Use of the Rhodopseudomonas palustris genome sequence to identify a single amino acid that contributes to the activity of a coenzyme A ligase with chlorinated substrates
Summary
Rhodopseudomonas palustris strain RCB100 degrades 3-chlorobenzoate (3-CBA) anaerobically. We purified from this strain a coenzyme A ligase that is active with 3-CBA and determined its N-terminal amino acid sequence to be identical to that of a cyclohexanecarboxylate-CoA ligase encoded by aliA from the R. palustris strain (CGA009) that has been sequenced. Strain CGA009 differs from strain RCB100 in that it does not use 3-CBA as a sole carbon source. The aliA gene from the 3-CBA degrading strain differed by a single nucleotide from the aliA gene from strain CGA009, causing the substitution of a serine for a threonine at position 208. Both AliA enzymes, purified as His-tagged fusion proteins, had comparable activities with cyclohexanecarboxylate. However, AliA from the 3-CBA degrading strain was 10-fold more active with 3-CBA (kcat/Km of 4.3 × 104 M−1 s−1) than the enzyme from the sequenced strain (kcat/Km 0.32 × 104 M−1 s−1). The CGA009 enzyme was not sufficiently active with 3-CBA to complement an RCB100 aliA mutant for growth on this compound. Here, whole genome sequence information enabled us to identify a single nucleotide among 5.4 million nucleotides that contributes to the substrate preference of a coenzyme A ligase.
Introduction
Public concerns about environmental contamination with halogenated organic compounds have stimulated work with microbes able to catalyse dechlorination reactions under anaerobic conditions. Many halorespiring bacteria have been described that reductively dehalogenate compounds such as trichloroethylene, tetrachloroethylene, chlorobenzoates or chlorobenzenes in the course of using them as terminal electron acceptors (Holliger et al., 1999; Adrian et al., 2000; Gibson and Harwood, 2002). Some Thauera and Azoarcus species completely degrade 3-chlorobenzoate (3-CBA) as a carbon source for growth when nitrate is present as a terminal electron acceptor (Song et al., 2000; 2001). A few strains of the phototrophic bacterium Rhodopseudomonas palustris, among them RCB100, have been described that degrade 3-CBA as a sole carbon source anaerobically in light (van der Woude et al., 1994; Egland et al., 2001). Strain RCB100 converts the substrate to 3-chlorobenzoyl-CoA by a 3-CBA-CoA ligase reaction (Egland et al., 2001). After a reductive dehalogenation which has not yet been demonstrated in cell extracts (Egland et al., 2001), benzoyl-CoA is degraded to acetyl-CoA and CO2 through a central aromatic ring reduction pathway that has been well described (Harwood et al., 1999). Many other strains of R. palustris cannot grow when only 3-CBA is present. However, if benzoate is also present in the growth medium, cell yields indicate that both compounds are completely utilized for growth (Kamal and Wyndham, 1990; Egland et al., 2001; Oda et al., 2001).
The genome of R. palustris strain CGA009 has recently been sequenced (Larimer et al., 2004). This strain will not degrade 3-CBA as a sole carbon source (Egland et al., 2001). We therefore investigated the enzymatic basis of 3-CBA degradation by further work with strain RCB100 including purification of its 3-CBA-CoA ligase to obtain N-terminal amino acid sequence for use in designing primers to clone the structural gene. In the course of carrying out these studies we found that the sequenced strain and the 3-CBA degrading strain have almost identical cyclohexanecarboxylate-CoA ligase genes that differ by just a single nucleotide. This results in a single amino acid difference between the encoded enzymes that confers on the RCB100 CoA ligase, good activity with 3-CBA and other halogenated compounds.
Results
Purification of a ligase active with 3-CBA from the 3-CBA-degrading R. palustris strain
We purified a CoA ligase active with 3-CBA from a mutant strain of R. palustris RCB100 that had a disrupted benzoate CoA ligase (badA) gene (Egland et al., 2001). We used this strain (RCB101) because benzoate-CoA ligase has been reported to have slight activity with 3-chlorobenzoate (Geissler et al., 1988) and we wanted to avoid the possibility of purifying benzoate-CoA ligase. The mutant strain grew as well as the parent with 3-CBA as a carbon source (Egland et al., 2001), and crude cell extracts of strain RCB101 had 3-CBA-CoA ligase activities that were comparable to those seen in its wild-type parent. A partial purification of the 3-CBA-CoA ligase was carried out as outlined in Table 1 and Experimental procedures. The active fraction that eluted from the size exclusion column had a dominant polypeptide of about 60 000 molecular weight, but a large number of other minor bands were also present (Fig. 1). Attempts at further purification resulted in loss of activity. The N-terminal sequence of the dominant polypeptide was determined as M-E-F-D-A-V-L-L-P. When searched against the R. palustris CGA009 genome sequence, this matched exactly the predicted N-terminal sequence of gene rpa0651, which we had previously named aliA. The aliA gene lies near a cluster of genes involved in anaerobic benzoate degradation and has been annotated as a cyclohexanecarboxylate-CoA ligase (Larimer et al., 2004). Cyclohexanecarboxylate is an alicylic acid comprised of a saturated six-carbon ring with a carboxyl substituent. It is catabolized by conversion to cyclohexanecarboxyl-CoA and oxidation to cyclohex-1-ene-carboxyl-CoA, a compound that is an intermediate of anaerobic benzoate degradation (Küver et al., 1995; Harwood et al., 1999). AliA has a predicted molecular weight of 59 508 and has 547 amino acids.
| Purification step | Volume (ml) | Protein (mg) | Sp. activitya (U mg−1 protein) | Total activity (U) | Activity recovered (%) | Purification (fold) |
|---|---|---|---|---|---|---|
| Cell extract | 95 | 1261.4 | 0.009 | 11.71 | 100 | |
| Q-Sepharose | 90 | 79.52 | 0.021 | 1.89 | 16.14 | 2.26 |
| Phenyl-Sepharose | 50 | 51.28 | 0.021 | 1.05 | 8.96 | 2.26 |
| Ultrafiltration | 5 | 5.1 | 0.162 | 0.826 | 7.05 | 17.41 |
| Hydroxyapatite | 8.8 | 2.5 | 0.204 | 0.522 | 4.45 | 21.94 |
| Superose 12 | 0.1 | 0.5 | 0.138 | 0.069 | 0.59 | 14.83 |
- a . Units are given in µmol 3-CBA-CoA formed per minute at 25°C. The isotopic assay was used to measure activity with 3-CBA.
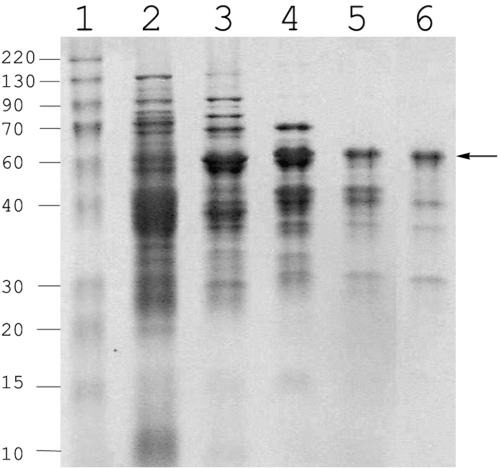
Purification of a CoA ligase active with 3-CBA from R. palustris RCB101. Active fractions from sequential stages of purification were analysed by SDS-PAGE. Lanes: 1, Protein molecular mass standards (indicated in kDa on the left); 2, Crude cell extract; 3, Q-Sepharose-pooled fractions; 4, Phenyl-Sepharose-pooled fractions; 5, Hydroxyapatite-pooled fractions; 6, Gel-filtration-pooled fractions. The arrow indicates the 60 kDa polypeptide that was N-terminal sequenced.
Cloning and sequencing the aliA gene from R. palustris RCB100
We used R. palustris CGA009 genome sequence information to design primers complementary to the nucleotides overlapping the ends of rpa0651 and amplified the corresponding gene from R. palustris RCB100. A single polymerase chain reaction (PCR) product of the expected size of 1.7 kb was obtained. The aliA gene from RCB100 was then sequenced and found to differ from the CGA009 aliA gene by a single nucleotide. A C at position 623 was substituted by a G in the RCB100 gene, a difference predicted to result in the substitution of a serine for a threonine at amino acid 208 of the expressed RCB100 protein.
Construction and complementation of an RCB100 aliA mutant
To determine whether the CoA ligase encoded by the RCB100 aliA (aliA100) gene contributes to 3-CBA degradation, we disrupted aliA100 with a Km resistance cassette to generate strain RCB102. Whereas the wild-type strain, RCB100, had a doubling time of 21 h with 3-CBA as a carbon source, the aliA100 mutant grew extremely slowly on 3-CBA with a doubling time of about 144 h. The RCB100 aliA100 mutant also grew slowly on cyclohexanecarboxylate. The mutant phenotype was complemented by the introduction of the RCB100 aliA gene into strain RCB102 on a plasmid in trans. The complemented mutant had a growth rate on 3-CBA of 40 h. As expected the aliA gene (aliA009) from CGA009 did not complement the 3-CBA growth defect of RCB102 when supplied in trans. The aliA100 gene did not confer on strain CGA009 the ability to grow on 3-CBA, indicating that the inability of the sequenced strain to degrade 3-CBA is not solely due to lack of expression of the appropriate CoA ligase activity.
Comparison of AliA enzymes from the sequenced strain and the 3-CBA-degrading strain
N-terminal His-tagged versions of the AliA proteins from the two R. palustris strains were expressed in Escherichia coli and purified in a single chromatographic step. Each of the purified proteins ran as a single band on SDS-PAGE gels. The enzyme expressed from the RCB100 gene was designated as AliA100, and the enzyme expressed from the CGA009 gene was designated AliA009. The activity of each enzyme was tested against a range of chlorinated and unchlorinated fatty acids, alicylic acids and aromatic acids. Kinetic constants were determined with the coupled enzyme assay (Experimental procedures) for a subset of substrates that gave good activity (Table 2). Kinetic constants determined with the isotopic assay with benzoate and 3-CBA as substrates were comparable to those determined with the coupled assay. As expected, cyclohexanecarboxylate was the substrate best utilized by both the AliA100 and AliA009 enzymes. The AliA100 enzyme was about twice as active with this substrate as the AliA009 enzyme. When we compared the catalytic efficiencies of the two enzymes with halogenated substrates we observed differences that were much greater. The enzyme from the 3-CBA degrading strain was over 10 times more active with 3-CBA as a substrate than the corresponding ligase from the sequenced strain. AliA100 was also more active with several other halogenated substrates including 3-bromobenzoate, 4-flurobenzoate and 2-fluorobenzoate than AliA009.
| Substrate | K m (µM) | k cat (s−1) | k cat/Km (M−1 s−1) | |||
|---|---|---|---|---|---|---|
| AliA100 | AliA009 | AliA100 | AliA009 | AliA100 | AliA009 | |
| 3-CBA | 88 | 49 | 3.8 | 0.16 | 4.3 × 104 | 0.32 × 104 |
| Cyclohexanecarboxylate | 3.9 | 19 | 1.8 | 4.1 | 4.6 × 105 | 2.2 × 105 |
| Benzoate | 144 | 372 | 6.6 | 3 | 4.6 × 104 | 0.8 × 104 |
| 3-Bromobenzoate | 83 | 38 | 1.8 | 0.07 | 2.1 × 104 | 0.2 × 104 |
| 4-Fluorobenzoate | 347 | 345 | 3.8 | 0.3 | 1.1 × 104 | 0.08 × 104 |
| 2-Fluorobenzoate | 154 | 525 | 4.9 | 2 | 3.2 × 104 | 0.4 × 104 |
| Cyclopentane carboxylate | 26 | 31 | 4 | 6.6 | 1.6 × 105 | 2.2 × 105 |
| Butyrate | 236 | 358 | 4.8 | 4.8 | 2.1 × 104 | 1.3 × 104 |
- All experiments were performed at least in duplicate. Measurements were made with variable substrate concentrations (0.2×−10×Km) keeping ATP (0.5 mM), CoASH (1 mM) and NADH (0.14 mM) fixed and all velocities were determined at points at which no more than 10% of the substrate had been consumed. The Km and kcat values were calculated by fitting the data to the Cleland's program (1979). The standard errors of the fitted values are usually <25%, which indicates the values are well determined. The data are means from at least two separate experiments. Values remain within twofold among replicate experiments.
The AliA100 enzyme had a Km of 13.5 µM for adenosine triphosphate (ATP) and 280 µM for CoASH, whereas AliA009 had a Km of 41 µM for ATP and 119 µM for CoASH. The values were determined in the presence of saturating concentrations of cyclohexanecarboxylate. Both enzymes had broad pH optima of 8.5–9.0 and neither was sensitive to oxygen.
Identification of AliA amino acids important for activity
The AliA enzymes belong to a large family of structurally related acyl-adenylate-forming enzymes which includes non-ribosomal peptide synthetases involved in antibiotic production and in iron-siderophore production, as well as firefly luciferases and acyl CoA ligases. In the case of CoA ligases, a two-step reaction occurs in which an acyl-adenylate that is formed from ATP and a carboxylic acid substrate in an initial reaction serves as the substrate for a subsequent CoA thioesterification reaction in which the acyl-group is transferred to the sulphhydryl of CoA with the accompanying release of adenosine monophosphate (AMP). The overall amino acid sequence identities among members of the acyl-adenylate-forming enzyme family can be as low as 15%. However when the sequences of family members are aligned, three stretches of highly conserved sequence become apparent. These conserved sequences have been designated motifs I, II and III (Chang et al., 1997) (Fig. 2). Motif I resembles a p-loop (phosphate-binding loop) motif that is commonly found in ATP-binding proteins. This conserved sequence includes the S/T 208 of the AliA enzymes. To further investigate the presumed importance of the p-loop and conserved motifs II and III for the activity of AliA CoA ligase, we constructed a series of site-specific aliA mutants and tested the activities of the expressed mutant enzymes with 3-CBA and benzoate.
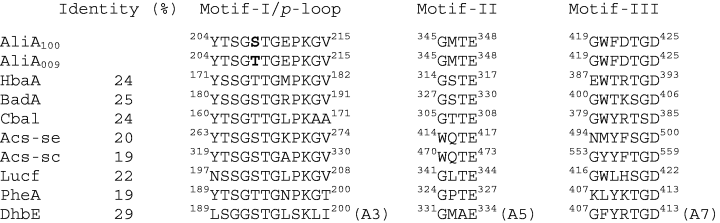
Sequence alignment of the three conserved motifs of selected members of the acyl-adenylate enzyme superfamily. Numbers represent the amino acid position in a given protein. AliA100, cyclohexanecarboxylate-CoA ligase from R. palustris RCB100 (this work); AliA009, cyclohexanecarboxylate-CoA ligase from R. palustris CGA009 (Larimer et al., 2004); HbaA, 4-hydroxybenzoate-CoA ligase from R. palustris CGA009 (Gibson et al., 1994); BadA, benzoate-CoA ligase from R. palustris CGA009 (Egland et al., 1995); Cbal, 4-chlorobenzoate CoA ligase from Alcaligenes sp. AL3007 (Gulick et al., 2004); Acs-se, acetyl CoA synthetase from S. enterica (Gulick et al., 2003); Acs-sc, acetyl CoA synthetase from Saccharomyces cerevisiae (Jogl and Tong, 2004); Lucf, luciferase from Photinus pyralis (Conti et al., 1996); PheA, Gramicidin S synthetase I from B. brevis (Conti et al., 1997); and DhbE, A-domain of 2,3-dihydroxy benzoate-AMP ligase from B. subtilis (May et al., 2002). The 208S/T of AliA ligases are shown in bold. The amino acid sequence identities are in comparison with AliA and were determined by using clustal w multiple alignment program (Thompson et al., 1994).
We started by making an S208 to T208 change in the AliA100 enzyme and a T208 to an S208 change in the AliA009 enzyme. As expected, the S208 enzyme was much more active with 3-CBA than the T208 enzyme (Table 3). This verified our initial finding that a single amino acid difference between two naturally occurring AliA enzymes from two different R. palustris strains is responsible for the ability of one but not the other of the enzymes to efficiently utilize 3-CBA as a substrate. Changing the amino acid at position 208 to an aspartate resulted in a dramatic drop in activity with 3-CBA and benzoate. A G207F change in the p-loop completely abolished activity, but a P212V change caused only a modest decrease of activity. We determined that the conserved glutamine at position 348 in motif II (Fig. 2) was important for enzyme activity. A conserved aspartate at position 425 in motif III was absolutely required for AliA activity as was a lysine at position 532 (Table 3). This lysine lies outside the three motifs but is conserved in all acyl-adenylate family members. Amino acids 207, 208, 348 and 532 correspond to residues that are located near the active site in the crystal structure of DhbE, an aryl-activating protein domain of a non-ribosmal peptide synthetase from Bacillus (May et al., 2002).
| Amino acid substitution | Substrate: 3-CBA | Substrate: benzoate |
|---|---|---|
| AliA100 (wt) S208 | 214 ± 24 | 293 ± 49 |
| AliA009 (wt) T208 | 25.3 ± 2.5 | 174 ± 40 |
| T208S (Thr-Ser) | 258 ± 38 | 385 ± 28 |
| S208T (Ser-Thr) | 17 ± 3.4 | 233 ± 56 |
| S208D (Ser-Asp) | 0.52 ± 0.18 | 3.3 ± 0.8 |
| G207F (Gly-Phe) | <0.04 | <0.04 |
| P212V (Pro-Val) | 36.3 ± 9.7 | 58.5 ± 8 |
| E348L (Glu-Leu) | 0.3 ± 0.04 | 7.5 ± 2 |
| D425V (Asp-Val) | <0.04 | <0.04 |
| K532Q (Lys-Gln) | <0.04 | <0.04 |
| Negative control | <0.04 | <0.04 |
| JM109(DE3)/pET-16b | ||
- The isotopic assay was used to measure activities in crude cell extracts of E. coli JM109(DE3) cells expressing various His-tagged AliA proteins. AliA expression was induced with 1 mM IPTG. The substrate concentration used was 1 mM.
- Specific activity is expressed as nmol CoA thioester formed per minute per milligram protein. Values are the averages of three separate determinations ± SD.
Discussion
The R. palustris genome sequence enabled us to discover that a single amino acid, residue 208, of cyclohexanecarboxylate-CoA ligase influences the degree to which this enzyme is active with halogenated substrates. A T208S change made the ligase sufficiently active with 3-CBA to permit R. palustris strain RCB100 to grow on this chlorinated compound at a reasonable rate. One would not necessarily have predicted that this amino acid would have an influence on substrate preference based on structural studies with homologous enzymes. To date crystal structures for acetyl-CoA synthetases from Salmonella enterica and yeast and for 4-chlorobenzoate-CoA ligase (CBAL) from Alcaligenes sp. AL3007 have been reported (Gulick et al., 2003; 2004; Jogl and Tong, 2004). Crystal structures are also available for three other acyl-adenylate-forming enzymes; firefly luciferase (Conti et al., 1996), the phenylalanine-activating domain (PheA) of gramicidin S synthetase I from Bacillus brevis (Conti et al., 1997) and the aryl acid-activating domain (2,3-dihydroxy benzoate-AMP ligase, DhbE) of a non-ribosomal peptide synthetase from Bacilius subtilis (May et al., 2002). These structures each reveal an overall protein topology consisting of a large N-terminal domain and a small C-terminal domain. Conserved motifs I, II and III (Chang et al., 1997) (Fig. 2) all lie in close proximity in the crystal structures at the interface between the two domains and contribute to the active site. Motif I, or the p-loop is also referred to as region A3 in some enzymes. Motif II is sometimes referred to as region A5. The amino acid corresponding to AliA residue 208, which is always conserved as either a serine or a threonine in acyl-adenylate-forming enzymes including CoA ligases (Turgay et al., 1992; Chang et al., 1997; Weimar et al., 2002; Schneider et al., 2003), is part of the p-loop, a feature that has been postulated to be involved in ATP/AMP binding. This residue is not part of the carboxy acid-binding pocket that has been proposed based on the structures of DhbE or CBAL (May et al., 2002; Gulick et al., 2004). Nor does it correspond to any of the 12 amino acids of 4-coumarate CoA ligase that are proposed to line its substrate-binding pocket and determine substrate specificity (Schneider et al., 2003).
Some of the acyl-adenylate enzyme crystal structures show a disordered conformation for the p-loop, indicating that this is a dynamic region and that the p-loop is flexible. Structural data have been obtained for the p-loop regions of the DhbE enzyme and the bacterial and yeast acetyl-CoA synthetases. DhbE has been crystallized in the absence of substrates, with bound 2,3-dihydroxybenzoate-adenylate intermediate and with 2,3-dihydroxybenzoate and AMP, the products of the dihydroxybenzoate-adenylate intermediate (May et al., 2002). Analysis of each of the enzyme structures indicates that the p-loop is located at the entrance to the catalytic cavity and that it moves as substrate is bound. May et al. (2002) have suggested that the p-loop interacts with ATP and moves it towards the carboxylate substrate to facilitate a nucleophilic attack (May et al., 2002). They have also suggested that after the adenylation reaction occurs, the p-loop of DhbE may close up and help to protect the adenylate phosphate intermediate from surrounding water molecules. By analogy with the DhbE data we suggest that amino acid 208 of AliA has an indirect effect in influencing catalytic efficiency with halogenated substrates, possibly by subtly altering the interactions between ATP and the carboxylate substrate or by influencing the efficiency with which the second part of ligase reaction, CoA thioesterification, occurs. It is also possible that residue 208 affects the movement or configuration of the p-loop sufficiently to alter the efficiency with which different carboxylate substrates can gain access to the substrate-binding pocket. However, there is no suggestion, from the DhbE structure at least, that the p-loop influences carboxylate substrate binding. In this context we note that in DhbE, the residue (serine 193) that corresponds to AliA amino acid residue 208 is over 11 Å distant from both bound 2,3-dihydroxybenzoate and bound AMP. The smaller amino acid (serine instead of threonine) at position 208 of AliA led to higher catalytic efficiency with all substrates but the improvement was more marked with halogenated substrates, which are more polar than cyclohexanecarboxylate. It is interesting in view of the proposed role of the p-loop in ATP binding, that AliA009 and AliA100 have about equal catalytic affinities for ATP.
A cyclohexanecarboxylate-CoA ligase has been previously purified and characterized from R. palustris strain CGA001 [the chloramphenicol sensitive parent of CGA009 (Kim and Harwood, 1991)] and although N-terminal amino acid sequence was not reported for this enzyme, it has biochemical characteristics that match those of the AliA enzyme (Küver et al., 1995). The previous work on the cyclohexanecarboxylate-CoA ligase provides the additional information that the enzyme is present in cells grown anaerobically on cyclohexanecarboxylate or benzoate, but not on succinate.
Rhodopseudomonas palustris strains are easily enriched and isolated from soil and water samples that are incubated anaerobically in light with benzoate as a sole carbon source. Strains isolated from such enrichments are usually able to degrade 3-CBA, but only when it is supplied together with benzoate as a co-substrate (Oda et al., 2004). However, such R. palustris strains will often acquire the ability to utilize 3-CBA as a sole carbon source after prolonged incubation in medium containing only 3-CBA (Oda et al., 2001). Typically strains first grow very slowly with 3-CBA as a sole carbon source (doubling times of five to 20 days), but variants then appear that degrade 3-CBA at much faster rates with doubling times of 23–37 h. R. palustris strain RCB100 is unusual in that it was enriched and isolated using a growth medium that contained only 3-CBA as a carbon source (Egland et al., 2001). It may be that strain RCB100 was proficient to degrade 3-CBA as a sole carbon source in the damp soil from which it was isolated, but it is also possible that it acquired this ability during the enrichment process. Regardless, it is clear that the AliA enzyme of this particular strain contributes to its ability to degrade 3-CBA. It is possible that different amino acid variations in other CoA ligases contribute to the ability of other R. palustris strains to grow with 3-CBA. The sequenced strain encodes 41 acyl-CoA ligases, two of which, benzoate-CoA ligase and 4-hydroxybenzoate-CoA ligase, react slowly with chlorinated benzoates (Geissler et al., 1988; Gibson et al., 1994).
Experimental procedures
Microorganisms and growth conditions
Bacterial strains and plasmids used in this study are listed in Table 4. R. palustris cultures were grown anaerobically in light at 30°C in defined mineral medium (photosynthetic medium, PM) (Kim and Harwood, 1991) prepared as described previously (Harwood and Gibson, 1988). Carbon sources were added to a final concentration of 3 mM except succinate, which was used at a final concentration of 10 mM. E. coli cultures were grown in Luria–Bertani (LB) medium at 37°C. Culture absorbance (OD) at 660 nm was used to monitor growth. Antibiotics were used at the following concentrations (in µg ml−1): for R. palustris, gentamycin, kanamycin (Km) and tetracycline, 100; and for E. coli, ampicillin, 100; gentamycin, 20; Km, 100; and tetracycline, 25.
| Bacterial strains and plasmids | Relevant characteristicsa | Reference or source |
|---|---|---|
| R. palustris | ||
| RCB100 | Wild-type, 3-CBA+ | Egland et al. (2001) |
| RCB101 | KmR; badA of RCB100 inactivated with Km cassette | Egland et al. (2001) |
| RCB102 | KmR; aliA of RCB100 inactivated with Km cassette | This work |
| CGA009 | Wild-type strain, spontaneous CmR derivative | Kim and Harwood (1991) |
| Plasmids | ||
| pBBR1MCS-5 | GmR; broad-host-range mobilizable vector | Kovach et al. (1994) |
| pET-aliA100 | AmpR; pET-16b with a 1.7 kb fragment from pCR2.1 containing aliA100 inserted at NdeI-BamHI sites | This work |
| pBB-aliA100 | GmR; pBBR1MCS-5 with a 1.7 kb fragment containing His-tagged aliA100 from pET-aliA100 inserted at HindIII-BamHI sites | This work |
| pET-aliA009 | AmpR; pET-16b with a 1.7 kb fragment from pCR2.1 containing aliA009 inserted at NdeI-BamHI sites | This work |
| pBB-aliA009 | GmR; pBBR1MCS-5 with a 1.7 kb fragment containing His-tagged aliA009 from pET-aliA009 inserted at HindIII-BamHI sites | This work |
- a . 3-CBA+, growth on 3-CBA.
Coenzyme A ligase assays
Ligase activities were measured by an isotopic or an enzyme coupled spectrophotometric (coupled) assay as previously described (Gibson et al., 1990). For the isotopic assay, the enzymatic conversion of [14C]-3-CBA or [14C]-benzoate to [14C]-3-chlorobenzoyl-CoA or [14C]-benzoyl-CoA, compounds that remain hydrophilic at acidic pH, was measured by scintillation counting. The reaction mixture contained 2.5 mM MgCl2, 0.5 mM ATP, 0.25 mM reduced CoA (CoASH) and [14C]-3-CBA or [14C]-benzoate (50 µM in standard assays; 0.05 µCi in a 0.5 ml reaction volume) in 20 mM triethanolamine-HCl, pH 7.5. In the coupled assay, AMP formed from ATP as a product of CoA ligase activity was measured by coupling the reaction via a series of auxiliary enzymes to NADH oxidation. Reaction mixtures contained 20 mM TEA-HCl (pH 7.5), 2.5 mM MgCl2, 0.5 mM ATP, 1 mM CoASH, 1 mM KCl, 10 mM phosphoenol pyruvate, 0.14 mM NADH, substrate and the following auxiliary coupling enzymes: 2 U pyruvate kinase, 2 U lactate dehydrogenase and 4 U myokinase (adenylate kinase) in a total volume of 1 ml. All assays were carried out at 25°C. The isotopic assay can be used to measure ligase activity in crude extracts, but the coupled assay can only be used with pure enzyme because of the extremely high background activity for NADH oxidation in crude cell extracts.
Preparation of cell extracts
For the purification of a ligase active with 3-CBA, R. palustris strain RCB101 (badA::Km) was grown anaerobically with 5 mM succinate in 13 l carboys illuminated with 60 W incandescent light bulbs. The culture was supplemented with 1 mM 3-CBA when it reached an OD660 of about 0.3. After an additional 24 h of growth the cells were harvested by centrifugation. Approximately 40 g of (wet weight) cell paste was suspended in 100 ml of TEA buffer (containing 20 mM triethanolamine-HCl, 1 mM MgCl2, 0.1 mM EDTA, 0.1 mM dithiothreitol and 5% glycerol; pH 7.5) and frozen at −70°C until used. Thawed cells were lysed in a French Press, cell debris and unbroken cells were removed by centrifugation, and the resulting supernatant was centrifuged at 100 000 g for 1 h at 4°C. The supernatant from this centrifugation step was then passed through a 0.22 µm filter (Millipore) unit and the filtrate was termed the crude cell extract. All procedures were carried out in air.
Purification of a CoA ligase with activity towards 3-CBA
A CoA ligase active with 3-CBA was partially purified from crude cell extracts as follows. All steps were carried out at 4°C.
Q-Sepharose chromatography. The clear, pale orange crude extract was loaded onto a Q-Sepharose column (2.6 by 10 cm) (Pharmacia) equilibrated with TEA buffer (composition as described above). After the column was washed with 100 ml of TEA buffer, protein was eluted with a linear gradient of 0–1 M KCl (in TEA buffer). Fractions were assayed for ligase activity using [7-14C]-3-CBA as substrate. Nine fractions (90 ml total) containing the ligase activity were pooled.
Phenyl-Sepharose chromatography. Solid ammonium sulphate was added slowly with stirring to the Q-Sepharose-pooled fractions to give a final concentration of 1 M (NH4)2SO4 keeping the protein solutions on ice. This solution was loaded onto a Phenyl-Sepharose column (2.6 by 10 cm) (Pharmacia) equilibrated with TEA buffer containing 1 M (NH4)2SO4. The column was developed with a linear gradient of 1.0–0 M (NH4)2SO4 in TEA buffer at a flow rate of 2.5 ml min−1. The active fractions were pooled and then concentrated and desalted with TEA buffer to 5 ml by ultrafiltration through a YM-10 membrane (molecular weight, MW, 10 000; Amicon, Beverly, Mass) under N2-positive pressure.
Hydroxyapatite chromatography. Samples were loaded onto a hydroxyapatite column (1.5 by 12 cm) that had been equilibrated with TEA buffer amended with 20 mM potassium phosphate (pH 7.5). The column was developed with a linear gradient of 20–400 mM potassium phosphate. The active fractions were concentrated to one-fifth the volume by ultrafiltration using YM-30 membranes (MW, 30 000).
Gel filtration chromatography. Combined fractions were concentrated further to 0.275 ml and loaded onto a Superose 12 column (1.0 by 30 cm) (Pharmacia) equilibrated with TEA buffer containing 150 mM KCl. The column was run at a flow rate of 0.2 ml min−1. The active fraction that eluted was concentrated by ultrafiltration using YM-30 membranes and then frozen and stored at −70°C.
Other analytical procedures
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out with 10% acrylamide gel by standard procedures (Ausubel et al., 1990). Gels were stained with Coomassie blue R250 to visualize separated protein bands. Molecular weight standards were from Amersham. Proteins separated by electrophoresis were transferred to a ProBlott membrane (Applied Biosystems). The band of interest was excised and subjected to automated Edman degradation in an Applied Biosystems Precise Sequencer at the protein sequence facility of the University of Iowa. The quantity of protein was estimated by dye-binding assay (Bio-Rad) with bovine serum albumin as standard.
Cloning of aliA
Rhodopseudomonas palustris genomic DNA was isolated from RCB100 and CGA009 strains using the DNeasy tissue kit (QIAGEN) with the modification that cells were broken by sonication. Oligonucleotides complementary to the 5′ and 3′ ends of the R. palustris aliA (1644 bp) coding sequence as it appears in the strain CGA009 genome sequence were synthesized. The 5′ primer (NdeI-aliA) (5′-GGAATTCCATATG GAGTTCGACGCCGTTCTGC-3′) contained an NdeI recognition site (underlined) at the 5′-terminus and spanned the initiating ATG codon. The 3′ primer (aliA-BamHI) (5′-CGCG GATCCGTGATCGGTGTGCACTTTAC-3′) contained a terminal BamHI recognition site and spanned the termination codon. PCRs were carried out by standard reaction conditions using Pfu polymerase. The amplified PCR product was directly cloned into the TA cloning vector pCR2.1 (Invitrogen) and then subcloned into the pET-16b His-tag expression vector (Novagen). The recombinant plasmids pET-aliA100 (where the gene was taken from RCB100) and pET-aliA009 (where the gene was taken from CGA009) were transformed into E. coli JM109(DE3) (Yanisch-Perron et al., 1985) for expression of the His-tagged enzymes. The recombinant plasmids direct the synthesis of proteins with the structure Met-Gly-His10-Ser2-Gly-His-Ile-Glu-Gly-Arg-His-AliA. The sequences of the cloned genes were verified by DNA sequencing.
Purification of His-tagged AliA proteins
His-tagged AliA enzymes were overexpressed in E. coli JM109(DE3) cells. Cultures (1 l) were grown to early log phase (OD600, 0.3–0.4) at 25°C in Terrific broth (Ausubel et al., 1990) supplemented with 100 µg ml−1 ampicillin. Gene expression was induced with 1 mM IPTG (isopropyl-1-thio-β-D-galactopyranoside) and cultures were further incubated with shaking until they reached an OD600 0.7–0.8. The cells were harvested by centrifugation at 4°C. The cell pellet was washed and suspended in 20 ml of binding buffer (20 mM TEA, pH 7.9, 500 mM NaCl and 5% glycerol). The cell suspension was lysed in a French Press after adding 200 µM phenylmethylsulfonyl fluoride. The lysed cell suspension was utracentrifuged at 100 000 g for 1 h at 4°C following the addition of RNase and DNase (final concentrations, 1 µg ml−1). The supernatant was passed through a 0.22 µm filter unit. The filtrate was loaded onto HiTrap chelating column (1.6 by 2.5 cm; Amersham/Pharmacia) that had been charged with a Ni+2 solution (400 mM NiSO4) and equilibrated with binding buffer. The column was first washed with 40 ml of 100% binding buffer and then with 30 ml of 95% binding buffer with 5% elution buffer (20 mM TEA, pH 7.9, 500 mM NaCl, 1 M imidazole) at 1 ml min−1. Protein was eluted with a linear gradient of 5% to 50% elution buffer over 25 ml, followed by 15 ml of 50% elution buffer, followed by a linear gradient from 50% to 100% elution buffer over 10 ml. Active fractions (2 ml) were pooled, washed with binding buffer to remove imidazole and concentrated using YM-30 ultrafiltration. This pure enzyme stock was stored at −70°C. A single protein band was found after SDS-PAGE.
Kinetic constant determinations
Kinetic parameters were determined by measuring enzyme activity with the coupled assay using His-tagged pure enzyme. Enzyme activities were measured at a range of substrate concentrations keeping ATP (0.5 mM) and CoASH (0.25 mM) fixed. At least eight substrate concentrations were tested. The initial velocity data were analysed by using the following equation and the Fortran HYPER computer program (Cleland, 1979): v0 = Vmax·[S]/([S] + Km), where v0 is the initial velocity, Vmax is the maximum velocity, [S] is the substrate concentration, and Km is the Michaelis constant for substrate. These values were used to calculate relative kcat (enzyme turnover rate), determined from Vmax/[E] where [E] is the enzyme concentration determined by the Bio-Rad dye binding assay and the calculated molecular mass of 62.04 kDa. kcat/Km is the catalytic efficiency. The reported kinetic constants are the averages of values obtained from at least two different experiments.
Mutant construction and complementation
To generate an aliA deletion mutant, a 1323 bp fragment of internal aliA sequence was PCR amplified from RCB100, and cloned into pJQ200KS (Quandt and Hynes, 1993). After digesting with PstI to remove a 350 bp fragment from the middle of the gene a 1236 bp Km cassette (from pACYC184::Km, Pharmacia) was introduced the PstI site. This construct was introduced into R. palustris RCB100 by conjugation from E. coli S17-1 (Simon et al., 1983). Sucrose resistant, Km resistant colonies were selected as described previously (Egland and Harwood, 1999) and were screened for the loss of the wild-type aliA and the presence of the aliA-Km construct by PCR and sequencing. The aliA gene cloned into the broad-host-range plasmid pBBR1MCS-5 vector (Kovach et al., 1994), was used to complement the R. palustris aliA mutant, RCB102. Growth was tested in liquid medium with 3-CBA under anaerobic conditions in light.
Site-directed mutagenesis
Various site-directed aliA mutants were generated by overlap extension PCR (Horton et al., 1993). The full-length mutated versions of aliA were cloned into the pET-16b expression vector. Two mutant genes were created by using the QuikChange site-directed mutagenesis kit of Stratagene and transformed into E. coli XL-1Blue strain (Stratagene). Finally all the recombinant plasmids were transferred into E. coli JM109(DE3) for the expression of His-tagged mutated proteins. The cloned aliA (wild-type and mutated) genes were sequenced to verify that they carried the expected mutations.
Radiochemicals
[7-14C]-3-CBA (0.55 mCi mmol−1) and [7-14C]-BA (0.57 mCi mmol−1) were purchased from American Radiolabeled Chemicals (St. Louis, MO).
Acknowledgements
This work was supported by the Division of Energy Biosciences, US Department of Energy (grant DE-FG02–95ER20184), and by the US Army Research Office (grant W911NF-04-1-0123). We thank B. Plapp for advice in the determination of the enzyme kinetic characteristics and J. Gibson for critical reading of the manuscript.




