cell wall protein, but it does not share any homology to the EPA genes in the N-terminal ligand-binding domain. From fosmids containing EPA6, we obtained approximately 1 kb of non-coding sequence 3′ to the EPA6 ORF and used chromosome walking to clone additional sequence downstream of EPA6 (Experimental procedures). We found that EPA6, like EPA1–5 (De Las Peñas et al., 2003) is subtelomeric, being located 2.435 kb from the telomere (Fig. 3).
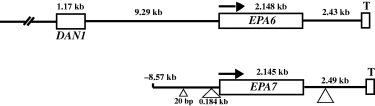
Structural map of the EPA6 and EPA7 loci. The overall structure of the EPA6 and EPA7 loci are very similar; both genes are transcribed toward their respective telomeres, and have very large intergenic regions. For the EPA6 locus, we have sequenced up to the next ORF (DAN1). For the EPA7 locus, we have sequenced 3.9 kb upstream of the EPA7 ORF. No ORF was found in this interval. Two insertions in the EPA7 promoter relative to EPA6 are indicated.
We cloned and sequenced the EPA7 locus from strain BG2 as well (GenBank Accession No. AY646926). EPA7 is highly related to EPA6, sharing 94% identity over the entire length of the gene. We expressed either full length EPA7 or a chimeric protein in which the Epa7 N-terminal domain was fused to the epitope-tagged C-terminal domain of Epa1 in S. cerevisiae and tested how well the strains could adhere to epithelial cells. In both cases, the constructs conferred on S. cerevisiae the ability to adhere avidly to Lec2 epithelial cell (Table 2 and data not shown). The genomic EPA7 locus is highly homologous to the EPA6 locus. We sequenced 3.94 kb upstream of EPA7 in strain BG2, and over this region there are only 80 single nucleotide differences with the EPA6 locus (97.9% homology). In addition, there are two insertions present in the EPA7 promoter region relative to EPA6: a 20 bp insertion 2.21 kb upstream, and a 0.184 kb insertion 409 bp upstream of the start site of translation. EPA7, like EPA6, is subtelomeric, located 2.496 kb away from the telomere (Fig. 3). The sequence between EPA7 and its telomere is also highly homologous to the EPA6 locus (97.43% identity). In this region, there is also a 63 bp insertion (1296 bases downstream of the stop codon) in EPA7 relative to EPA6.
EPA6 and EPA7 mediate epithelial adherence in the rif1Δ and sir3Δ strains
We showed above that EPA6 and EPA7 can both function as adhesins when expressed in S. cerevisiae, and that they are overexpressed in a sir3Δ or rif1Δ background. We were interested in whether they were necessary for the hyperadherent phenotype of sir3Δ and rif1Δ strains, or whether there might be other adhesins also derepressed in the sir3Δ or rif1Δ strains. We made a comprehensive series of EPA gene deletions in rif1Δ or sir3Δ backgrounds and measured the ability of the multiply mutant strains to adhere in vitro to Lec2 epithelial cells. Deletion of EPA1 alone or EPA7 alone in a rif1Δ did not reduce the adherence to Lec2 cells, while deletion of EPA6 in the rif1Δ background reduced adherence by about half (Table 3). When we deleted both EPA1 and EPA7 in the rif1Δ background, adherence to Lec2 cells was not decreased, whereas deleting EPA1 and EPA6 in this background strongly reduced adherence, showing that EPA1 and EPA6 both contribute significantly to the hyperadherent phenotype (Table 3). A rif1Δ strain in which EPA1, EPA6 and EPA7 are all deleted was approximately twofold less adherent than the rif1Δepa1Δepa6Δ strain, and adhered at levels comparable to the background adherence of the wild type strain.
Our analysis of the sir3Δ mutant yielded somewhat different results. As shown in Table 3, in vitro adherence to Lec2 cells did not decrease when either EPA1, EPA6 or EPA7 was deleted in a sir3Δ background. Even deletions of any two of these three EPA genes did not reduce adherence. However, the triple deletion of EPA1, EPA6 and EPA7 decreased adherence of a sir3Δ strain 2.4-fold. We conclude that in the sir3Δ background, the EPA1, EPA6 and EPA7 genes all do contribute to adherence, but that there are probably other adhesins also induced that mediate adherence even in the absence of EPA1, EPA6 and EPA7.
Subtelomeric localization of EPA6 and EPA7 is required for silencing
As described above, we initially isolated three hyperadherent mutants: the first two were the insertions in RIF1 and SIR3, described above; the third hyperadherent strain (strain BG550) contained a large insertion 423 bp downstream of EPA7. The large insertion in the original hyperadherent mutant increased the distance between EPA7 and its telomere, placing its promoter around 20 kb away from the telomere. The phenotype of this insertion mutant suggested that the distance between EPA7 and the telomere was critical for its repression. We therefore first tested whether EPA6 and EPA7 loci are subject to subtelomeric silencing and second whether that silencing depends on proximity to the telomeric sequences.
We integrated the URA3 gene at the EPA6 and EPA7 loci and used a 5-FOA plate assay to assess if it was silenced (Fig. 4A). We found that the EPA6 and EPA7 loci, like the EPA1−3 and EPA4−5 loci (De Las Peñas et al., 2003) were subject to transcriptional silencing because strains carrying the URA3 gene integrated at the EPA6 and EPA7 loci were able to grow in the presence of 5-FOA showing that URA3 gene at these positions is transcriptionally repressed (Fig. 4B). The subtelomeric silencing at both the EPA6 and EPA7 loci is dependent on some of the proteins known to be involved in subtelomeric silencing in S. cerevisiae. As shown in Fig. 4B we generated the same URA3 insertions at the EPA6 and EPA7 loci in three mutant strains – a rif1Δ strain, a sir3Δ strain and a rap1–21 strain in which the C-terminal 28 amino acids of Rap1 have been deleted rendering it unable to interact with the Sir complex (De Las Peñas et al., 2003). In all three backgrounds, and at both loci, the silencing of the URA3 gene was eliminated, demonstrating that repression of the loci requires intact RIF1, RAP1 and SIR3. Transcriptional repression of the EPA6 and EPA7 genes themselves depends on RIF1, RAP1 and SIR3 as well, because EPA6 and EPA7 transcription was derepressed in all three mutant backgrounds (Fig. 2).
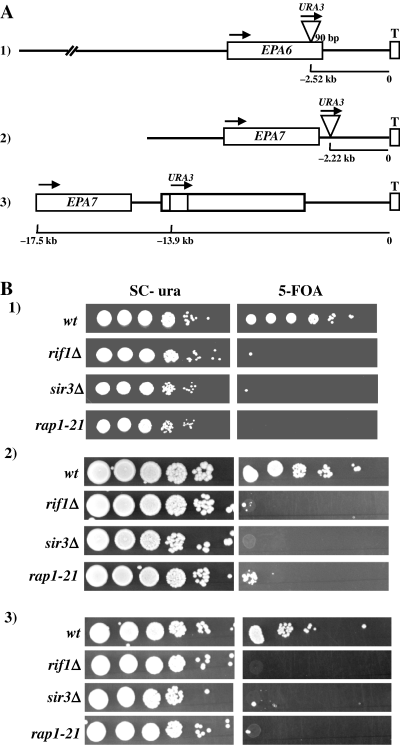
EPA6 and EPA7 are subject to subtelomeric silencing. A. Schematic representation of the position of three different insertions of Tn7 carrying the URA3 gene. In all cases, this gene is transcribed toward the telomere. B. URA3 placed between EPA6 or EPA7 and their telomeres, is subject to transcriptional silencing, which is dependent on RIF1, RAP1 and SIR3. Tn7 insertions 1–3 (Fig. 4A) were constructed in the wild-type, rif1Δ, sir3Δ and rap1–21 background strains. Equal numbers of cells were spotted in 10-fold serial dilutions onto SC-Ura and SC plates containing 5-FOA and the plates incubated for 48 h.
Consistent with silencing at the EPA7 locus being determined by distance from the telomere, we found that the extent of silencing, as measured by URA3 repression, changes as a function of distance from the telomere. We constructed strains with URA3 at two different positions at the EPA7 locus. Strain BG733 and BG826 contains the URA3 marker 13.9 kb and 4.89 kb from the telomere, respectively (see Fig. 4A insertions 2 and 3). As shown in Fig. 4B, the silencing of the URA3 gene, as measured by growth on 5-FOA plates, decreased as the URA3 marker was moved further from the telomere.
Deletion of RIF1 or SIR3 alters virulence in a systemic model of candidiasis
Because deletion of RIF1 and SIR3 altered expression of the EPA gene family, which we believe to be important in host colonization, we examined the role of these two genes in virulence using a systemic model of infection. We infected groups of 10 mice with five isogenic strains: BG462, a wild type C. glabrata strain, BG562, a derivative of BG462 in which the RIF1 ORF has been deleted, BG1033, a derivative of BG562 in which the RIF1 gene has been restored, BG808, a derivative of BG462 in which the SIR3 ORF has been deleted, and BG1043, a derivative of BG808 in which the wild-type SIR3 has been restored. The animals were sacrificed after 7 days and colony-forming units (cfu) from liver, kidney and spleen were determined. Interestingly, colonization of kidney by the rif1Δ or sir3Δ strains was significantly increased relative to the wild type parent strain (Fig. 5A and B). For assessing statistical significance, we calculated pooled P-values for pairwise comparisons (Mann–Whitney test). Strain BG562 (rif1Δ) showed significantly higher colonization of kidney than the wild-type strain BG462 (P = 0.023); restoration of RIF1 (strain BG1033) reversed the rif1Δ (BG562) mutant phenotype (P = 0.0065). To test if hypercolonization of kidney in the rif1Δ strain was related to derepression of EPA1 or EPA6, we compared colonization of the rif1Δ strain to strain BG1040, a derivative of the rif1Δ strain in which EPA1 and EPA6 were deleted. This strain showed some decrease in kidney colonization relative to the rif1Δ strain but this difference was not significant (P = 0.41). Deletion of SIR3 also resulted in increased colonization of kidney because strain BG808 (sir3Δ) showed significantly higher kidney colonization than the wild-type strain BG462 (P = 0.0004). No increase in kidney colonization was seen in which the SIR3 gene was restored (strain BG808) (P = 0.016). The hypercolonization of kidney in the sir3Δ strain depends, at least in part, on derepression of EPA genes because deletion of EPA1, EPA6 and EPA7 in the sir3Δ strain (strain BG1027), significantly decreased (P = 0.003) kidney colonization relative to the sir3Δ strain (strain BG808).
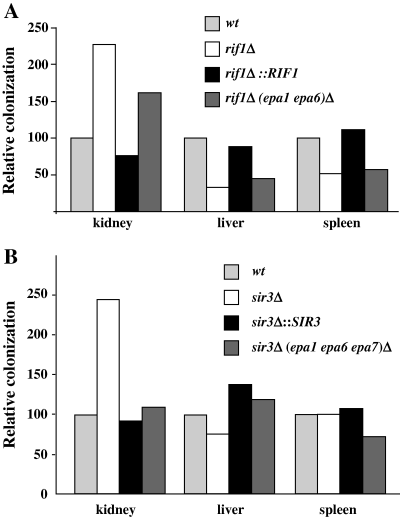
rif1Δ and sir3Δ mutants display increased colonization of the kidney relative to the wild-type strain. Groups of 10 mice were infected with ∼8 × 107 yeast cells and sacrificed at day 7 after infection. Kidney, liver and spleen were recovered and recoverable cfu assessed for each organ. Cfu for each mutant tested are expressed relative to those found for the wild-type strain. Numbers represent the mean of three independent experiments (30 mice total). A. Strains are: BG462 (wild-type), BG562 (rif1Δ), BG1033 (rif1Δ::RIF1) and BG1040 [rif1Δ (epa1 epa6)Δ]. B. Strains are: BG462 (wild-type), BG808 (sir3Δ), BG1043 (sir3Δ::SIR3) and BG1027 [sir3Δ (epa1, epa6, epa7)Δ].
There was no difference in recovered cfu from liver and spleen between the wild type and sir3Δ strains. By contrast, there was a reproducible and significant reduction in colonization of liver and spleen in the rif1Δ strain. Strain BG562 (rif1Δ) showed significantly lower colonization of liver (P = 0.0004) and spleen (P = 0.0004) relative to the wild-type strain (BG462). Restoration of the RIF1 gene reversed this reduction in colonization of both liver (P = 0.04) and spleen (P = 0.09); however, the reduction in liver and spleen colonization was not reversed by deletion of EPA1 and EPA6 in the rif1Δ background suggesting that the decreased colonization of liver and spleen in vivo may not be related to inappropriate derepression of EPA6 and EPA1 in the rif1Δ strain.
Discussion
Candida glabrata is an important agent of mucosal and systemic candidiasis. Adherence of Candida species to host cells is thought to be an important initiating step in virulence. In C. glabrata, in vitro adherence seems to be mediated largely by one adhesin encoded by the EPA1 gene. Deletion of this gene eliminates in vitro adherence. From our own studies (this article and De Las Peñas et al., 2003) as well as from the C. glabrata genome project, it is apparent that the genome encodes many EPA-related sequences, although the exact complement of intact EPA genes (as opposed to pseudogenes, for example) is not known. Assembly of the genome sequence of strain CBS138 is largely complete but unambiguous assembly of the repeat-rich subtelomeric regions and of the highly homologous EPA family remains to be done; as a result, the complement of full-length EPA genes in this strain is still unclear. In strain BG2, we have confirmed the presence of 16 sequences related to EPA1 in the ligand-binding domain, and have further confirmed that seven of these (EPA1–7) correspond to ORFs predicted to encode full-length GPI-anchored proteins. We have also assigned provisional names for EPA8−16 (see Table 4), but it remains to be seen if these correspond to full-length genes.
| Gene | Primer No. | Sequence |
|---|---|---|
| EPA1 | 1435 | GCCAGTTCTAGGGTAATTGGGATCTAAATATGCTGCATCCCAACATGGGTACGAACCCTTCTTCCGAAAATCTATCC |
| EPA2 | 1691 | GAATGATTTCCTTATTAAATTCTGTACTGAAAGAAAAATCTAAAACTGCATTGTTGTCCCTATTCACATAGAACAATCTAT ATCC |
| EPA3 | 1692 | GGATGAGTCCTTACCAATATTGTTAAAAAACATCCGAATTGGATAATAGAGATCCTTTTCCAAAAATAGACTTACTAATAA |
| EPA4/5 | 1558 | CTCTCTCTGTGTCAAATTCTGTAGTGAAAGAAAAATCTATAACAGCATTGTTATCCCTATTCACATAGAACATAGAC |
| EPA6 | 2143 | GCTTGTGGTAATGTATCAAACAGCGAAGTACACCCCATTGGGGCATGATAATAAAAATTTTATTTCCAGCAACATTTA GTTG |
| EPA7 | 2149 | GCTTGCCGGTAAATGATCTAATTCGGGTGTGCATCCTTTGGTTGCATGATAATAGAAATTTATATTACCGGTAACACCAA GTTG |
| EPA8 | 1548 | GGTTCCGTCGCACCATTTAAGTAATATGAAAAGTCCAAAGCTGCCCTACCATCTCTGTTGTTGAAAAACAATCTTAACC CTAT |
| EPA9 | 1551 | CTGTTGTTGTAAAACAACCTGATAGGATAATATGAATCCTCGAACAAGTACACTTCCAATTCTTGGTCAGCCTCATTT CCGG |
| EPA11 | 1553 | GGGAACCACCATCTCTGTTGTTGAAGAAAAGCCTTAGTGGGTAATAAACACCTTTTTCAAGGTAGACTGATTGT |
| EPA12 | 1554 | CCGTAGTAATCTCTGTTATTGAAAAAAAGTCTTAATGGATAGTATATCCCTTTATCTAAACGCACAGATTCA |
| EPA13 | 1555 | GCGATAGCATCTCTGTTGTTGAAAAACAACCGCAATGGATAATAAATGCCGCTTTCAAGATAGACAGTGACGTTTAACAA |
| EPA14 | 1552 | CTCGGAGTCTTTACCGATGTTATTATAGAACATCCTGATTGGATAGTAAATGTCTTTTTCAAGATTCACACTGTGGA |
| EPA15 | 1559 | GCAATATAATCTCGATTATTGTAGAAAAGTCTGATTGGATAATAAACACCCGTATGTAAATAAACAGTAAGTTCTAAAA |
| EPA16 | 1556 | CTTCACTAAATTCTGTCATCAATGTAAAACTTAAAGCACCATAATAATCTCTATTATTGAAAAATACTCACTAT |
- Because EPA4 and EPA5 are almost identical (De Las Peñas et al., 2003), the S1 primers cannot distinguish them and they are treated as one (EPA4/5).
We show here that EPA6 and EPA7, like EPA1, encode adhesins able to mediate adherence to epithelial cells. This represents the first evidence that the EPA family encodes a number of adhesins capable of binding mammalian cells. We do not know if the EPA2−5 genes also encode adhesins but if they do, then the ligands are likely not expressed on Lec2 cells because adherence to this cell type was mediated only by Epa1, Epa6 and Epa7 and not by Epa2, Epa3, Epa4 or Epa5. Notably, of the EPA genes analysed in detail to date, EPA1, EPA6 and EPA7 are more closely related to each other than to EPA2–5, and may form a subclass among all the EPA genes (data not shown). Epa1 is a lectin, regognizing LacNac-containing glycoconjugates (Cormack et al., 1999). It seems probable (based on sequence homology between EPA family members) that Epa6 and Epa7 (and potentially other members of the family) are lectins as well, but this remains to be tested.
If the C. glabrata genome encodes multiple adhesins, why does deletion of EPA1 essentially eliminate in vitro adherence to epithelial cells? The mutational analysis carried out here, combined with the subtelomeric location of EPA1−7, reveals that some EPA adhesins (notably EPA6 and EPA7) are subject to chromatin based silencing that normally represses their transcription. Stationary phase rif1Δ and sir3Δ cells are hyperadherent to epithelial cells and show an increase in transcription of EPA1 and induction of the normally silent EPA6 and EPA7. Thus, multiple genes in the EPA family in C. glabrata do encode adhesins, but at least some of them are normally kept transcriptionally repressed by the Sir-dependent chromatin silencing machinery. We show in this article that telomeric proximity is required for repression of EPA6 and EPA7 because insertions between each gene and the telomere result in derepression of the locus and a hyperadherent phenotype. In this regard, it is worth noting that the EPA6 and EPA7 loci are telomeric both in our strain BG2 (data from this article) and in the CBS138 strain whose genome was recently completely sequenced (http://cbi.labri.fr/Genolevures/elt/CAGL/). This suggests that the chromatin-based regulation of adherence described for strain BG2 may apply at least to some additional clinical isolates. While we have demonstrated that telomeric proximity seems to be important for silencing of EPA6 and EPA7, our experiments do not distinguish between silencing initiating from the telomeric repeats themselves or alternatively from a putative (and distal) silencer element.
What is the mechanistic basis for derepression of EPA6 and EPA7 in the rif1Δ and sir3Δ backgrounds? We can infer much about the function of C. glabrata Sir3 and Rif1 from S. cerevisiae, because the two species are closely related. This phylogenetic relatedness is reflected in the high degree of synteny between the two genomes; perhaps most pertinently, our preliminary inspection of the C. glabrata sequence shows that C. glabrata has homologues of essentially all the S. cerevisiae genes (with the exception of SIR1) implicated in transcriptional silencing. In S. cerevisiae, Sir3 is recruited to chromatin by the DNA binding protein Rap1 and is thought to play a structural role in silent chromatin. Thus, the requirement for SIR3 and RAP1 in silencing of EPA6 and EPA7 is interpretable in the context of current mechanistic models from S. cerevisiae.
What is the role of Rif1 in silencing of EPA6 and EPA7? Rif1 was originally identified in S. cerevisiae as a binding partner for Rap1. S. cerevisiae rif1Δ strains show an increase in telomere length, a loss of silencing at HMR and HML and an increase in silencing of marker genes placed adjacent to the telomeric repeats in S. cerevisiae (Hardy et al., 1992; Wotton and Shore, 1997). The rif1Δ silencing defects suggest a model in which the longer telomeres in a rif1Δ strain titrate Sir proteins from other chromosomal sites, reducing silencing at those sites, and increasing silencing of telomerically positioned marker genes. To our knowledge, the role of Rif1 in silencing of natural S. cerevisiae telomeres has not been analysed. In C. glabrata, rif1Δ strains show increased telomere length and a derepression of subtelomeric EPA expression (similar to the derepression of the S. cerevisiae HMR or HML loci). We cannot say whether EPA6 derepression in a rif1Δ strain is resulting from the loss of Rif1 directly (implying a mechanistic role for Rif1 in establishing or maintaining silent chromatin) or indirectly (for example, by titration of Sir proteins to the elongated telomeres). That said, if the effect of RIF1 on subtelomeric silencing is indirect in C. glabrata, it might suggest the existence of EPA gene-associated silencers similar to those that normally silence the HMR and HML loci in S. cerevisiae.
Does the regulation of the EPA genes by silencing imply that environmental conditions leading to derepression of one EPA gene would necessarily derepress the others as well? We do not think so, and believe that normal regulation of different EPA loci is likely to be complex. In support of this contention, our mutant analysis shows that EPA genes are differentially sensitive to loss of particular components of the silencing machinery. In particular, even though the EPA6 and EPA7 loci are highly homologous to each other, including near identity across 4 kb of upstream and 2.5 kb downstream non-coding sequence, EPA7 is only modestly induced in a rif1Δ background while EPA6 is strongly induced. Similarly, EPA7 is strongly induced in the sir3Δ background while EPA6 is less strongly induced. The same pattern can be seen with EPA1 and EPA4/5. In a sir3Δ background EPA1 is induced strongly and EPA4/5 only modestly, while in a rif1Δ background, EPA4/5 are induced strongly and EPA1 only modestly (see Fig. 2). The differential derepression of different adhesins in the various mutant backgrounds implies a complex regulatory system controlling the expression of individual members of the EPA family and suggests the possibility that transcription of different EPA family members might respond differently to particular environmental signals.
We show for the first time that multiple members of the EPA family actually encode functional adhesins. Epa1, Epa6 and Epa7 all function as adhesins when heterologously expressed in S. cerevisiae (Table 2). How many adhesins are present in C. glabrata and how many of these are regulated by chromatin-based silencing? The strains deleted for multiple EPA genes allow us to begin to address this question rigorously. While in a rif1Δ background, the in vitro adherence is primarily because of derepression of EPA1 and EPA6 (and, to a lesser degree, EPA7) in a sir3Δ strain, there are apparently other adhesins derepressed as well because deletion of EPA1, EPA6 and EPA7 reduces adherence of the sir3Δ strain by 2.5-fold but not to background levels. These data point to the existence of additional (perhaps EPA-related) adhesins also regulated by silencing.
The hypercolonization of kidney in the sir3 and rif1 strains implicates the EPA genes in the colonization of this tissue. It is apparent that the hypercolonization of kidney seen for the sir3Δ strain depends on the overexpression of EPA1, EPA6 and EPA7, because deletion of EPA1, EPA6 and EPA7 in the sir3Δ background reduces colonization of kidney. For the rif1Δ deletion strain, deletion of EPA1 and EPA6 did not significantly reduce hypercolonization of kidney. It is certainly possible, even likely, that a rif1Δ strain deleted for EPA1, EPA6 and EPA7 would show reduced colonization of kidney, similar to the effect seen in a sir3Δ background, but this remains to be tested. Nonetheless, we have shown clearly that silencing mutants show increased colonization of kidney and that in the case of sir3Δ mutants, that increase depends on inappropriate expression of certain EPA genes. This implicates Sir-mediated silencing in the normal regulation of EPA expression and in virulence. Consistent with our findings in this article, we have previously shown that a (epa1,2,3,4,5)Δ strain shows decreased colonization of kidney in systemic infections (De Las Peñas et al., 2003).
We do not yet understand the molecular basis for the decrease in colonization of liver and spleen seen for the rif1Δ strain. Because deletion of EPA1 and EPA6 in the rif1Δ background has no effect on recoverable cfu from liver or spleen, we would argue that derepression of EPA1 and EPA6 is not responsible for the decreased colonization of liver and spleen. We favour a model in which silencing-independent pleiotropic effects of the RIF1 deletion (perhaps by titration of Rap1 from essential genes) results in loss of fitness in liver and spleen. This view is consistent with the fact that there is no decrease in liver or spleen colonization in the sir3Δ background, which has telomeres of normal length and exhibits complete loss of subtelomeric silencing.
In an accompanying article, Iraqui et al. show that EPA6 is implicated in formation of model biofilms. Mutants disrupted for SIR4 or RIF1 show increased adherence to plastic, while mutants that disrupt EPA6 show decreased adherence to plastic. Their results are overall highly consistent with our own and suggest that induction of EPA6 might normally have a role in adherence to plastic or to other yeast during biofilm formation. Some differences between their article and ours as to the normal expression levels of EPA6 and EPA7 in stationary phase (they suggest that EPA6 and EPA7 are normally both expressed in late stationary phase) are likely attributable to the different methods used for detection of RNA. For example, we too can detect transcript for EPA6 and EPA7 in wild type strains during in vitro growth using RT-PCR (TableS1). However, when quantitative S1 analysis is used (Fig. 1), large differences in expression levels, not easily detected by RT-PCR, become apparent.
Lastly, while we have shown that EPA1, EPA6 and EPA7 are subject to chromatin-based silencing, and that disruption of silencing by deletion of SIR3 or RIF1 impacts virulence, we do not yet know when and where these two genes are normally transcribed at appreciable levels. It will be of considerable interest to understand the role of EPA6 and EPA7 in normal C. glabrata infections as well as the environmental cues (particularly those in the host) that normally serve to induce EPA6 and EPA7.
Experimental procedures
Strains
All strains used in this study are listed in TableS2. Strain BG676 (sir3Δ) has been previously described (De Las Peñas et al., 2003).
Plasmids
All plasmids used in this work are summarized in TableS3.
Primers
All primers used for cloning, RT-PCR, and sequencing are described in TableS4.
Epithelial cell lines and growth and fixing conditions
Lec2 cells are CHO-derived epithelial cells (ATCC No. CRL-1736). A-498 cells are human epithelial cells from kidney (ATCC No. HTB-44), and T24 cells are human epithelial cells from bladder (ATCC No. HTB-4).
All epithelial cell lines were grown according to supplier's instructions, in media supplemented with 10% fetal bovine serum. Cells were seeded in 24-well plates and allowed to grow for 24–48 h at 37°C until the monolayer was confluent (∼105cells per well). The media was aspirated and cells were fixed with 2% formaldehyde in PBS for 1–2 h. Each well was washed 4–5 times with PBS. One millilitre of PBS supplemented with Pen/Strep (Penicillin 100 U ml−1, Streptomycin 100 µg ml−1) was added to each well for storage at 4°C for several months.
Media and growth conditions
Yeast cells were grown on standard yeast media as described (Sherman et al., 1986), and 2% agar was added for plates. Synthetic complete (SC) contains YNB without NH2SO4, 1.7 g l−1, NH2SO4 5 g l−1, and supplemented with 0.6% of casamino acids and 2% glucose. When needed, SC was supplemented with 25 mg l−1 of uracil. To score for resistance to 5-fluorotic acid, 1.1 g l−1 5-FOA and 25 mg l−1 of uracil was added to SC. YPD contains yeast extract 10 g l−1, peptone 20 g l−1, supplemented with 2% glucose. To plate tissue homogenates, YPD plates were supplemented with Pen/Strep (Penicillin 100 U ml−1, Streptomycin 100 µg ml−1). Bacterial media were prepared as described (Ausubel et al., 1987), and 1.5 g l−1 of agar was added for plates. Luria-Bertani (LB) media contains bactopeptone 10 g l−1, yeast extract 5 g l−1 and NaCl 10 g l−1. When needed LB plates were supplemented with 30 mg l−1 of kanamycin, or 100 mg l−1 of carbenicillin. Phosphate buffered saline (PBS) contains 8 g l−1 NaCl, 0.2 g l−1 KCl, 1.15 g l−1 Na2HPO4.7H2O, 0.2 g l−1 KH2PO4.
Escherichia coli strain BW23473 (Table S2) was used for maintenance of conditional replicons carrying R6Kγ origin of replication. Otherwise, strain DH10 (Gibco BRL) was used routinely for electroporation of plasmids.
Media for growth of the CHO-derived epithelial cell line Lec2 (ATCC CRL-1736) is Alpha minimum essential medium (α-MEM)supplemented with 10% fetal bovine serum. Media for growth of A498 cell line was α-MEM supplemented with 2% sodium pyruvate, non-essential aminoacids and 10% fetal bovine serum. T24 cells were grown in McCoy medium supplemented with 10% fetal bovine serum.
Transformation
Yeast transformation with linear or supercoiled plasmids was done as previously described (Castaño et al., 2003).
Sequence
To retrieve DNA flanking the insertion mutation from either the library made by non-homologous insertion of YIplac211 (Cormack et al., 1999), or the library generated by homologous recombination of in vitro generated Tn7 insertions in C. glabrata genomic clones (Castaño et al., 2003), genomic DNA from the mutants of interest was prepared, digested with either HindIII or EcoRI for the YIplac21I library, or with SpeI or XbaI for the Tn7 library, ligated and transformed into E. coli strain BW23473. Plasmid DNA from the recovered mutants was prepared using Qiagen minipreps and then used as templates for sequencing with universal primers for YIplac211-derived vectors, and primers ♯661 and ♯662 (TableS4) for Tn7 vectors. These primers anneal to the Tn7 ends and face outward.
Retrieving telomeres downstream of EPA6 and EPA7
To clone the fragment downstream from EPA6 containing the telomere, we first cloned a 1.025 kb PCR fragment containing the last 448 nt of the coding sequence of EPA6 and 577 bp downstream from the EPA6 ORF into vector pNEB193 using the fosmid 6–77 (which contains EPA6) as template and primers ♯1693 and ♯1694. The resulting plasmid was mutagenized with Tn7 as described (Castaño et al., 2003) and one insertion (pSP121, TableS3) was selected to integrate by homologous recombination back into C. glabrata to generate strain BG582; insertion at the correct site was verified with primers external to the cloned and mutagenized fragment. This insertion is 98 bp upstream of the stop codon of EPA6. Genomic DNA from BG582, was digested with KpnI and blunted with T4 polymerase and religated. This plasmid was sequenced using primers ♯1685, ♯1791, ♯1799 and ♯1844; and shown to contain at least 12 repeats of the C. glabrata telomere repeats.
To clone the fragment downstream from EPA7 containing the telomere, we integrated plasmid pIC62 by homologous recombination at the NheI site of EPA7 generating a partial duplication of 1420 bp of the EPA7 locus. Insertion at the correct chromosomal site was verified by PCR as above. Genomic DNA from this strain (BG748) was prepared and digested with BstEII, blunted with T4 polymerase, religated and transformed into BW23473 electrocompetent cells. The resulting plasmid (pIC83) was sequenced with primers ♯ 1824, ♯1799 and ♯1844 and the sequence revealed at least 12 repeats of the telomere repeat of C. glabrata.
Cloning of EPA7
Because EPA7 is not represented in our C. glabrata genomic library (it is very close to the telomere and therefore under-represented), we cloned the full length EPA7 gene from strain BG826 which contains an insertion of Tn7 273 bp downstream of EPA7. Genomic DNA from this strain was digested with BseRI and BsrBI, blunted with T4 polymerase and ligated and transformed into electrocompetent BW23473 cells. The resulting plasmid (pIC90) contained full length EPA7 plus 3.944 kb of upstream sequences and 1.087 kb of downstream sequences. pIC90 was sequenced with primers ♯1617, ♯1648, ♯1697, ♯1868 and ♯1873.
Construction of deletion strains
We used the two-step gene replacement strategy to generate the deletion strains used in this work. Briefly, we cloned two fragments, one immediately upstream and one immediately downstream of the gene to be deleted in the integrative URA3 plasmid pRS406 (Sikorski and Hieter, 1989) so that they are contiguous. The plasmid was linearized with an enzyme that cuts in either of the two cloned fragments and then transformed into C. glabrata selecting for Ura+ transformants on SC-Ura plates. Integration at the correct locus was verified by PCR analysis of the integrant using a primer external to the cloned fragment and one primer that anneals with the plasmid. The integrants were streak purified on SC-Ura plates and then grown in YPD overnight and appropriate dilutions were plated on SC + 5-FOA plates to select for recombinants that had lost the integrated plasmid and therefore were rendered Ura–. The resulting Ura– segregants have either regenerated the wild-type locus, or have left the deletion constructed in the plasmid (the 5′-and the 3′ fragments adjacent to each other). Genomic DNA was prepared from several 5-FOAR (Ura–) segregants and PCR analysis was carried out using primers outside from the cloned fragments to confirm the genomic structure of the deletion derivatives. Strains generated by this procedure are listed in TableS2, and the plasmids used to make them are described in TableS3.
Southern blot for telomere length
Genomic DNA was isolated from wild-type strain BG14, rif1Δ (BG509), sir3::Tn7 (BG542) and EPA7::Tn7 (BG550) and digested with either ApaIl or SauIIIA, and about 5 µg of DNA were run on a 0.8% agarose gel and transferred to a Hybond-N membrane (Amersham-Pharmacia Biotech). The probe was a 32-mer oligonucleotide containing two copies of the C. glabrata telomere repeat (primer ♯1682, TableS4) end-labelled with [γ-32P]-ATP using T4 polynucleotide kinase (New England Biolabs). Conditions for the Southern were performed as previously described (Castaño et al., 2003).
In vitro adherence assays
Yeast cells (S. cerevisiae or C. glabrata) were grown in 3 ml of SC + uracil overnight at 30°C, 0.6 µl of this overnight were inoculated in 3 ml of fresh media with [35S] Express protein labelling mix (New Life Sciences Products Perkin Elmer Cat No. NEG 072), 132 µCi per culture. After 24 h growth at 30°C, cells were spun and washed three times with Hanks Balanced Salt Solution (HBSS) supplemented with 5 mM CaCl2. Cells were resuspended in the same solution with 5 mM CaCl2 and OD600 was adjusted to 0.1. One millilitre of labelled yeast cell suspension was added to each well of 24-well plates containing previously fixed and washed epithelial cells in 500 µl of HBSS with CaCl2 was added. The plates were spun down for 1 min at 1000 r.p.m. and incubated at room temperature for 10 min. Non-adherent yeast cells were washed four to five times with HBSS with CaCl2. Adherent cells were recovered by lysing the epithelial cells with 500 µl of 0.1% triton 0.5% SDS in PBS. The cells were scraped off the plate and counted in 4 ml of scintillation liquid. Adherence is calculated as the radioactive counts that remain associated with the epithelial cells expressed as a percentage of the total counts added. Most experiments were carried out using Lec2 cells and the data shown are the result of at least two experiments performed in triplicate.
RT-PCR
RNA was extracted from the stationary phase cells (grown for 24 h) for wild-type (BG14) and the rif1Δ (BG509) strains and used for RT-PCR synthesis using the appropriate primers, shown below. cDNA synthesis and PCR were carried out as previously described (De Las Peñas et al., 2003). The following primers and temperatures that were used in this study: RT primers are EPA1, TAACAGTGTTTTCGTTTGAT; EPA2, GAATGATTTCCTTATTAAAT; EPA3, TAATTTGATCAG TAGCACCG, EPA4, GTCAAAATTCTGTAGTGAAAG; EPA4/5, GTCAAATTCTGTAGTGAAAG; EPA6 TTACAGGGTTCG GATCTGAC; EPA7, TTACAGGGTTCGAATCTGAC EPA8, TTCCTGGTTCCGTCGCACCA; EPA9, GGCCTAATGAG GCTGACCAA; EPA11, TTAGTGGAGCCGGAGAATCG; EPA12, TTATGCGCTCTGATCCATGT; EPA13, TTTGCCGA ATAGGCGAGTCA; EPA14, TGATACGTGTAGGCGCACCA; EPA15, TTATTATTGCATTTGAATGC; EPA16, TAACAATT TCTTCACTAAAT and ACT1, GGCTTTCGATTTCTCACC. The cDNA synthesis reaction was carried out at 55°C for EPA1, EPA2, EPA3, EPA4/5, EPA6, EPA7, EPA13, EPA14, EPA15, EPA16; and 62°C for EPA8, EPA9, EPA11, EPA12. The PCR primers were the same for all the reverse primers, and the forward primers were: EPA1, GGGCTCAAAAA CAGCTAAAG; EPA2, GGGATCAGATTATGCAAAAG; EPA3, GCATGTTGATAGTTCCAAAA; EPA4/5, GCTAACATTACTG TATTTCT; EPA6, GGGTTCTCAAACAGCTAAGG, EPA7, GGGTTCTCAAACAGCTAAGG; EPA8, GGGCGGGTACCA GAAAAGAC; EPA9, GGCCTAATGAGGCTGACCAA; EPA11, GGCCCAATCAAGATAAGAAT; EPA12, GGGAAGGCGA ATAGTGCGTA; EPA13, GGCCAGGCGTGAACAAAAAC; EPA14, GCATGTTGACCATTCCAAGA; EPA15, GGGCAA AAAAAGCCTCAAAA, EPA16, GGGGCTCAAAAAATGCAA AAA and ACT1, GTGGCAACGGTTTGATGC.
S1 nuclease protection assay
For the assay in Fig. 1, cells were grown for 24 h into stationary phase. RNA was extracted as previously described (De Las Peñas et al., 2003). Probes (listed in Table 4) were end labelled using [γ-32P]-ATP with T4 polynucleotide kinase. Thirty microgram of RNA from each strain (wild-type, rif1Δ and sir3Δ) was hybridized with each end-labelled probe at 55°C overnight. The mix was digested at room temperature with 150 units of S1 nuclease (Invitrogen) for 30 min The samples were then extracted with phenol, precipitated and resuspended in 17 µl of 1X loading buffer. Five microlitre of each sample was separated by electrophoresis on a 10% acrylamide gel, and the signal detected using a phosphorimager.
Animal studies
Yeast cells from each strain were grown overnight in YPD at 30°C. Cells were collected by centrifugation and washed once with PBS. Cells were then resuspended in PBS at a concentration of ∼8 × 108 cells ml−1. The number of cells was determined by OD600, by counting the cells in a hemacytometer. Total viable counts were confirmed by plating appropriate dilutions on YPD plates and assessing colony number after 24 h incubation (30°C). Eight to nine week old Balb/C mice (Taconic) were injected with ∼8 × 107 yeast cells in a total volume of 0.2 ml of the cell suspension in PBS by tail vein injection. In each experiment, groups of 10 mice were infected per strain. These were sacrificed after 7 days and the kidneys, liver and spleen were harvested and homogenized. Dilutions of the homogenates were plated on YP Agar supplemented with Pen/Strep. Cfu were scored the following day. Geometric means for all the groups were calculated and wild-type values for each organ were standardized to 100%. Numbers for each mutant strain tested are expressed relative to wild-type. Each graph represents the mean of three experiments (30 mice total per strain). Pooled P-values were calculated for pair-wise comparisons from the primary data using the Mann–Whitney test.
Acknowledgements
We thank Rich Hebel for help with the statistical analyses. We thank Guilhem Janbon for communication of results prior to publication. We thank members of the Cormack Lab and Scott Erdman for careful reading of the manuscript. This work was supported by Grants RO1 AI46223 and 2PO1 DK49720 to B.P.C.
Supplementary material
The following material is available from http://www.blackwellpublishing.com/product/journals/suppmat/mmi/mmi4465/mmi4465sm.htm
TableS1. RT-PCR analysis of the RNA expression of EPA family members.
TableS2. Strains used in this work.
TableS3. Plasmids used in this study.
TableS4. Oligonucleotides used in this study.




