Retinal biosynthesis in Eubacteria: in vitro characterization of a novel carotenoid oxygenase from Synechocystis sp. PCC 6803
Summary
Retinal and its derivatives represent essential compounds in many biological systems. In animals, they are synthesized through a symmetrical cleavage of β-carotene catalysed by a monooxygenase. Here, we demonstrate that the open reading frame sll1541 from the cyanobacterium Synechocystis sp. PCC 6803 encodes the first eubacterial, retinal synthesizing enzyme (Diox1) thus far reported. In contrast to enzymes from animals, Diox1 converts β-apo-carotenals instead of β-carotene into retinal in vitro. The identity of the enzymatic product was proven by HPLC, GC-MS and in a biological test. Investigations, of the stereospecifity showed that Diox1 cleaved only the all-trans form of β-apo-8′-carotenal, yielding all-trans-retinal. However, Diox1 exhibited wide substrate specificity with respect to chain-lengths and functional end-groups. Although with divergent Km and Vmax values, the enzyme converted β-apo-carotenals, (3R)-3-OH-β-apo-carotenals as well as apo-lycopenals into retinal, (3R)-3-hydroxy-retinal and acycloretinal respectively. In addition, the alcohols of these substrates were cleaved to yield the corresponding retinal derivatives.
Introduction
Retinal and its derivatives (retinol, retinoic acid) are carotenoid cleavage products with essential biological functions. They are common in animals, green algae and archaebacteria. In addition, recently discovered rhodopsins (Spudich et al., 2000; Jung et al., 2003) suggest the occurrence of retinal in Eubacteria and fungi. However, there is no report of the formation of retinal or its derivatives in the eubacterial domain of life.
The retinal derivative retinoic acid exerts important signalling functions in vertebrates, influencing developmental processes by binding nuclear receptors (Giguere et al., 1987; Pektovich et al., 1987). There are two known families of retinoic acid receptors (Leid et al., 1992). Members of the retinoic acid receptor (RAR) family bind all-trans as well as 9-cis-retinoic acid isomers. In contrast, retinoic acid Xreceptors (RXRs) show narrow isomer specificity, binding only 9-cis-retinoic acid (Levin et al., 1992; Mangelsdorf and Evans, 1995). Retinal functions as a chromophore of the photoreceptor rhodopsin in animals and green algae as well as of the archaeal rhodpsins in Halobacteria.
In animals, the 11-cis-retinal chromophore is converted to the all-trans isomer upon light stimulation. This leads via the G-protein transducin to a hyperpolarization of the plasma membrane in rod cells (Spudich et al., 2000).
The primary phototransduction event in archaeal rhodopsins is the photoisomerization of all-trans-retinal to the 13-cis form (Spudich et al., 2000). There are four archaeal rhodopsins in Halobacterium salinarum. Two of them, bacteriorhodopsin and halorhodopsin, act as light-driven ion pumps utilizing light energy to transport protons and chloride, respectively, across the cell membrane. The other two, sensory rhodopsin I and II, are phototaxis receptors that control via tightly bound transducers a phosphorylation cascade modulating the cell's flagellar motors (Spudich, 1998).
In green algae, e.g. Chlamydomonas, the all-trans-retinal polyene chromophore isomerizes upon light stimulation to 13-cis and activates a photoreceptor channel, leading to a rapid Ca2+ influx into the eyespot region. The Ca2+ fluxes represent the molecular basis for phototaxis (Hagemann, 1997). Recently, a new type of rhodopsin, channelrhodopsin, has been reported from Chlamydomonas reinhardtii. Channelrhodopsin-1 (Chr1) represents a combined photoreceptor and ion channel exhibiting mechanistic similarities to the proton pump bacteriorhodopsin (Nagel et al., 2002).
Database information for sequenced genomes have recently revealed archaeal rhodopsin homologues in eubacterial species. For instance, Jung et al. (2003) reported on a sensory opsin from the cyanobacterium Nostoc sp. PCC 7120. The heterologously expressed protein was capable of binding all-trans-retinal and produced a visibly absorbing pigment. Recent data also suggest that retinal biosynthesis is common in non-photosynthetic Eubacteria. Béjàet al. (2000) identified a new type of rhodopsin, proteorhodopsin, in the genome of the uncultivated γ-proteobacterium SAR86. Proteorhodopsin shares significant homology with archaebacterial rhodopsin, and when expressed in Escherichia coli, it constituted an active, light-driven proton pump that contained bound retinal. This previously unrecognized retinal-dependent phototrophic pathway was directly demonstrated through photochemical analyses of native cell membranes of bacterioplancton (Béjàet al., 2001). Genes encoding proteorhodopsin homologues are not restricted to γ-proteobacteria and have been recently identified in divergent marine bacterial taxa (Torre et al., 2003).
In animals, the C20-compound retinal is formed through symmetrical oxidative cleavage of β-carotene (C40) at the central C15–C15′ double bond. The corresponding enzyme 15,15′ BCO (β-β-carotene-15,15′-oxygenase), also named BCO I, has been identified from Drosophila melanogaster (von Lintig and Vogt, 2000) and mammals (Redmond et al., 2001). It has been also shown that the 15,15′ BCO acts as a monooxygenase (Leuenberger et al., 2001). A second BCO (β-β-carotene-9′,10′-oxygenase), also named BCO II, has been reported more recently from animals. It catalyses the asymmetric cleavage of β-carotene at the C9′–C10′ position, forming the volatile compound β-ionone (C13) and β-apo-10′-carotenal (C27) (Kiefer et al., 2001). β-apo-10′-carotenal may represent a precursor of retinoic acid, which is thought to arise through β-oxidation-like reactions (Barua and Olson, 2000). In addition to BCO I and BCO II, the carotene oxygenase family from animals contains a third enzyme that is abundant in retinal pigment epithelial cells, but the RPE65 (retinal pigment epithelium 65) protein seems to be an all-trans-retinyl ester-binding protein rather than an oxygenase (Mata et al., 2004).
The identification of carotenoid oxygenases has become possible through their sequence homology to VP14 (viviparous14) from maize, the first carotenoid cleavage enzyme ever described. VP14 represents a non-haem iron oxygenase that catalyses the oxidative cleavage of 9-cis-violaxanthin and 9′-cis-neoxanthin, leading to xanthoxin, the precursor of the phytohormone abscisic acid ABA (Schwartz et al., 1997). Based on their substrate specificity, VP14 and its orthologues have been classified as NCEDs (nine-cis-epoxy-carotenoid dioxygenases). This cleavage reaction seems to be key regulatory in the biosynthesis of ABA. For instance, the in vivo mRNA and protein levels of PvNCED1 (Phaseolusvulgaris NCED1) correlated well with the ABA content (Qin and Zeevaart, 1999). In addition, the overexpression of AtNCED3 (Arabidopsisthaliana NCED3) caused an increase of endogenous ABA amounts and promoted the expression of ABA- and drought-inducible genes (Iuchi et al., 2001). Similar results were obtained by transforming Nicotiana plants either with LeNCED1 from Lycopersiconesculentum (Thompson et al., 2000) or with PvNCED1 (Qin and Zeevaart, 2002).
The cloning of VP14 also led to the identification of additional carotenoid cleavage enzymes from plants which are involved in the synthesis of apo-carotenoid pigments, such as bixin in Bixa orellana (Bouvier et al., 2003a) and saffron in Crocus sativus (Bouvier et al., 2003b), or of volatile compounds like β-ionone in A. thaliana (Schwartz et al., 2001).
Database information for sequenced genomes reveals the occurrence of homologous enzymes with unknown function in many species (Guiliano et al., 2003). For instance, the genome of A. thaliana encodes nine putative carotenoid oxygenases (Tan et al., 2003). Apart from AtNCED3 and AtCCD, there are no reports on their enzymatic characterization.
Enzymes with striking homology are also common in many Eubacteria. For example, the genome of the freshwater unicellular cyanobacterium Synechocystis sp. PCC 6803 encodes two such proteins [open reading frames (ORFs): sll1541, slr1648]. Based on significant homology to the bacterial enzyme lignostilbene-α,β-dioxygenase (LSD) from Pseudomonas paucimobilis (Kamoda and Saburi, 1993), the ORF sll1541-encoded protein (Accession No. BAA18428, here named Diox1) is annotated as a lignostilbene dioxygenase in the databases. LSD catalyses the cleavage of the Cα–Cβ bond in dimeric lignin to yield two molecules of vanillin. However, lignin degradation activity has not been reported for Synechocystis sp. PCC 6803 so far. But carotenoid cleavage activity is found in several cyanobacterial species. For instance, Jüttner and Höflacher (1985) showed the cleavage of β-carotene at the C7–C8, C7′–C8′ positions in the cyanobacterium Microcystis. This reaction led to the formation of two molecules of the volatile compound β-cycloctitral (C10) and one molecule of the di-apo-carotenal crocetindial (C20). In addition, some carotenoid cleavage products represent biologically active compounds that inhibit the growth of several cyanobacterial species (Jüttner, 1979a). In the light of these findings, we investigated the ability of Diox1 from Synechocystis sp. PCC 6803 to cleave carotenoids. Here, we report on Diox1 as the first retinal-forming enzyme from Eubacteria and the first apo-carotenoid-cleaving enzyme known to date.
Results
Diox1 homologues are widespread in cyanobacteria
Diox1 from the cyanobacterium Synechocystis sp. PCC 6803 shows significant homology to the animal β-carotene-cleaving enzymes BCO I and BCO II. Moreover, all sequenced cyanobacterial genomes reveal at least one gene with striking similarity to Diox1. The similarity at the amino acid sequence level (see Fig. 1) varies between about 67% (BAB75983, from Nostoc sp. PCC 7120) to about 55% (CAE22054 from Prochlorococcus marinus str. MIT 9313). Indicating their biological relevance, Diox1 homologues are common in cyanobacterial taxa independent of their ecotype and cellular organization. They occur in filamentous, freshwater Anabaena species, as well as in the unicellular, marine Synechococcus sp. WH 8102. Cyanobacterial Diox1 homologues have been annotated as LSD (lignostilbene-α,β-dioxygenase) or RPE65 (retinal pigment epithelium 65) in the databases.
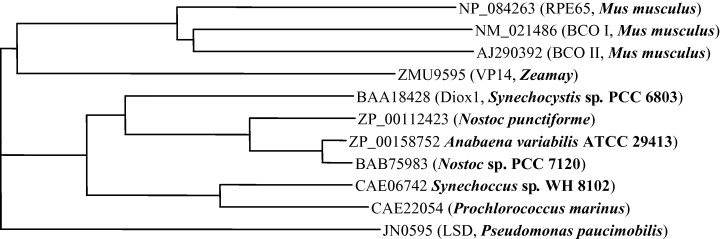
Phylogenetic tree of selected oxygenases. BCO I, β-β-carotene-15,15′-oxygenase; BCO II, β-β-carotene-9′,10′-oxygenase; RPE65, retinal pigment epithelium 65. Diox1 and putative retinal forming enzymes from other cyanobacteria group together. Alignments were performed using Vector NTITM Suite (InforMax, UK).
Apo-carotenoids but not carotenoids are cleaved by Diox1
To investigate its ability to cleave carotenoids, we expressed Diox1 in carotenoid-accumulating E. coli cells. While no enzymatic activity was detectable in β-carotene- or lycopene-accumulating cells, the expression of Diox1 in zeaxanthin background led to the disappearance of biosynthetic side-products that are present in small amounts in these cells. The UV/Vis spectrum of these side-products showed apo-carotenal characteristics, e.g. lacking UV/Vis spectral fine structure and eluting in relatively polar fractions in RP-HPLC separations (data not shown). The small amount did not permit the elucidation of the molecular identity, so we investigated the substrate specificities in vitro using the overexpressed protein with added carotenoid and apo-carotenoid substrates.
Diox1 was expressed as a GST (gluthatione-S-transferase) fusion protein, purified using gluthatione-sepharose and released by the protease thrombin. In vitro assays with purified individual carotenoids from Synechocystis as well as total carotenoid extracts revealed that none of the major carotenoids such as echinenone or zeaxanthin was cleaved. However, when total carotenoid extracts were applied as substrates, we observed again the disappearance of minor compounds that were similar to the ones from E. coli mentioned above. But again, the amounts present did not allow their purification, structural elucidation or individual use in vitro. As a consequence, we resorted to testing the enzymatic activity of Diox1 with synthetic β-apo-carotenals.
A striking activity was observed with β-apo-8′-carotenal. The red colour of the substrate in the assay turned to a light yellow, indicative of cleavage (Fig. 2). Diox1 converted β-apo-8′-carotenal into a compound that resembled retinal in its UV/Vis spectrum and its chromatographic behaviour on HPLC. To prove its identity, GC-MS analyses were carried out using retinal as a standard, which showed an identical retention time. The mass spectrum obtained was identical with published data (Robinson et al., 1998) and with spectra in the NIST database, including the presence of the correct molecular ion of m/z = 284 (Fig. 2), thus proving that Diox1 converted the C30 compound β-apo-8′-carotenal into retinal (C20). The catalytic activity of Diox1 must also lead to a second product(s) with a chain-length of C10 or shorter. Indeed, as can be seen in Fig. 2, a second product appeared in the HPLC analysis. Given that Diox1 cleaves only at the C15–C15′ double bond (see Fig. 5), the formation of apo-8′, 15-apo-carotene-dial (2,6-dimethyl-octa-2,4,6-trien-dial, C10O2H11) must be expected. The product spectrum obtained with a maximum at 327 nm underscored the presence of a pentaene. To verify this structure, the compound was isolated by TLC and applied to GC-MS (Fig. 2). The chromatographic characteristics and the obtained chemical ionization (CI) mass spectrum were identical to those obtained with the authentic reference compound kindly provided by BASF (Ludwigshafen, Germany), including the correct molecular ion of m/z = 165.1 [M+H]+. These data indicate that the cleavage site of Diox1 is the 15–15′ double bond (see Fig. 5).
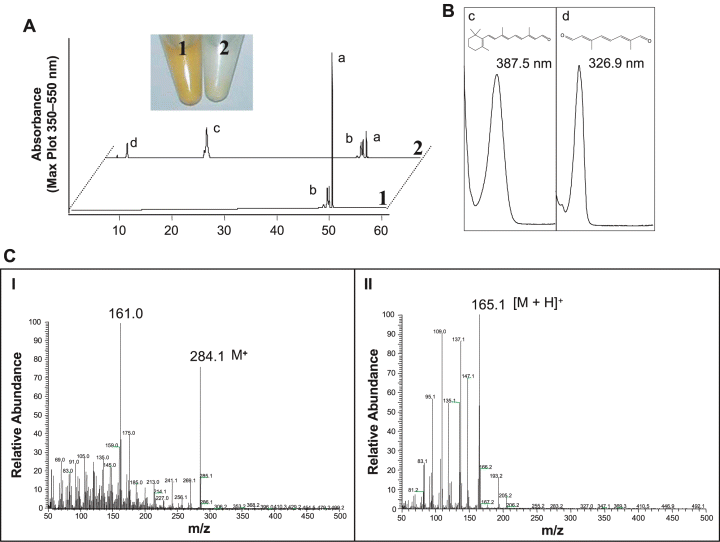
Analysis of in vitro assay products using β-apo-8′-carotenal as substrate. Assays were incubated for 2 h. A. The incubation of Diox1 with β-apo-8′-carotenal led to cleavage of the substrate as visualized by the disappearance of the colour (2), compared with the control assay (1). HPLC analysis revealed that all-trans-β-apo-8′-carotenal (peak a) was converted into novel compounds (peaks c and d). Peak b: a mixture of β-apo-8′-carotenal cis-isomers that were not converted by Diox1. B. UV/Vis spectra of the products (c and d). The UV/Vis spectrum of compound c was identical with the spectrum of the reference retinal. C. GC-MS analysis of the products c (I) and d (II). The EI mass spectrum of compound c showed identity with the reference spectrum of retinal and exhibited the expected molecular ion of m/z 284 as well as the typical m/z 161 fragment indicative for loss of the ionone ring from the parent ion. The CI mass spectrum of compound d was identical to that obtained with synthetic apo-8′,15-apo-carotene-dial (C10O2H11) and showed the expected molecular ion of m/z 165.
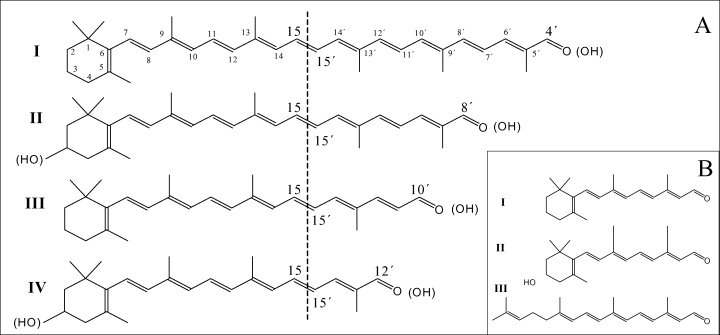
Structures of substrates used (A) and products obtained (B). (A) The substrates β-apo-4′- (I), β-apo-8′- (II), β-apo-10′- (III) and β-apo-12′-carotenal (IV) were converted into retinal (I, B). The use of the (3R)-3-hydroxy-derivatives of II and IV led to the formation of (3R)-3-hydroxy-retinal (II, B). Apo-lycopenals (structures not shown) were cleaved to acycloretinal (III, B). The same C20 products were obtained using the corresponding alcohols of these substrates. As indicated by the dashed line, all substrates were cleaved at the C15–C15′ double bond.
Investigation of the stereospecificity of Diox1
Some homologous dioxygenases show stereospecificity with respect to the state of geometric isomerism of their substrates. As can be seen from the HPLC analysis (Fig. 2), the substrate used consisted mainly of all-trans-β-apo-8′-carotenal accompanied by small amounts of a mixture of cis-isomers. These cis-isomers were not converted by Diox1, indicating specificity for the all-trans form. To test this inference, β-apo-8′-carotenal mixtures composed of different all-trans/cis ratios were used in the enzymatic reactions. For this purpose, cis-isomers were purified by TLC and increasing relative amounts were then added to the all-trans-β-apo-8′-carotenal substrate. Figure 3 shows that the amount of the retinal produced decreased by increasing the cis/trans ratio. These data indicate that cis-isomers are not cleaved by Diox1.

Determination of the stereospecificity of Diox1. A. β-Apo-8′-carotenal substrate preparations containing different all-trans/cis ratios were applied to standard in vitro assays. The retinal formed was quantified by HPLC. The amount of retinal decreased concomitantly with an increased proportion of cis-isomers. B. β-Apo-8′-carotenal consisting of about 95% all-trans-isomer was used in a standard in vitro assay. The formed product was derivatized into retinaloxime and analysed by normal phase HPLC. Diox1 formed all-trans-retinal separated as syn (1) or anti (2) form of the respective oximes. Traces of 13-cis-retinaloxime (3) were also obtained.
Supporting results were obtained by determining the configuration of the retinal produced in vitro. HPLC analysis of its oxime derivative performed according to von Lintig and Vogt (2000) is represented in Fig. 3. The retinal produced occurred mainly in the all-trans form accompanied by only traces of the 13-cis-isomer that were probably formed spontaneously due to acetone extraction.
The in vitro product of Diox1 is biologically active
To test for the biological activity of the C20 product obtained in vitro, it was fed to the Drosophila mutant ninaB360d as the only retinal source. These flies are defective in BCO I and are therefore not able to utilize carotenoids as retinal precursors (von Lintig et al., 2001) leading to a blind phenotype. Because of the absence of the chromophore, the major opsin Rh1-opsin occurs as an unripe glycosylated dimer with a molecular mass of 72 kDa. Feeding of retinal leads to its disappearance because of its conversion into mature opsin with a molecular mass of 32 kDa (De Couet and Tanimura, 1987; Huber et al., 1994). As seen in a Western blot analysis (Fig. 4), the product of Diox1 rescued the mutation, as indicated by the formation of this mature opsin. These data prove that the product represents biologically active retinal.
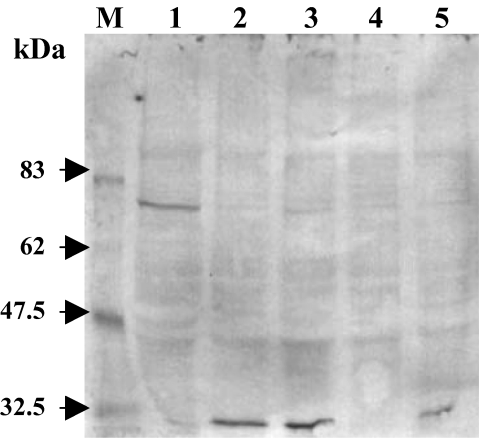
Western blot analysis of Rh1 opsin from ninaB mutant after feeding with Diox1 product. M: molecular mass marker. 1: ninaB mutant fed with solvent- (EtOH) treated yeast on standard corn medium (control). 2: ninaB mutant fed with standard retinal (400 µg in 200 µl of EtOH) 3: ninaB mutant fed with Diox1 product (400 µg in 200 µl of EtOH). 4: ninaE mutant, not expressing Rh1 (control for the signal specificity). 5: wild type containing the mature Rh1. Feeding with standard retinal as well as with the Diox1 in vitro product rescued the bcoI phenotype and led to mature Rh1, as indicated by the signal shift from the dimer with 72 kDa to the monomer with 32 kDa.
Substrate specificity of Diox1
To investigate the end-group specificity, several 8′-apo-carotenoids formally derived from different carotenes and xanthophylls were used in vitro as described. In all cases, Diox1 converted these substrates into retinal or into the corresponding C20 compounds, e.g. acycloretinal from apo-8′-lycopenal, (3R)-3-hydroxy-retinal from (3R)-3-OH-β-apo-8′-carotenal, and 4-oxo-retinal from 4-oxo-β-apo-8′-carotenal. We also tested β-apo-8′-carotenol and the corresponding alcohols of the above-mentioned substrates. Here again, we obtained retinal or the corresponding C20 compounds. The identity of the products was shown by HPLC as well as by GC-MS (data not shown). The molecular structures of the substrates and products obtained are shown in Fig. 5.
However, the cleavage reactions exhibited very different velocities depending on the substrate employed. For instance, 4-oxo-β-apo-8′-carotenal as well as apo-8′-lycopenal was converted at a very slow rate. In contrast, β-apo-8′-carotenal, (3R)-3-OH-β-apo-8′-carotenal and their corresponding alcohols were converted more quickly. Thus, incubations with these latter substrates were completed after 2–3 h.
Table 1 gives apparent Km and Vmax values determined in this biphasic in vitro incubation system (see also Fig. 6). The lowest Km values were obtained for (3R)-3-OH-β-apo-8′-carotenal, followed by β-apo-8′-carotenal. In addition, the Vmax of (3R)-3-OH-β-apo-8′-carotenal was higher than that of β-apo-8′-carotenal. However, both substrates showed lower Vmax values when compared with the corresponding alcohols, i.e. Diox1 exhibited a higher affinity to aldehydes but converted alcohols faster. The determined Km values for the alcohols lay in the same order as for the aldehydes, i.e. lower for (3R)-3-OH-β-apo-8′-carotenol as compared with β-apo-8′-carotenol.
| Substrate | K m (µM) | V max (pmol min−1) |
|---|---|---|
| β-Apo-8′-carotenal | 2.5 | 40 |
| β-Apo-8′-carotenol | 43 | 650 |
| (3R)-3-OH-β-apo-8′-carotenal | 1.4 | 71 |
| (3R)-3-OH-β-apo-8′-carotenol | 31 | 705 |
| (3R)-3-OH-β-apo-12′-carotenal | 2.6 | 11.5 |

Michaelis-Menten plots for the formation of the C20 products [retinal or (3R)-3-hydroxy-retinal] from different substrates. A. Substrates with different functional end-groups: β-apo-8′-carotenal (◆), β-apo-8′-carotenol (▪), (3R)-3-OH-β-apo-8′-carotenal (▴) and (3R)-3-OH-β-apo-8′-carotenol (X). B. Substrates with different chain-lengths: (3R)-3-OH-β-apo-8′-carotenal (▴) and (3R)-3-OH-β-apo-12′-carotenal (X).
To investigate the enzyme's preference for chain-lengths of the substrate, we compared different (3R)-3-OH-apo-carotenals, namely (3R)-3-OH-β-apo-8′-carotenal (C30) with (3R)-3-OH-β-apo-12′-carotenal (C25). In another series different apo-carotenals were used, namely β-apo-4′-carotenal (C35), β-apo-8′-carotenal (C30), β-apo-10′-carotenal (C27) and β-apo-12′-carotenal (C25). Here again, the cleavage products were determined using GC-MS as retinal or (3R)-3-hydroxy-retinal (data not shown). The kinetic parameters varied significantly. For instance, the conversion of β-apo-4′-carotenal (C35) took place at a very slow rate, so that overnight incubations were required to accumulate a sufficient amount of product for identification. In contrast, the determined Km and Vmax values for β-apo-10′-carotenal (C27; data not shown) were very similar to those for β-apo-8′-carotenal (C30). The determined values (Table 1) indicate that the preferred chain-length of Diox1 is very likely in the range of C27 or C30, because a further reduction in chain-length increased the apparent Km value, as shown with (3R)-3-OH-β-apo-12′-carotenal (C25).
Discussion
Here we report on Diox1 from Synechocystis as a novel eubacterial oxygenase capable in synthesizing retinal in vitro. But in contrast to known BCOs from animals, Diox1 converts β-apo-8′-carotenal and related apo-carotenoids instead of β-carotene. The nature of the products was determined by HPLC, GC-MS as well as in a biological assay. The catalysis is stereospecific, converting all-trans-β-apo-8′-carotenal into all-trans-retinal. It also showed a narrow regional specificity of cleavage at the 15–15′ double bond. In contrast, Diox1 showed a wide substrate specificity, accepting β-apo-carotenals with different chain-lengths, apo-carotenols, 3-hydrox-apo-carotenals as well as apo-8′-lycopenals. Depending on the nature of the end-groups, Diox1 produced retinal (3R)-3-hydroxy-retinal or acycloretinal (see Fig. 5). However, the apparent values determined for Km and Vmax differed markedly (Table 1). Given that low substrate concentrations prevail in vivo, one would assume the preferred natural substrate to be a C3-hydroxylated apo-carotenal with a C27 or C30 chain-length.
In fact, although the capability to produce retinal (3R)-3-hydroxy-retinal and even acycloretinal was proven, the identity of the natural substrate and the reaction(s) leading to its formation remain obscure.
HPLC analyses of carotenoid extracts from Synechocystis revealed the occurrence of small amounts of compounds which resembled (3R)-3-OH-β-apo-carotenals in their UV/Vis spectra as well as in their elution characteristics (data not shown), but these were too low in abundance to allow an investigation of their molecular structure. Such precursor molecules may be formed through the action of a different dioxygenase. For instance, the genome of Synechocystis encodes a second homologous, putative carotenoid-cleaving enzyme (ORF: slr1648, here named Diox2), which may be a candidate. It appears likely that Diox2 catalyses this initial reaction delivering the apo-carotenoid substrate. In this case, the catalytic specificities of Diox2 would determine not only the substrate but also the end-product of Diox1, given the broad substrate specificity of the latter. Unfortunately, we have not been able to show any catalytic activity with the overexpressed Diox2 so far, so that the possibility exists that its substrate may not be a carotenoid. Alternatively, apo-carotenoids may arise from photodestruction processes mediated by reactive oxygen species (ROS) under light stress. In their microarray analysis of gene expression in Synechocystis during acclimation to high light, Hihara et al. (2001) reported that the expression of Diox1 was increased by fourfold within 15 min after exposure to high light. This increase was followed by a gradual decrease to the low light level within 15 h. This rapid induction was also shown for scavenging enzymes for reactive oxygen species such as gluthatione peroxidase (slr1992) and sodB (slr1516) as well as for psbA2 (slr1311), psbA3 (slr1867) and several heat-shock proteins. Considering this induction, it may be that ROS led to an inhomogeneous population of apo-carotenoids that were consecutively converted into homogeneous C20 compounds, into retinal, retinol and its in-ring hydroxylated derivatives.
This scenario may allow one to speculate on a possible signalling function of retinal or retinal-like compounds in Synechocystis. There are several reports on regulatory functions of carotenoid-cleavage products in cyanobacteria. For instance, β-ionone, methylheptenone and geranylacetone exert an inhibitory effect on the growth of several cyanobacterial species such as Synechococcus, Anabaena and Cyanidium (Jüttner, 1979a). In addition, the C12-compound geranylacetone acts as a strong inhibitor of carotenoid biosynthesis in Synechococcus 6911 (Jüttner, 1979b).
Retinal is a common chromophore in animals, Archaea and green algae. But so far, there is no report on retinal biosynthesis in any eubacterial species. There are several lines of evidence indicating the occurrence of this compound to act as a chromophore in cyanobacteria. Direct evidence has been recently provided by Jung et al. (2003) who reported on a rhodopsin from Nostoc sp. PCC 7120, which is supposed to act as a photosensory receptor. The Nostoc rhodsopsin was capable of binding 3H-labelled all-trans-retinal in E. coli as well as in isolated Nostoc membranes. Interestingly, the genome of Nostoc sp. PCC 7120 encodes three polypeptides with a high similarity to carotenoid-cleaving enzymes. Among these, the enzyme BAB75983 (ORF: all4284) shares about 67% similarity with Diox1. Our preliminary data indicate that this Nostoc enzyme also catalyses the synthesis of retinal from apo-carotenals. Further evidence on the occurrence of retinal in cyanobacteria has been reported from Calothrix, where a rhodopsin was supposed to act as a green-light photoreceptor (Hoff et al., 1995). However, the genome of Synechocystis does not encode a polypeptide with significant homology to any known opsin. Synechocystis sp. PCC 6803 does not represent the sole cyanobacterium lacking opsins. Although the occurrence of Diox1 homologues indicates retinal biosynthesis (see Fig. 1), the genomes of Anabaena variabilis ATCC 294131, Nostoc punctiforme, Synecchococcus sp. WH8102 and P. marinus do not encode opsins either. Therefore, we assume that retinal and/or retinal derivatives may have an additional, opsin-independent function in Synechocystis as well as in other cyanobacteria. Diox1 knockout mutants of Synechocystis are currently being investigated in our laboratory to study the physiological role and significance of the Diox1 product(s).
The Synechocystis carotenoid oxygenase-dependent retinal pathway described here may also occur in other carotenoid-containing Eubacteria as well as in green algae. However, the sequenced genome of H. salinarum strain NRC-1, a carotenoid-synthesizing bacterium which also possesses retinal, does not encode carotenoid oxygenase homologues. Therefore, it must be postulated that retinal biosynthesis in Halobacteria is either mediated by an unrelated cleaving enzyme or based on a non-carotenoid precursor not necessitating a cleavage reaction. Recent evidence indicates that in H. salinarum retinal is synthesized from β-carotene via an unknown mechanism. The halobacterial retinal biosynthesis requires two enzymes (Brp and Blh) exhibiting significant homology to each other but not to known carotenoid oxygenases (Peck et al., 2001). An example for a carotenoid derivative produced through two different pathways is represented by the phytohormone abscisic acid (ABA). In plants, ABA biosynthesis is initiated by the oxidative cleavage of 9-cis-violaxanthin and 9′-cis-neoxanthin (Schwartz et al., 1997), while fungi utilize the so-called direct pathway involving cyclization of farnesyl diphosphate to produce the same C15 compound (Siewers et al., 2004). One may speculate that a similar direct pathway occurs for retinal biosynthesis in carotenoid-free Eubacteria.
Experimental procedure
Cloning of sll1541
The gene sll1541 coding for Diox1 was amplified using the oligonucleotides Dio1-backward: 5′-ATGGTCACTTCCCCC AACCAG-3′ and Dio1-forward: 5′-TCAAGTCTGGGCCCAG GAACCG-3′. The polymerase chain reaction (PCR) was carried out using 100 ng genomic DNA, 100 ng of each primer, 200 µM dNTPs and 1 µl Advantage® cDNA Polymerase Mix (BD Biosciences, CA, USA) in the buffer provided, as follows: 2 min initial denaturation at 94°C followed by 32 cycles (30 s 94°C, 30 s 62°C, 2 min 68°C) and 10 min final polymerization at 68°C. The obtained PCR product was purified using GFXTM PCR DNA and Gel Band Purification Kit (Amersham Biosciences, NJ, USA), and cloned into pCR®II-TOPO (Invitrogen, Paisley, UK) to yield pSyn-Diox1. The nature of the product was controlled by sequencing.
Protein expression and purification
To express Diox1 as a GST fusion protein, the corresponding gene was excised from pSyn-Diox1 using the restriction enzymes Ecl136 II and EcoRV, and ligated into SmaI-treated pGEX-4T-1 (Amersham Biosciences, NJ, USA) to yield pGESD1. Subsequently, Bl21 E. coli cells were transformed with pGESED, grown at 37°C and induced at an OD600 of 0.5 with 0.2 mM IPTG. After incubation for additional 4 h at 28°C, cells were harvested and extracts subjected to SDS-PAGE to control protein expression. The fusion protein was then purified using glutathione-sepharose, and Diox1 was released by treatment with the protease thrombin at room temperature overnight, according to the instructions of the manufacturer (Amersham Biosciences, NJ, USA).
Enzyme assays
Substrates were purified using thin-layer silica-gel plates (Merck, Darmstadt, Germany). Plates were developed in petroleum benzene (boiling range: 40–60°C)/diethylether/acetone (40:10:10, v/v/v) or with (40:10:4, v/v/v) to separate and isolate cis-isomers. Substrates were scraped off in dim daylight and eluted with MeOH. Synthetic apo-carotenals were kindly provided by BASF (Ludwigshafen, Germany). The corresponding alcohols were obtained by reducing the aldehydes with NaBH4 in EtOH. Enzyme assays were performed according to Schwartz et al. (2001) with some modifications. The incubation buffer contained 0.05% Triton X-100, 5 mM ascorbate, 1 mg ml−1 catalase (Sigma, Deisenhofen, Germany) in 100 µl of 100 mM Bis-Tris-HCl, pH 7.0. Ten microlitres of purified Diox1 corresponding to about 2 µg were then added, plus 5 µl of an EtOH solution of the appropriate substrate at a concentration of about 0.5 mM. Assays were incubated at 27°C for various time intervals, as given in the text. The assays were stopped by adding 1 volume of acetone and extracted using petroleum benzene/diethylether (1:4, v/v). The analysis was carried out as given below.
Kinetic analyses
Initial measurements were carried out photometrically at 27°C using a UV-2501PC spectrophotometer (Shimadzu, Japan). Time linearity was observed over 5–25 min depending on the different substrates. Based on these observations the initial velocities of the reactions were measured at 5 min (apo-carotenols) or 10 min (apo-carotenals). HPLC analyses and quantification by peak integration were used throughout to monitor product formation. Fifty micrograms of purified Diox1 were added to 2.8 ml of incubation buffer containing 20 µl of a saturated solution of Sudan 3 (Sigma, Deisenhofen, Germany) in EtOH as an internal standard. Aliquots (200 µl) of this solution were then mixed with 20 µl of EtOH containing the individual substrates at concentrations as given in the text.
Analytical methods
Substrates and products were quantified spectrophotometrically at their individual λmax using extinction coefficients calculated from E1% as given by Barua and Olson (2000) or as determined with the synthetic compounds (BASF, Ludwigshafen, Germany). Protein concentration was determined using the Bio-Rad protein assay kit (Bio-Rad, CA, USA). For HPLC, a Waters (Eschborn, Germany) system equipped with a photodiode array detector (model 996) was used. The separation was performed using a C30-reversed phase column (YMC Europe, Schermbeck, Germany) with the solvent systems B: MeOH/tert-butylmethyl ether/water (120:4:40, v/v/v) and A: MeOH/tert-butylmethyl ether (500:500, v/v). The column was developed at a flow rate of 1 ml min−1 with a linear gradient from 100% B to 43% B within 45 min, then to 0% B within 11 min, maintaining the final conditions for another 14 min. Determination of retinal isomers in the form of their corresponding oxims was performed by HPLC according to von Lintig and Vogt (2000). The reference substances all-trans, 13-cis, 11-cis-retinal and 9-cis-retinal were kindly provided by Dr Johannes von Lintig, University of Freiburg.
GC-MS analyses
To remove detergents present in the extracts, products were pre-purified by TLC using RP-18 F245s plates (Merck, Darmstadt, Germany). The plates were developed in MeOH/water (100:1, v/v). Products were then scraped off and eluted with CHCl3, evaporated and redissolved in acetone for GC-MS analyses. All plates were washed in a chromatographic step with CHCl3 before use. GC-MS analyses were performed using a Finnigan Trace DSQ mass spectrometer coupled to a Trace GC gas chromatograph. Separations were carried out using a 30 m Zebron ZB 5 column (5% phenyl−95% dimethylpolysilanoxane, 0.25 mm internal diameter and 0.25 µm film thickness; Phenomenex, Aschaffenburg, Germany). For the identification of retinal and (3R)-3-hydroxy-retinal a temperature programme was used with an initial temperature of 100°C for 5 min followed by a temperature ramp of 25°C per minute to a final temperature of 320°C, which was maintained for an additional 5 min. A constant He carrier gas flow was maintained at 1 ml min−1 using a split flow of 1:30. The splitless time was 3 min and the injector oven temperature was 220°C. Standard EI ionization was used at an ion source potential of 70 eV at 200°C. Identification of compounds was performed by chromatographic comparison with the authentic references and by comparing the mass spectra with the NIST Mass Spectral Search Program Version 2.0 (National Institute of Standards and Technology). Because of ambiguities with EI ionization in identifying the molecular ion of the second cleavage product (apo-8,15′-carotene-dial), CI was applied using CH4 as the ionizing agent at a flow rate of 1.5 ml min−1.
Feeding experiments
The Drosophila strains yw (wild type), ninaB360d and ninaE 17ol (Y19) were kindly provided by Dr Johannes von Lintig, University of Freiburg. The strains were kept on the retinal-free standard corn medium (http://flystocks.bio.indiana.edu/bloom-food.htm) at 25°C in 80% relative humidity and 16/8 h light-dark illumination cycle. For biological tests, yeast was dispersed on standard corn medium after being treated with 400 µg per bottle standard retinal or with similar amounts of retinal produced in vitro by Diox1, both dissolved in EtOH. The medium was air-dried and covered with paper towels. Flies were kept for 5 days until analysis. For protein extraction, three heads were homogenized in 50 µl of 3× urea-SDS-PAGE buffer using a mortar and pestle. After incubation for 10 min at room temperature, samples were then centrifuged at 13 000 g for 10 min. Supernatants were subjected to 10% SDS-PAGE. After transfer to a nitrocellulose membrane, Western blot analyses were performed using polyclonal anti-Rh1 antibodies (Huber et al., 1994) and secondary alkaline phosphatase-conjugated anti-mouse antibodies (Sigma, Deisenhofen, Germany). BCIP/NBT (Roth, Karlsruhe, Germany) were then employed for detection.
Acknowledgements
This work was supported by the European Commission, (contract: QLK3-CT2000-0809), ProVita, and by the HarvestPlus programme (http://www.harvestplus.org). We are indebted to Dr Johannes von Linting, Dr Vitus Oberhauser and Olaf Voolstra for providing support in the Drosophila experiment. We thank Dr Catherine Caris-Veyrat for valuable discussions and Dr Randall Cassada for correcting the English version of the manuscript.




