Indole induces the expression of multidrug exporter genes in Escherichia coli
Summary
Our comprehensive expression cloning studies previously revealed that 20 intrinsic xenobiotic exporter systems are encoded in the Escherichia coli chromosome, but most of them are not expressed under normal conditions. In this study, we investigated the compounds that induce the expression of these xenobiotic exporter genes, and found that indole induces a variety of xenobiotic exporter genes including acrD, acrE, cusB, emrK, mdtA, mdtE and yceL. Indole treatment of E. coli cells confers rhodamine 6G and SDS resistance through the induction of mdtEF and acrD gene expression respectively. The induction of mdtE by indole is independent of the EvgSA two-component signal transduction system that regulates the mdtE gene, but mediated by GadX. On the other hand, the induction of acrD and mdtA was mediated by BaeSR and CpxAR, two-component systems. Interestingly, CpxAR system-mediated induction required intrinsic baeSR genes, whereas BaeSR-mediated induction was observed in the cpxAR gene-deletion mutant. BaeR and CpxR directly bound to different sequences of the acrD and mdtA promoter regions. These observations indicate that BaeR is a primary regulator, and CpxR enhances the effect of BaeR.
Introduction
Some multidrug exporters including AcrAB in Escherichia coli and MexAB in Pseudomonas aeruginosa play a major role in bacterial intrinsic tolerance against various drugs and toxic compounds (Nikaido, 1996; 1998; Poole, 2001). In addition, multidrug resistance mutants because of the overexpression of these drug exporters have been clinically isolated.
Our previous comprehensive expression cloning studies revealed that the E. coli chromosome encodes 20 intrinsic drug exporter systems that actually confer drug resistance when they are overexpressed (Nishino and Yamaguchi, 2001a). However, most of them are poorly expressed under normal laboratory conditions except acrAB, mdfA and emrE (Sulavik et al., 2001), therefore to elucidate the expression mechanisms of these drug exporter genes is not only interesting as to the study of novel expression-control mechanisms but also important for conquering future potential multidrug resistance.
Recently, we found that overexpression of some response regulators of two-component signal transduction systems upregulates a variety of drug exporter genes including acrD, emrKY, mdtABC and mdtEF (formally named yhiUV ) in E. coli (Kato et al., 2000; Nishino and Yamaguchi, 2001b; 2002; Baranova and Nikaido, 2002; Nagakubo et al., 2002; Hirakawa et al., 2003a,b).
Two-component signal transduction systems are general mechanisms for environmental responses and adaptation of microorganisms (Mizuno, 1997; 1998). A typical two-component system consists of two types of signal transducers, a sensor kinase and its cognate response regulator. The sensor kinase monitors the environmental conditions and accordingly modulates the phosphorylation state of the response regulator (Parkinson, 1993; Hoch and Varughese, 2001). As a small part of a response regulator is naturally phosphorylated in the cell without a corresponding sensor kinase, the overexpression of a response regulator can mimic the activation by a two-component system (Aguilar et al., 2001). Our previous comprehensive cloning of 32 putative E. coli response regulator genes revealed that five of them, baeR, evgA, cpxR, ompR and rcsB, upregulate a variety of drug exporter genes (Hirakawa et al., 2003a,b). However, there is no information as to which signal induces the expression of these drug exporter genes.
Kawamura et al. reported that an E. coli multidrug exporter, AcrEF, excreted indole (Kawamura-Sato et al., 1999). Indole is metabolically synthesized in E. coli cells from tryptophan by a tryptophanase (Snell, 1975). The indole concentration in the culture medium increases with cell growth. Wang et al. reported that indole regulates the expression of some genes such as astD, tnaB and gabT (Wang et al., 2001). DeMoss reported that several Gram-negative species, including E. coli, Klebsiella oxytoca, Haemophilus influenzae and so on, showed indole production (DeMoss and Moser, 1969). Therefore, indole acts as a stationary-phase extracellular signal for many bacteria, whereas the physiological significance of indole sensing is poorly understood.
In this study, we found that indole induces the expression of several multidrug exporter genes, and confers acquired multidrug resistance to E. coli via both two-component signal transduction pathways and other two-component system-independent pathways.
Results
Indole induces the expression of various drug exporter genes
In the previous study, we found that the expression of some E. coli drug exporter genes was regulated by two-component signal transduction systems (Nagakubo et al., 2002; Nishino and Yamaguchi, 2002; Hirakawa et al., 2003a). However, the signals causing upregulation of the expression of these exporter genes remain unknown. Indole is a potential candidate for such a signal molecule because there has been a report that indole is excreted by a drug exporter, the AcrEF system (Kawamura-Sato et al., 1999). We investigated the effect of indole treatment on the transcript levels of 20 drug exporter genes by means of quantitative real-time reverse transcription polymerase chain reaction (RT-PCR). As a result, the expression of acrD, acrE, cusB, emrK, yceL and mdtE was found to be significantly increased at least fivefold by indole in a dose-dependent manner (Table 1). Among them, the induction level of the mdtE gene was greatest (about 22-fold). The second greatest induction was observed for acrD (6.5-fold). The induction levels increased in proportion to the indole concentration. The remaining genes (acrA, bcr, emrA, emrD, emrE, fsr, macA, mdfA, mdtA, yceE, ydgF, ydhE, yidY and yjiO) were not significantly induced by indole (lower than fivefold). The cell growth itself was not affected by the concentrations used in this experiment.
| Gene | Fold changea | ||
|---|---|---|---|
| 0.5 mMb | 1 mMb | 2 mMb | |
| acrA | 1.2 | 0.8 | 0.8 |
| acrD | 1.6 | 1.9 | 6.5 |
| acrE | 0.8 | 2.2 | 5.9 |
| bcr | 1.6 | 1.5 | 2.6 |
| cusB | 1.0 | 2.0 | 5.1 |
| emrA | 1.4 | 1.3 | 2.3 |
| emrD | 1.2 | 1.1 | 1.7 |
| emrE | 1.0 | 0.7 | 1.4 |
| emrK | 1.2 | 2.3 | 6.1 |
| fsr | 1.6 | 1.4 | 2.3 |
| macA | 1.5 | 1.3 | 2.7 |
| mdfA | 1.5 | 1.1 | 1.3 |
| mdtA | 1.7 | 1.3 | 2.7 |
| yceE | 1.0 | 1.0 | 2.3 |
| yceL | 1.5 | 2.2 | 6.4 |
| ydgF | 0.9 | 0.7 | 2.2 |
| ydhE | 1.6 | 1.0 | 1.6 |
| mdtE (yhiU) | 2.4 | 5.5 | 22.0 |
| yidY | 1.7 | 1.4 | 3.1 |
| yjiO | 1.2 | 1.4 | 4.6 |
- The average results of three independent experiments are shown. The amounts of mRNAs were determined in MC4100 cells as the host.
- a . Values indicates fold change of transcripts level of the cells cultured with indole against that of the host strain cultured only with the solvent (dimethylsulphoxide).
- b . Indole concentration.
Indole treatment confers drug resistance to E. coli via upregulation of drug exporter genes, acrD and mdtEF
When the expression of drug exporter genes is upregulated by indole, it is expected that indole-induced drug resistance should be observed in ΔacrB drug-hypersensitive mutants. ΔacrB E. coli cells showed a low survival rate in the presence of 12.5 mg l−1 rhodamine 6G (1.48%) or 100 mg l−1 SDS (0.75%) (Fig. 1A and B). When cells were treated with 2 mM indole, the survival rate greatly increased up to 60.2% with rhodamine 6G and 4.56% in SDS, while the survival rate with carbenicillin was moderately increased (2.8-fold) (Fig. 1A–C). We previously reported that the overexpression of E. coli multidrug exporters MdtEF, AcrD and AcrEF conferred resistance to rhodamine 6G and SDS (Nishino and Yamaguchi, 2001a). When the mdtEF gene was deleted, the indole-induced resistance to rhodamine 6G was significantly reduced but slightly remained (Fig. 1A). On the other hand, indole-induced SDS resistance was completely lost in the acrD gene-deletion strain (Fig. 1B). These results indicate that the expression of MdtEF and AcrD are major causes of indole-induced resistance against rhodamine 6G and SDS respectively. AcrEF did not contribute to the MdtEF-independent indole-induced rhodamine 6G resistance (data not shown).
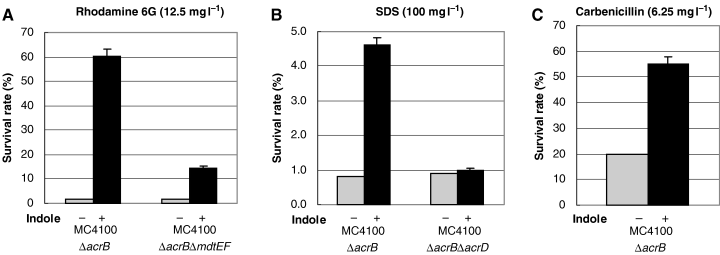
Survival rate assay for indole-induced drug resistance. The strains (MC4100ΔacrB, MC4100ΔacrBΔmdtEF and MC4100ΔacrBΔacrD) were grown to OD600 = 0.8 in LB broth with (black bars) or without (grey bars) 2 mM indole. Rhodamine 6G 12.5 mg l−1 (A), SDS 100 mg l−1 (B), or carbenicillin 6.25 mg l−1 (C) was added to the culture medium, followed by incubation for 30 min at 37°C. Then, an appropriate volume of the cell culture was spread on a YT agar plate. After overnight culture, the number of colonies was determined. At least three independent experiments were repeated.
Indole signalling is transmitted via BaeSR and CpxAR, two-component signal transduction pathways, to cause the induction of acrD and mdtABC gene expression
We previously reported that the acrD gene is upregulated by response regulators BaeR and CpxR (Hirakawa et al., 2003a), and that the mdtEF gene is upregulated by EvgA (Nishino and Yamaguchi, 2002). BaeR and EvgA also upregulate the mdtABC and emrKY genes respectively (Nishino and Yamaguchi, 2001b; Nagakubo et al., 2002). Therefore, we investigated whether or not these two-component signal transduction systems contribute to the indole signalling pathway. The lacZ reporter gene was fused to the promoter regions of acrD, mdtA, emrK and yhiE. The resulting plasmids were named pNNacrD, pNNmdtA, pNNemrK and pNNyhiE. As the mdtEF genes are transcribed in the same operon as the yhiE gene (Masuda and Church, 2003) (our unpubl. data), the yhiE promoter-fused lacZ is a reporter for MdtEF expression. This exporter gene expression was measured as reporter enzyme activity of the cells cultured in the presence of 2 mM indole until OD600 of 0.8. The indole effect on the reporter enzyme activities in wild-type cells supported the results of RT-PCR experiments (Fig. 2A–D). When the activation was tested at the earlier point in the growth phase, similar results were obtained in the case of acrD and mdtA, whereas the degree of the activation of yhiE (mdtE) was far lower than that of OD of 0.8 (data not shown). The acrD gene expression was about fivefold increased by indole treatment in the wild-type strain, while the induction was almost completely lost in the baeSR single and baeSR-cpxAR double-deletion mutants, and reduced to about one-half in the cpxAR-deletion mutant (Fig. 2A). The sensor kinase baeS mutant also lost the indole induction of the acrD gene (Fig. 2A). The indole induction rate for acrD in the cpxA sensor kinase-deletion mutant was also certainly diminished, though the absolute β-galactosidase activity of the cpxA-deletion mutant was somehow higher than that of the wild-type strain both in the presence and absence of indole. As to the mdtA gene, the expression was increased by indole treatment by a factor of about 4 (Fig. 2B). The baeS and baeSR deletion mutants showed significantly reduced indole induction rates for mdtA. In the cpxA and cpxAR deletion mutants, the indole induction rate was also decreased but the remaining induction level was greater than in the case of ΔbaeSR. The baeSR-cpxAR double-deletion mutant completely lost the indole induction of mdtA. In contrast to acrD and mdtA, the indole induction of the yhiE and emrK genes was not affected at all by deletion of the evgSA gene (Fig. 2C and D). These results indicate that the induction of acrD and mdtA by indole is mediated by the BaeSR and CpxAR systems, whereas that of yhiE and emrK is independent of the EvgSA system.

The effect of deletion of two-component signal transduction systems on the indole-induced expression of the acrD, mdtA, yhiE and emrK genes. The strains (wild-type, ΔbaeS, ΔbaeSR, ΔcpxA, ΔcpxAR, ΔbaeSRΔcpxAR and ΔevgSA) were grown to OD600 = 0.8 in LB broth with (black bars) or without (grey bars) 2 mM indole. The effects of indole on expression of lacZ fusions of acrD (A), mdtA (B), yhiE (C) and emrK (D) were monitored as β-galactosidase activity. At least three independent experiments were repeated.
The upregulation of the acrD and mdtA genes by the CpxAR system depends on the BaeSR system
We previously found that BaeR and CpxR overexpression caused upregulation of acrD and mdtA gene expression on RT-PCR analysis (Hirakawa et al., 2003a). In this study, we investigated the effects of BaeR and CpxR overexpression on the reporter enzyme activities of the acrD and mdtA genes (Fig. 3A–D). As shown in Fig. 3, the effect of BaeR overexpression on acrD and mdtA gene expression (about 20- and 146-fold increase) was greater than that of CpxR (about 7.3- and 2.5-fold increase) in wild-type cells. Interestingly, CpxR overexpression did not cause the upregulation of the acrD and mdtA genes in the baeS, baeR and baeSR deletion mutants (Fig. 3A and B). In contrast, the upregulation of these exporter genes by BaeR overexpression was not affected in the CpxAR deletion mutant (Fig. 3C and D). These results indicate that CpxAR signalling depends on the BaeSR system.
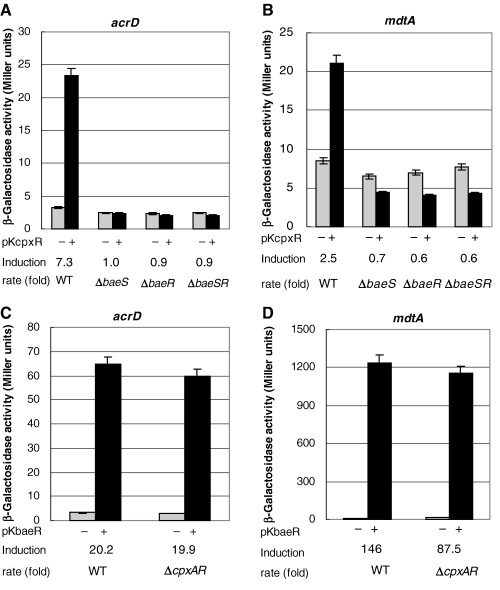
The effect of baeSR and cpxAR deletion on the CpxR and BaeR overexpression-induced upregulation of acrD and mdtA gene expression. The strains (wild-type, ΔbaeS, ΔbaeR, ΔbaeSR and ΔcpxAR) harbouring multicopy plasmid pKcpxR (A, B) or pKbaeR (C, D) were grown to OD600 = 0.4 in LB broth. Then, 0.5 mM IPTG was added, and the cells were incubated for 2 h at 37°C. Then, the β-galactosidase activity of lacZ fusions of acrD (A, C) and mdtA (B, D) was measured. At least three independent experiments were repeated.
Activation of BaeR by indole depends on its putative phosphorylation site
In a two-component system, a sensor kinase phosphorylates the conserved aspartic acid in a response regulator. The phosphorylation is a trigger for a signal transduction event. In the BaeR response regulator, aspartate 61 is postulated to be the phosphorylation site (Nagasawa et al., 1993). We replaced Asp61 with alanine. In order to determine the effect of indole, we used the low copy number plasmid pTH19kr (Hashimoto-Gotoh et al., 2000) as the baeSR cloning vector in the baeSR-deletion mutant. Transformation of ΔbaeSR strains with this vector encoding baeSR genes did not cause upregulation of the acrD gene without indole (Fig. 4). As shown in Fig. 4, the baeSR-deletion mutant transformed by the mock vector did not show indole-induced upregulation of the acrD gene, while pTHbaeSR transformation restored the indole induction similar to that in the wild-type cells. In cells transformed with the D61A mutant plasmid, pTHbaeD61A, indole induction of the acrD gene was greatly reduced. These results indicate a possibility that the indole-induced signal transduction is transmitted via phosphorylation of BaeR. The remaining low level induction in the pTHbaeD61A-transformed cells might be due to a possible secondary phosphorylation site of BaeR.
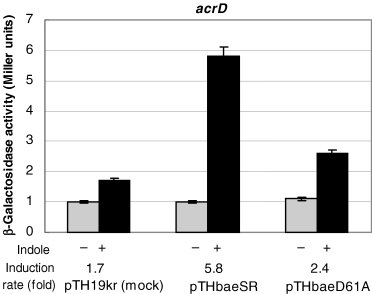
The effect of mutation of the BaeR phosphorylation site on the indole-induced acrD expression in ΔbaeR strains carrying plasmids encoding BaeR. The baeSR-deletion mutant strains harbouring a low copy plasmid, pTH19kr (mock), pTHSR (encoding BaeSR) or pTHD61A (encoding BaeS and D61A mutant of BaeR), were grown to OD600 = 0.8 in LB broth with (black bars) or without (grey bars) 2 mM indole. The effects of indole on expression of lacZ fusions of acrD were monitored as β-galactosidase expression. At least three independent experiments were repeated.
BaeR and CpxR directly bind to the promoter regions of the acrD and mdtA genes
As described under Experimental procedures, 276 bp, 321 bp and 250 bp DNA fragments of the acrD (−1 to −276), mdtA (−1 to −321) and emrK (−1 to −250) promoter regions were prepared by PCR. Polyhistidine-tagged BaeR and CpxR proteins were purified on Ni-chelated resin, followed by phosphorylation with acetylphosphate. The electrophoretic mobilities of the DNA fragments of the acrD and mdtA promoter regions shifted on the addition of phosphorylated BaeR and CpxR, whereas that of emrK (negative control) was not affected (Fig. 5), indicating the BaeR and CpxR proteins directly bind to the promoter regions of the acrD and mdtA genes. Purified BaeR and CpxR proteins without acetylphosphate treatment also bind to the promoter regions of these genes, while the concentrations of the non-treated purified proteins required for the electrophoretic mobility shifts were higher than those of phosphorylated ones, indicating that the significant part of the purified proteins might be intrinsically phosphorylated (data not shown).
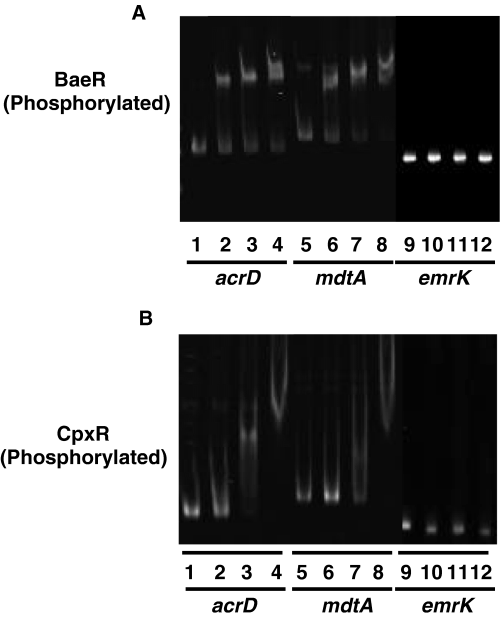
Electrophoretic mobility shift assay for the BaeR and CpxR binding to the acrD and mdtA promoter regions. A total of 0.15 pmol of DNA fragments including the acrD (276 bp), mdtA (321 bp), or emrK (250 bp) (control) promoter region were incubated without or with various concentrations of His6-BaeR (A) and His6-CpxR (B). Lanes 1, 5 and 9, no regulator; BaeR protein: lanes 2, 6 and 10, 0.5 µM; lanes 3, 7 and 11, 1.1 µM; lanes 4, 8 and 12, 2.3 µM. CpxR protein: lanes 2, 6 and 10, 0.25 µM; lanes 3, 7 and 11, 0.5 µM; lanes 4, 8 and 12, 1.1 µM. Samples were electrophoresed on a 7% non-denaturing poly acrylamide gel for the DNA-BaeR complex, and a 4% one for DNA-CpxR.
Then, the binding sites were determined by footprinting analysis. As to the acrD promoter region, BaeR bound to the 31 bp region between −75 and −105 from the acrD start codon, while CpxR bound to three separate sites, the 29 bp region between −97 and −125 (site I), the 31 bp between −127 and −157 (site II), and the 31 bp between −159 and −189 (site III) (Fig. 6A and B). It should be noted that the first binding site of CpxR (site I) partially overlaps that of BaeR. As to the mdtA promoter region, BaeR bound to the 33 bp between −65 and −97, while CpxR bound to two separate sites, the 30 bp between −87 and −116 (site I), and the 31 bp between −146 and −176 (site II) (Fig. 6A and C). The first binding site of CpxR again overlaps that of BaeR in the mdtA promoter region. The footprinting analysis on the reverse strands gave the same results (data not shown). Wulf et al. reported that CpxR-recognition requires two tandem 5′-GTAAA-3′ motifs separated by a 5 bp linker (Wulf et al., 2002). The homologous sequences actually exit in the CpxR binding sites of the acrD and mdtA promoter regions. The results suggest that BaeR primarily binds to the proximal region to play a major role in the upregulation of exporter gene expression, and CpxR binds to multiple distal regions to modulate the effect of BaeR.
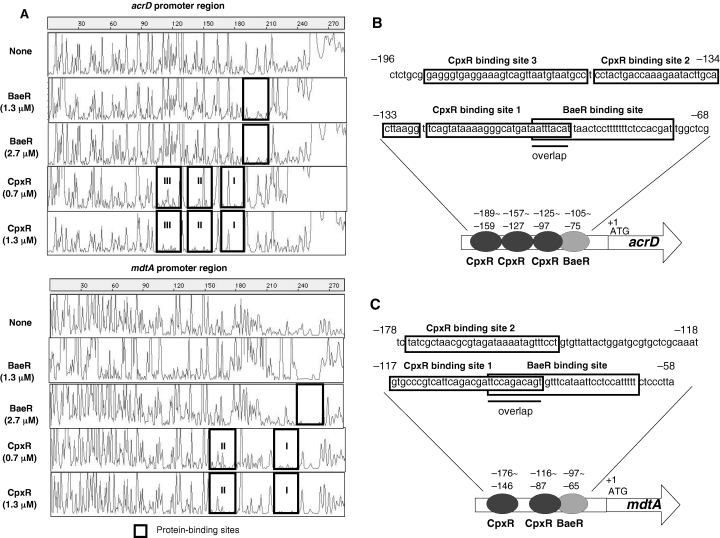
DNase I footprinting analysis of BaeR and CpxR binding to the acrD and mdtA promoter regions. A. DNA fragments (0.45 pmol) including the acrD and mdtA promoter regions were labelled with 6-FAM at the 5′ end, incubated with His6-BaeR (2.7 or 1.3 µM) or His6-CpxR (1.3 or 0.7 µM) and then subjected to DNase I footprinting assays using an ABI PRISM 310 Gene Scan. The fluorescence intensity (ordinate) of 6-FAM-labelled DNA fragments is plotted against the sequence length of the fragment (abscissa). Protein-binding sites are enclosed in squares. B, C. DNA sequences around the protein-binding sites of acrD (B) and mdtA (C). Protein-binding sites are enclosed in squares. The sequence numbers indicate the positions from the start codons.
Indole signalling to cause yhiE gene expression is transmitted via GadX
As shown in Fig. 2C, the expression of the yhiE gene induced by indole was independent of the EvgSA system. Recently, Tucker et al. reported that the yhiE gene is regulated by the GadX transcriptional activator that confers E. coli acid resistance (Tucker et al., 2003). In the gadX-deletion mutant, the indole induction of the yhiE gene was completely abolished, indicating that the indole signalling causing yhiE gene expression is transmitted via GadX (Fig. 7). In the real-time PCR experiment, the mRNA level of the gadX gene increased with 2 mM indole treatment by a factor of 4.5. Thus, the control of expression of the yhiE gene by indole might be regulated via the control of gadX gene expression.
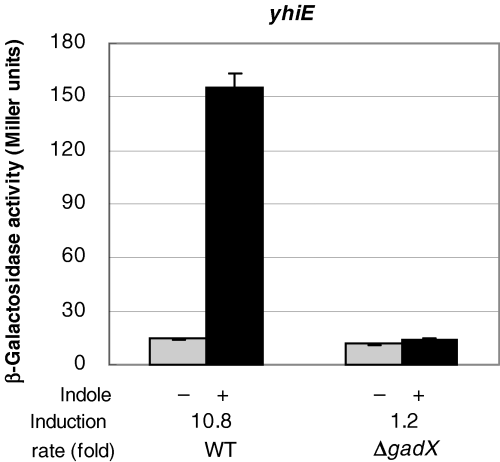
The effect of deletion of the gadX gene on the indole-induced upregulation of yhiE gene expression. The strains (wild-type and ΔgadX) were grown to OD600 = 0.8 in LB broth with (black bars) or without (grey bars) 2 mM indole. The effects of indole on the expression of lacZ fusions of yhiE genes were monitored as β-galactosidase expression. At least three independent experiments were repeated.
The effect of conditioned medium prepared from the wild-type and a tnaAB mutant E. coli strain on the expression of the acrD, mdtA and yhiE genes
The intracellular production of indole from tryptophan is catalysed by tryptophanase, TnaA. In order to investigate whether indole production by E. coli actually causes upregulation of the acrD, mdtA and yhiE genes or not, the tnaAB genes were deleted from the wild-type strain MC4100 by means of homologous recombination as described in Experimental procedures. Conditioned mediums were prepared from the wild-type strain MC4100 and the ΔtnaAB mutant at the stationary phase culture as described in Experimental procedures. Then, E. coli ΔtnaAB cells carrying reporter plasmids were cultured with the conditioned medium until the absorbance at 600 nm reached 2.5. The reporter enzyme activities of the acrD, mdtA and yhiE gene promoters of the cells cultured with conditioned medium from the wild-type strain were significantly higher than those of the cells cultured with conditioned medium from the ΔtnaAB mutant by a factor of about 2.1, 1.7 and 5.1 respectively (Fig. 8A–C). These results clearly indicate that the indole production by E. coli cells at the stationary phase actually controls the expression of the multidrug exporter genes.
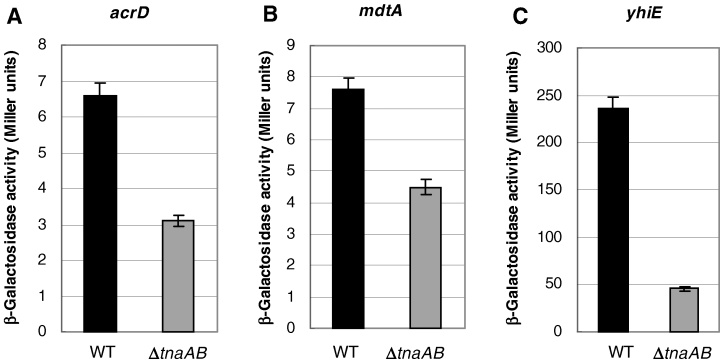
Activation of acrD, mdtA, yhiE gene expression by conditioned medium from the wild-type or a ΔtnaAB mutant E. coli strains. The MC4100ΔtnaAB cells carrying reporter plasmids were grown to OD600 = 2.5 in conditioned medium prepared from MC4100 (black bars) or MC4100ΔtnaAB (grey bars) supplemented with fresh YT medium. The expression of acrD, mdtA and yhiE genes was monitored as β-galactosidase activity expressed from lacZ fused to the promoter regions of these exporter genes in the reporter plasmids. At least three independent experiments were repeated.
Discussion
Grkovic et al. recently summarized the mechanisms for the regulation of drug exporter gene expression known at that time (Grkovic et al., 2002). For instance, the expression of acrAB in E. coli is controlled by repressor protein AcrR, global activator protein MarA, SoxS, Rob and quorum sensing regulator LuxR homologue protein SdiA. AcrR represses both itself and acrAB transcription, although the repression is very weak. The acrAB gene is induced by various environmental stresses, such as ethanol, osmotic shock and oxidizing agents via MarA and SoxS (Ma et al., 1996). Recently, it was reported that acrAB is also induced by bile salts and fatty acids via the Rob system (Rosenberg et al., 2003). However, these acrAB induction rates are low (about twofold increase), probably because of the semi-constitutive expression of acrAB caused by the leakiness of AcrR control. SdiA overexpression also increases the transcriptional level of acrA in addition to that of acrD (Wei et al., 2001). Furthermore, it has been reported that carbonyl cyanide m-chlorophenylhydrazone (CCCP), 2,4-dinitrophenol, tetrachlorosalicylanilide (TCS) and nalidixic acid upregulate the expression of emrAB, via the action of the EmrR repressor protein (Lomovskaya et al., 1995). As for the other E. coli drug exporter genes, signals that induce their expression remain unknown.
In this study, we found that indole highly upregulates the expression of various drug exporter genes. Among them, the mdtEF gene was the most induced by a factor of 22. Indole-induced expression of mdtEF requires the gadX gene, which encodes an AraC-type transcription factor. GadX is known to induce various genes including gadA, hdeAB and yhiE under low pH conditions (Masuda and Church, 2003; Ma et al., 2003). However, as indole obviously does not cause acidification of the medium, our results indicate that GadX responds to indole even under non-acidic conditions.
In this study, we also found that indole induces acrD and mdtABC gene expression via the BaeSR and CpxAR two-component signal transduction systems. We previously reported that the overexpression of BaeR and CpxR causes significant upregulation of acrD and mdtABC genes (Hirakawa et al., 2003a). In this study, we found that the CpxR-overexpression-mediated upregulation of acrD and mdtABC genes strictly depends on baeSR genes, while BaeR-overexpression-mediated upregulation of these exporter genes is independent to cpxAR genes (Fig. 3). These observations suggest the existence of cross-talk between BaeSR and CpxAR systems. As for the acrD and mdtABC upregulation, the former is primary and latter is secondary. In the case of acrD gene, the above finding is consistent with the indole signalling via BaeSR and CpxAR systems, that is the acrD upregulation by indole strictly depends on baeSR while only partially depends on cpxAR (Fig. 2A). On the other hand, in the case of mdtA gene, the upregulation by indole reduced but remained in the baeSR-deletion strains and completely abolished in the baeSR-cpxAR double-deletion strains (Fig. 2B), suggesting that the unknown indole signalling pathway via factor X from CpxA may also upregulate mdtABC independent to BaeR therefore indole can stimulate mdtABC in the baeSR knockout strain, whereas indole can not stimulate mdtABC in the baeSR-cpxAR double knockout strain because factor X does not work in the absence of CpxA.
Raffa and Raivio reported that indole induces the expression of spy gene via BaeSR and CpxAR (Raffa and Raivio, 2002). Thus, we also investigated the indole signalling to spy gene expression via BaeSR and CpxAR. Surprisingly, CpxR-overexpression-mediated spy gene upregulation was independent to baeSR gene as well as BaeR-overexpression-mediated spy gene upregulation which was independent to cpxAR gene (data not shown). Thus, the CpxAR and BaeSR are not likely to cross-talk in the upregulation of spy gene.
We propose a model for the indole-induced regulatory network of the drug exporter genes (Fig. 9). Indole first acts on the sensor kinases BaeS and CpxA, and then the signals are transmitted to the cognate response regulators BaeR and CpxR. The activated BaeR and CpxR directly bind to different sites in the acrD and mdtA promoter regions, resulting the upregulation of the expression of acrD and mdtABC. BaeR alone upregulates the expression of target genes without CpxR, whereas CpxR enhances the effect of BaeR by direct binding to the multiple regions upstream of the BaeR-binding site. BaeR may be a primary activator for the acrD and mdtABC genes, while CpxR may be a modulator for the BaeR action. This is the novel cross-talk between the response regulators of two-component signal transduction systems. In addition, the signalling pathway from CpxA via factor X might also upregulate mdtA. This pathway seems to be independent to BaeR. On the other hand, indole upregulates the mdtEF gene expression via GadX without EvgSA. We also confirmed that the deletion of baeSR and cpxAR genes did not affect the upregulation of mdtEF by indole (data not shown). In addition, gadX deletion also did not affect the upregulation of acrD and mdtA by indole at all. Therefore, GadX pathway of indole signalling is clearly independent to these two-component signal transduction systems.
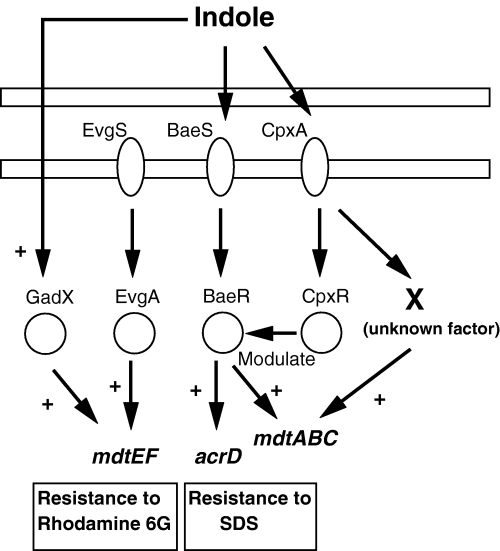
Proposed regulatory network of drug exporter genes induced by indole. The induction of the acrD and mdtABC genes by indole is mediated via two-component signal transduction systems (BaeSR and CpxAR). First, indole acts on the sensor kinases, BaeS and CpxA, and then the signal was transmitted to the cognate response regulators, BaeR and CpxR. BaeR and CpxR activated by indole upregulate target genes by direct binding to the promoter regions, while BaeR is essential and CpxR modulates the BaeR action. There may be an additional signalling pathway from CpxA via unknown factor X to upregulate the mdtA gene. The factor X pathway seems to be independent to BaeR. The induction of the mdtEF gene by indole is not mediated by EvgSA but mediated by GadX, one of the regulators that confer acid-induced resistance. Symbol ‘+’ represents upregulation.
Do BaeS, CpxA and GadX directly sense the indole molecule? High concentration of indole is toxic to E. coli, possibly because of injury to the plasma membrane causing superoxide generation (Garbe et al., 2000). As CpxA is an envelope stress sensor (Raivio and Silhavy, 1999), it might sense the indole-induced membrane injury. However, indole concentration used in this study was sufficiently lower than the concentration affecting the cell growth. In addition, physiologically produced indole by E. coli actually upregulated these exporter gene expression. Thus, the induction of E. coli drug exporter genes by indole is not likely to be a result of the envelope stress response. Recently, it was reported that indole acts as an extracellular signal like N-acyl homoserine lactone and AI-2 (Baca-DeLancey et al., 1999; Fuqua et al., 2001; Wang et al., 2001; Bassler, 2002; Chen et al., 2002). The indole concentration increases with cell growth as a byproduct of pyruvate production in E. coli cells (Wang et al., 2001). Therefore, indole might be an extracellular signal acting in the stationary phase to induce drug exporter genes. Besides, it has been reported that indole regulates biofilm formation by E. coli associated with the virulence of H. influenzae (Martin et al., 1998; Martino et al., 2003). Anyway, we believe that the indole sensing mechanism revealed in this study must be useful for drug development to overcome possible multidrug resistance pathogens.
Experimental procedures
Bacterial strains and plasmids
The bacterial strains and plasmids used in this study are presented in Table 2. Chromosomal DNAs of E. coli MC4100 (Casadaban, 1976) and W3104 (Yamamoto et al., 1981) were used as PCR templates. The construction of drug exporter genes and two-component system gene-deletion mutants of E. coli MC4100 was performed by the gene replacement method previously described, using the pKO3 plasmid (Link et al., 1997). Basically, the entire genes targeted for inactivation were replaced to an appreciate 33 bp linker sequence remaining the start and stop codons. However, when the baeS gene was deleted, the 21 bp of the baeS C-terminal including ribosomal binding site was remained to maintain the expression of the downstream baeR gene. The primers used for the deletion experiments were listed in Table 3. E. coli cells were grown in Luria–Bertani (LB) medium, supplemented with appreciate antibiotics when necessary, under aerobic conditions at 37°C.
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| E. coli strains | ||
| W3104 | Wild type, chromosomal DNA used for PCR amplification | Yamamoto et al. (1981) |
| MC4100 | F–araD139 Δ(argF-lac) U169 rpsL150 (Strr) relA1 flbB5301 deoC1 ptsF25 rbsR | Casadaban (1976) |
| MC4100ΔacrB | Derivative of MC4100 that lacks a restriction system and acrB | This study |
| MC4100ΔacrBΔacrD | Derivative of MC4100ΔacrB that lacks acrD | This study |
| MC4100ΔacrBΔmdtEF | Derivative of MC4100ΔacrB that lacks mdtEF | This study |
| MC4100ΔbaeSR | Derivative of MC4100 that lacks baeSR | This study |
| MC4100ΔcpxAR | Derivative of MC4100 that lacks cpxAR | This study |
| MC4100ΔbaeS | Derivative of MC4100 that lacks baeS | This study |
| MC4100ΔbaeR | Derivative of MC4100 that lacks baeR | This study |
| MC4100ΔcpxA | Derivative of MC4100 that lacks cpxA | This study |
| MC4100ΔbaeSRΔcpxAR | Derivative of MC4100 that lacks baeSR and cpxAR | This study |
| MC4100ΔevgSA | Derivative of MC4100 that lacks evgSA | This study |
| MC4100ΔgadX | Derivative of MC4100 that lacks gadX | This study |
| Plasmids | ||
| pTrc99K | Expression vector; Kmr; multiple cloning site downstream of trc promoter | Hirakawa et al. (2003a) |
| pKbaeR | Overexpresses BaeR | Hirakawa et al. (2003a) |
| pKcpxR | Overexpresses CpxR | Hirakawa et al. (2003a) |
| pNN387 | Cmr; single-copy vector containing promoter less lacZY | Elledge and Davis (1989) |
| pNNacrD | pNN387 (acrD promoter –lacZ) | This study |
| pNNmdtA | pNN387 (mdtABC gene promoter –lacZ) | This study |
| pNNyhiE | pNN387 (yhiEmdtEF gene promoter –lacZ) | This study |
| pNNemrK | pNN387 (emrKY gene promoter –lacZ) | This study |
| pUCbaeSR | High level expresses BaeS and BaeR | Nagakubo et al. (2002) |
| pTH19kr | Kmr; low-copy vector | Hashimoto-Gotoh et al. (2000) |
| pTHbaeSR | Low level expresses BaeS and BaeR | This study |
| pTHbaeD61A | Low level expresses BaeS and mutated BaeR (substituted Asp 61 for alanine) | This study |
| pKO3 | repA(Ts) CmrsacB+ | Link et al. (1997) |
| Oligonucleotide | Oligonucleotide sequence (5′ to 3′) |
|---|---|
| Used for gene deletion | |
| acrB-No | cgcggatccctgcgcctgaaacaggaactg |
| acrB-Ni | cacgcaataaccttcacactccaaatttataaccatgtcttaacggctcctgtttaag |
| acrB-Ci | cacgcaataaccttcacactccaaatttataactgatacaacgtgtaatcactaaggcc |
| acrB-Co | cgcggatccatggaaaaaacttactgacctggac |
| baeS-No | cgcggatccgtgcttggcagcatgggaa |
| baeS-Ni | cacgcaataaccttcacactccaaatttataaccttcattgcgcgctcctttt |
| baeS-Ci | gttataaatttggagtgtgaaggttattgcgtgacagtagagttaccgctggaacgg |
| baeS-Co | gcgggatccatggattttggcgcgtcggcc |
| baeR-No | cgcggatccgtcggtacactgaccaaactg |
| baeR-Ni | cacgcaataaccttcacactccaaatttataaccatacttctctctgtaaatcccgttc |
| baeR-Ci | gttataaatttggagtgtgaaggttattgcgtgtagttttagcgacattattttgttag |
| baeR-Co | cgcggatcccccggcatcgtaattacg |
| cpxA-No | cgcggatccgcgcctgcgtttggtgaaaac |
| cpxA-Ni | cacgcaataaccttcacactccaaatttataactaaacttcgcatttgcaggcagg |
| cpxA-Ci | gttataaatttggagtgtgaaggttattgcgtgcatgaagcagaaaccatcagatagcc |
| cpxA-Co | cgcggatcccttgacgtaatgatgccgaag |
| cpxR-Ci | gttataaatttggagtgtgaaggttattgcgtgcattgtttaaatacctccgaggcag |
| cpxR-Co | cgcggatcctaaaaccgcttgctgctccgg |
| evgSA-No | cgcggatccgaaaacgcaataaataaaactaccgcc |
| evgSA-Ni | cacgcaataaccttcacactccaaatttataaccatagattattccctttgcaatga |
| evgSA-Ci | gttataaatttggagtgtgaaggttattgcgtgtaaatagcggctcccaca |
| evgSA-Co | cgcggatcccatggcaccttttgatgttttcaatact |
| gadX-No | cgcggatcccctaaattgcgtggtagctga |
| gadX-Ni | cacgcaataaccttcacactccaaatttataacggaataagattatagagttttactcag |
| gadX-Ci | gttataaatttggagtgtgaaggttattgcgtgcatagttgacttaatattacataaaca |
| gadX-Co | cgcgtcgactgtacgacctctctgaacgtc |
| tnaAB-No | cgcggatcctttctccagcttctgtattgg |
| tnaAB-Ni | cacgcaataaccttcacactccaaatttataaccatttattttaattacagtgatccctg |
| tnaAB-Ci | gttataaatttggagtgtgaaggttattgcgtgtaaatccttcaagaagccagccattcg |
| tnaAB-Co | cgcgtcgacgacagcactttagcccgacg |
| Used for reporter plasmid construction, gel shift assay and footprinting | |
| acrD-PF | gcggcggccgcaacgcgcggaacggctagg |
| acrD-PR | gcgaagctttaaaagaggacctcgtgtttc |
| mdtA-PF | gcggcggccgcagcttatgactaagagcac |
| mdtA-PR | gcgcgttaagagtttctcttcctg |
| yhiE-PF | cgcgcggccgcttaccccggttgtcacccg |
| yhiE-PR | cgcaagcttaacttgctccttagccgttat |
| emrK-PF | cgcgcggccgcttggcactataagtcttct |
| emrK-PR | cgcaagctttattatctctcatttctcata |
| Used for expression cloning | |
| pQEbaeR-F | gggggatccaccgagttaccaatcgacg |
| pQEbaeR-R | cccaagcttctactaaacgatgcggcaggc |
| pQEcpxR-F | cgggatccaataaaatcctgttagttgatg |
| pQEcpxR-R | gcaagctttcatgaagcagaaaccatcag |
Transcriptional analysis of drug exporter genes
Cells were grown at 37°C in LB broth with indole (final concentration is 0.5, 1 or 2 mM) or only with the solvent (dimethylsulphoxide) as a control until the absorbance at 600 nm reached 0.8. The purification of total RNAs and cDNAs was performed by the method described previously (Hirakawa et al., 2003a). Specific primer pairs were listed in Table 4. Real-time PCR was performed with each specific primer pair, using SYBR Green PCR master mix (PE Applied Biosystems). E. coli rrsA gene was chosen as a control for the normalization of cDNA loading in each PCR. The reactions were performed with an ABI PRISM 7000 Sequence Detection System (PE Applied Biosystems).
| Oligonucleotide (gene name) | Forward primers (5′ to 3′) | Reverse primers (5′ to 3′) |
|---|---|---|
| rrsA | cggtggagcatgtggtttaa | gaaaacttccgtggatgtcaaga |
| acrA | gtctatcaccctacgcgctatctt | gcgcgcacgaacatacc |
| acrD | gtaccctggcgattttttcatt | cggtcactcgcacattcg |
| acrE | cgtgattgccgcaaaagc | ttggcgcagtgactttggta |
| bcr | tgtttttctgttcgtgatgaccat | ggaacatatttaacgcgccaat |
| cusB | cgcttaccgtgggcgata | ttccacccagtcaggaatgg |
| emrA | gcgaatattgaggtgcagaaaa | ggcacacggcggttgta |
| emrD | gtggatccccgactggttt | cccggcaccgaaaaaga |
| emrE | ggtattgtcctgattagcttactgtcat | gcacaaatcaacatcatgcctataa |
| emrK | gcgcttaaacgtacggatattaaga | actgtttcgccgacctgaac |
| fsr | tggtgttggcgcaaatca | tcgtcgctttgggttttcc |
| macA | cggtgattgccgcacaa | ttaccagcatggcgctcat |
| mdfA | cttgctgttagcgcgtctga | gccagccgcccataataat |
| mdtA | cgccgtagaacaggcagttc | tgcgcaccgtaacggtatta |
| mdtE | cccccggttcggtcaa | ggacgtatctcggcaacttcat |
| yceE | cggtattgtcttcagcattacatttt | ggcgagtccaccccaaa |
| yceL | ttttcaccctgatttgtctgttttat | cagcgaagcacttaaggtttca |
| ydgF | tgatgaaaattgccgggttaa | cgctttacgggtacctgatttta |
| ydhE | ccggttatcgcgcaattaaat | gaaaccttgtcgcacctgatg |
| yidY | tatcccgccgggattgatat | cgcttcgctggcattga |
| yjiO | cgtgattttaatgccgatgtca | gccataccgccagcaagat |
Construction of ΔtnaAB mutant and preparation of conditioned medium
The ΔtnaAB mutant was constructed on the basis of the method described above using tnaAB-No, tnaAB-Ni, tnaAB-Ci and tnaAB-Co as primers. Conditioned medium was prepared by inoculating 30 ml of LB broth with 300 µl of an overnight culture of E. coli MC4100 or MC4100ΔtnaAB followed by shaking (170 r.p.m.) at 37°C for 24 h (OD600 = 5.1). Cells were then removed by centrifugation and 30 ml of the resulting supernatants were supplemented with 750 µl of fresh 20× YT broth (without NaCl) and the pH was adjusted to 7.5. Supplemented conditioned medium was then sterilized by filtration through a 0.2 µm pore filter and used for cell culture.
Construction of reporter plasmids of drug exporter genes
The reporter plasmids were constructed as follows. DNA fragments including the putative promoter region (276 bp upstream of the start codon for acrD, 250 bp upstream of that for emrK, 321 bp upstream of that for mdtA and 798 bp upstream of that for yhiE) was amplified by PCR using primers listed in Table 3. The primer pairs were listed in Table 3. These DNA fragments were cloned in front of the lacZ reporter gene in a single-copy pNN387 vector (Elledge and Davis, 1989). The resulting plasmids were introduced into host cells for the β-galactosidase activity measurements.
Reporter gene assays
To determine the effect of indole on the transcription of various reporter constructs, each bacterial strain was grown at 37°C in LB broth containing indole and chloramphenicol until the absorbance at 600 nm reached 0.8. β-Galactosidase activity in cell lysates was assayed using o-nitrophenyl-β-D-galactopyranoside (ONPG) as a substrate as described by Miller with a slight modification (Miller, 1992). To determine the effect of overexpression of BaeR and CpxR, 0.5 mM IPTG was added to induce these response regulator genes when cultures reached an OD600 of 0.4. Then induction was performed for 2 h. Finally, the β-galactosidase activity in cell lysates was assayed.
Drug resistance assay
Each bacterial strain was grown at 37°C in LB broth containing indole. When the cultures reached an OD600 of 0.8, rhodamine 6G, SDS or carbenicillin was added. After incubation for 30 min, the percentage survival was calculated as the number of cfu ml−1 remaining after drug treatment per that before drug treatment. Each experiment was repeated at least three times.
Site-directed mutagenesis
The putative phosphorylation site, Asp 61, of BaeR was replaced with alanine by site-directed mutagenesis using pUCbaeSR as a template (Nagakubo et al., 2002). The mutagenesis was performed with mutagenic oligonucleotide (5′-ctaggacaatcgcgactacgagggacc-3′) containing mismatches for the desired amino acid replacements and silent mismatches producing new restriction enzyme sites by a method of Kunkel (Kunkel, 1985). Mutations were first detected by restriction enzyme (Aor51HI) analysis, and then verified by DNA sequencing.
Purification of BaeR and CpxR, and electrophoretic mobility shift assay
The baeR and cpxR genes were cloned into the pQE30 vector (Qiagen) using primers listed in Table 3. BaeR and CpxR were overproduced as His6-BaeR and His6-CpxR by IPTG induction, and then purified on Ni affinity resin (Amersham Pharmacia Biotech). DNA (the promoter region of the acrD, mdtA and emrK genes) probes were generated by PCR using E. coli MC4100 genomic DNA as a template using same primers described in construction of reporter plasmids. Then, the His6-BaeR and His6-CpxR proteins (final concentration, 200 µg ml−1) were phosphorylated with 50 mM acetylphosphate by incubation for 1 h at 30°C in a buffer containing 100 mM Tris (pH 7.4), 10 mM MgCl2 and 125 mM KCl. The probe DNA fragments (0.15 pmol) were mixed with phosphorylated His6-BaeR and His6-CpxR (0–23 pmol) in a 10 µl reaction mixture containing 100 mM Tris (pH 7.4), 10 mM MgCl2, 100 mM KCl, 10% Glycerol and 2 mM dithiothreitol. After incubation for 20 min at room temperature, samples were then electrophoresed on a 4% or 7% non-denaturing acrylamide Tris borate/EDTA gel in Tris borate/EDTA buffer at 4°C. The gel was soaked in 10 000-fold-diluted SYBR Green I nucleic acid stain (Cambrex), and DNAs were visualized under blue incident light at 460 nm (Luminescent Image Analyzer LAS-3000, FUJIFILM).
DNase I footprinting analysis
DNase I footprinting analysis was performed by means of non-radiochemical capillary electrophoresis, which was established by Wilson et al. (Wilson et al., 2001). The 6-FAM DNA fragments were synthesized by PCR primers listed in Table 3. The forward primers were labelled by 6-FAM prior to PCR. The 6-FAM labelled acrD and mdtA fragments (0.45 pmol) were mixed with purified His6-BaeR (0–138 pmol) and His6-CpxR (0–69 pmol) in a 50 µl reaction mixture containing the same buffer as above. After incubation for 20 min at room temperature, 0.3 units of DNase I (Promega) was added. After incubation for 60 s at room temperature, samples were purified for GeneScan sequencing analysis (PE Applied Biosystems). The prepared samples were electrophoresed using an ABI PRISM 310 Sequencer/Genetic Analyzer equipped with an ABI PRISM 310 Gene Scan, and fragment sizes were determined with Gene Scan Analysis software.
Acknowledgements
We wish to thank George M. Church for the plasmid pKO3. H. Hirakawa was supported by a research fellowship from the Japan Society for the Promotion of Science for Young Scientists. This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Zoonosis Control Project of the Ministry of Agriculture, Forestry and Fisheries of Japan.




