A membrane metalloprotease participates in the sequential degradation of a Caulobacter polarity determinant
Summary
Caulobacter crescentus assembles many of its cellular machines at distinct times and locations during the cell cycle. PodJ provides the spatial cues for the biogenesis of several polar organelles, including the pili, adhesive holdfast and chemotactic apparatus, by recruiting structural and regulatory proteins, such as CpaE and PleC, to a specific cell pole. PodJ is a protein with a single transmembrane domain that exists in two forms, full-length (PodJL) and truncated (PodJS), each appearing during a specific time period of the cell cycle to control different aspects of polar organelle development. PodJL is synthesized in the early predivisional cell and is later proteolytically converted to PodJS. During the swarmer-to-stalked transition, PodJS must be degraded to preserve asymmetry in the next cell cycle. We found that MmpA facilitates the degradation of PodJS. MmpA belongs to the site-2 protease (S2P) family of membrane-embedded zinc metalloproteases, which includes SpoIVFB and YluC of Bacillus subtilis and YaeL of Escherichia coli. MmpA appears to cleave within or near the transmembrane segment of PodJS, releasing it into the cytoplasm for complete proteolysis. While PodJS has a specific temporal and spatial address, MmpA is present throughout the cell cycle; furthermore, periplasmic fusion to mRFP1 suggested that MmpA is uniformly distributed around the cell. We also determined that mmpA and yaeL can complement each other in C. crescentus and E. coli, indicating functional conservation. Thus, the sequential degradation of PodJ appears to involve regulated intramembrane proteolysis (Rip) by MmpA.
Introduction
Aside from removing abnormal or misfolded proteins from the bacterial cell, proteolysis plays a key role in many regulatory processes that contribute to homeostasis and developmental changes (Gottesman, 2003; Jenal and Hengge-Aronis, 2003). In Caulobacter crescentus, temporally and spatially specific degradation of proteins helps ensure normal cell cycle progression (Quardokus and Brun, 2003; Ryan and Shapiro, 2003). During every round of the cycle, this α-proteobacterium executes an orchestrated cell differentiation program involving two distinct cell types: a motile swarmer cell and a sessile stalked cell (see Fig. 2D) (Ausmees and Jacobs-Wagner, 2003; Skerker and Laub, 2004). Only the stalked cell can initiate DNA replication, whereas the swarmer cell cannot replicate its chromosome until it differentiates into a stalked cell. During this swarmer-to-stalked transition, the flagellum, pili and chemotaxis machinery at one pole of the cell are lost and replaced by a stalk with holdfast material at its distal tip. As the stalked cell elongates to form a predivisional cell, it replicates its chromosome and assembles a new flagellum, chemotaxis machinery and pilus secretion apparatus at the pole opposite the stalk. An asymmetric cell division then produces a stalked cell and a new swarmer cell, which forms pili at the flagellated pole.
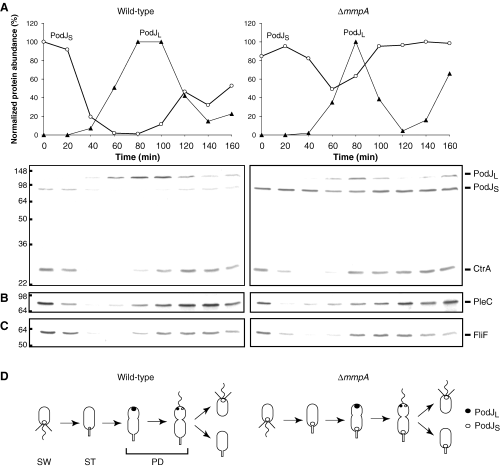
Comparison of wild-type (NA1000) and ΔmmpA (LS3706) strains over the course of the Caulobacter cell cycle. Swarmer cells were isolated by Ludox gradient centrifugation and released into M2G media. Samples were collected from the synchronous cultures every 20 min and subjected to immunoblotting. The blots were probed with antibodies against PodJ and CtrA (A), PleC (B), or FliF (C). The top graphs in (A) show relative levels of PodJL and PodJS during the cell cycle, as determined from corresponding immunoblots below the graphs. Protein positions are indicated on the right, while molecular weight standards in kDa are indicated to the left of the blots. (D) Schematic representation of the Caulobacter cell cycle showing swarmer (SW), stalked (ST) and predivisional (PD) cells and the subcellular positions of PodJL and PodJS in wild-type and ΔmmpA strains, as demonstrated below in Fig. 3.
Many structural and regulatory components have well-defined degradation patterns over the course of this cycle. For example, the loss of polar organelles during the swarmer-to-stalked cell transition is coupled to proteolytic turnover of their constituent proteins, such as FliF, a central motor component that anchors the flagellum in the cell membrane (Jenal and Shapiro, 1996; Grunenfelder et al., 2004). Of particular importance is the oscillating degradation pattern of master regulatory protein CtrA, which controls multiple processes that accompany cell cycle progression, including DNA replication and polar morphogenesis (Quon et al., 1996; Domian et al., 1997). This essential response regulator is cleared from the cell by the ClpXP protease during the swarmer-to-stalked transition; after being resynthesized in the stalked cell, it is degraded again in the stalked compartment of the predivisional cell once a diffusion barrier has been formed between the two daughter compartments (Domian et al., 1997; Jenal and Fuchs, 1998; Judd et al., 2003).
PodJ has been shown to be a localization factor involved in polar morphogenesis (Viollier et al., 2002a; Hinz et al., 2003) whose expression is regulated by the cyclic variation in CtrA and GcrA levels (Crymes et al., 1999; Laub et al., 2002; Holtzendorff et al., 2004). PodJ undergoes defined proteolytic processing (Viollier et al., 2002a; Hinz et al., 2003), with both the long form, PodJL, and the short form, PodJS, controlling different aspects of polar organelle development (Viollier et al., 2002a). The podJ gene is transcribed in the early predivisional cell, producing the full-length form of the protein (PodJL). This form contains a cytoplasmic N-terminal domain (residues 1–638), a single transmembrane segment (residues 639–662), and a periplasmic C-terminal domain (residues 663–974) (Fig. 1A). PodJL localizes to the incipient swarmer pole (Fig. 2D) and, by recruiting the CpaE pilus assembly protein and the PleC histidine kinase to that pole, provides the positional information required for pili biogenesis. As the cell divides, the periplasmic domain of PodJL is degraded, converting it to a truncated form (PodJS) that stays at the flagellated pole and segregates with the swarmer progeny cell when cytokinesis is completed. PodJS is needed for chemotaxis and holdfast formation, possibly by recruiting required components in a manner similar to PodJL (Viollier et al., 2002a). After serving its function at the swarmer cell pole, this truncated form is then degraded during the swarmer-to-stalked transition (Viollier et al., 2002a; Hinz et al., 2003). The cycle restarts in the early predivisional cell with synthesis and polar localization of new PodJL. In short, PodJ is sequentially proteolyzed at specific times and locations during the cell cycle that correspond to its assigned functions.

Steady-state levels of PodJ in protease mutants. A. Schematic representation of PodJ, CC1916 (MmpA) and CC3381, showing their membrane topology as predicted using MEMSAT 2 (McGuffin et al., 2000). Stars indicate the locations of HExxH and NPDG motifs conserved in the putative proteolytic active sites of MmpA and CC3381. N and C at the ends of the proteins are the amino and carboxyl termini respectively. MmpA contains a PDZ domain in its periplasmic region (Marchler-Bauer et al., 2003). B. Schematic representation of E. coli YaeL and B. subtilis SpoIVFB and their respective substrates, RseA and pro-σK (Rudner et al., 1999; Green and Cutting, 2000; Kanehara et al., 2001; Alba et al., 2002; Drew et al., 2002; Kanehara et al., 2002; Alba and Gross, 2004). YaeL and SpoIVFB belong to the S2P family of membrane-bound zinc metalloproteases (Lewis and Thomas, 1999; Rudner et al., 1999; Brown et al., 2000). Stars indicate the locations of conserved active site motifs. YaeL also contains a PDZ domain. IM space is the intermembrane space between mother cell and forespore. C. Immunoblots probed with antibodies against the cytoplasmic domain of PodJ, which recognize both PodJL and PodJS (top panel), or with antibodies against MmpA (bottom panel). The PodJ blot was also probed with CtrA antibodies as a control. Relevant genotypes of the strains being examined are shown above the blots. G indicates growth in the presence of glucose to repress expression from the Pxyl promoter, whereas X indicates growth in xylose to induce expression. The positions of various proteins are labelled on the right. The lower band (*) in the anti-MmpA blot results from non-specific cross-reaction. Numbers on the left represent molecular weight markers, in kDa. Cell cultures were grown in rich media and harvested for immunoblot analysis when OD600 reached 0.8–0.9. Strains used: NA1000, LS3706, LS3712, LS4034, LS3706/pMR10, LS3706/pJC210.
We have embarked on a systematic study of candidate proteases to identify components involved in the PodJ proteolytic pathway. A conserved clan of membrane-embedded zinc metalloproteases, with mammalian site-2 protease (S2P) and Bacillus subtilis SpoIVFB as its founding members, all share the active site motifs HExxH and NPDG (Fig. 1A and B) (Rawson et al., 1997; Lewis and Thomas, 1999; Rudner et al., 1999). Characterized members of this clan participate in what is termed regulated intramembrane proteolysis (Rip) (Brown et al., 2000; Urban and Freeman, 2002; Weihofen and Martoglio, 2003). These proteases appear to cleave within or near the transmembrane segments of their respective substrates, releasing the resulting polypeptides from the membrane. For instance, SpoIVFB converts its substrate pro-σK to active σK by removing the N-terminal extension of pro-σK that tethers it to the membrane (Fig. 1B) (Rudner et al., 1999; Yu and Kroos, 2000). We report that a member of the S2P/SpoIVFB clan in Caulobacter, encoded by CC1916, also participates in Rip. The CC1916 protease cleaves PodJS during the swarmer-to-stalked transition, releasing it from the membrane for subsequent degradation. In the absence of CC1916, PodJS remains at the nascent stalked cell pole, resulting in a bipolar distribution of PodJ isoforms. Thus, the CC1916 membrane metalloprotease serves to maintain the polar asymmetry of the PodJ protein.
Results
Membrane zinc metalloprotease MmpA participates in PodJ processing
To identify proteases responsible for the processing and degradation of PodJ, we assessed the levels of PodJL and PodJS in strains with mutations in genes encoding proteins that belong to the S2P clan of membrane zinc metalloproteases (Lewis and Thomas, 1999; Rudner et al., 1999). Members of this clan – including mammalian S2P, Escherichia coli YaeL, Enterococcus faecalis Eep, and B. subtilis SpoIVFB and YluC – cleave membrane-bound substrates (Fig. 1B) (Rawson et al., 1997; An et al., 1999; Rudner et al., 1999; Zelenski et al., 1999; Ye et al., 2000; Yu and Kroos, 2000; Alba et al., 2002; Kanehara et al., 2002; Schobel et al., 2004). Like their homologues, the predicted sequence of the Caulobacter proteins CC1916 and CC3381 contain HExxH and NPDG motifs that overlap or reside next to two of several transmembrane segments (Fig. 1A), as determined by topology prediction method MEMSAT 2 (McGuffin et al., 2000). CC1916 also contains a putative PDZ domain (Pallen and Ponting, 1997; Ponting, 1997; Marchler-Bauer et al., 2003), which has been shown in other systems to play a regulatory role in protein–protein interactions (van Ham and Hendriks, 2003; Schlieker et al., 2004).
We constructed strains with in-frame deletions of CC1916 and CC3381 and examined PodJ levels in the deletion mutants by immunoblot analysis, using polyclonal antibodies against the cytoplasmic domain of PodJ (Fig. 1C). As a control, we also observed the cumulative levels of the CtrA master regulatory protein (Quon et al., 1996). The steady-state level of PodJS increased in the CC1916 mutant, whereas it remained similar to the wild-type level in the CC3381 mutant, suggesting that CC1916 participates in the degradation of PodJS. In wild-type cells the levels of both CtrA (Domian et al., 1997; Jenal and Fuchs, 1998) and PodJL (Viollier et al., 2002a; Hinz et al., 2003) vary during the cell cycle. However, the levels of CtrA and PodJL did not change in either mutant, indicating that the activity of CC1916 is specific to PodJS. In addition, PodJL, PodJS and CtrA levels in the CC1916 CC3381 double mutant were similar to those in the CC1916 mutant; thus, CC1916 and CC3381 do not appear to play redundant roles in the PodJS proteolytic pathway.
We were able to complement the CC1916 deletion by placing the CC1916 gene under the control of a xylose-regulated promoter (Pxyl) on a low-copy plasmid (Fig. 1C). Growth in the presence of xylose induced CC1916 expression and reduced the level of PodJS back down to the wild-type level. No complementation occurred when the strain was grown with glucose, preventing expression from the Pxyl promoter. Growth in either glucose or xylose with the vector alone also failed to reduce the level of PodJS in the CC1916 mutant. Based on these results and homology to other proteases, we name the CC1916 gene mmpA, for membrane metalloprotease A.
The homologue of MmpA in E. coli, YaeL, cleaves its substrate RseA after RseA is first truncated by periplasmic protease DegS (see below) (Alba et al., 2002; Kanehara et al., 2002). By analogy, we asked if periplasmic proteases such as DegP, DegQ and DegS (Strauch and Beckwith, 1988; Strauch et al., 1989; Bass et al., 1996; Waller and Sauer, 1996; Alba et al., 2001) cleave the C-terminal domain of PodJ first before MmpA cleaves PodJS. Accordingly, we constructed strains with deletions in genes that encode putative homologues of these proteases, including CC0151, CC0702, CC1282 and CC2758. PodJ levels were similar to those in wild-type cells in all mutants examined, even one in which all four genes were deleted (data not shown). In addition, no obvious physiological defects were detected in these deletion strains. Thus, DegP, DegQ and DegS homologues are not necessary for PodJ processing in Caulobacter.
MmpA facilitates PodJS degradation during swarmer-to-stalked transition
We examined the effect of an mmpA deletion on PodJS levels during the cell cycle by immunoblot analysis (Fig. 2A). As reported previously (Viollier et al., 2002a; Hinz et al., 2003), in wild-type cells PodJL is synthesized in the early predivisional cell and converted to the shorter PodJS form around the time of cell division; PodJS becomes the only form present in the swarmer cell, and it is degraded as the swarmer cell differentiates into a stalked cell. In the mmpA mutant, PodJL is still converted to PodJS, but PodJS is not fully degraded during the swarmer-to-stalked transition. The pattern of CtrA proteolysis during the cell cycle was observed to be the same in wild-type and mmpA mutant strains (Fig. 2A). Thus, MmpA appears to show specificity in degrading PodJS at the swarmer-to-stalked transition. In addition to CtrA, cell cycle-dependent variation in the levels of representative proteins, such as FliF, McpA, PleC and CpaE, were similar in wild-type and mmpA cells (Fig. 2B and C, and data not shown). Hence, while PleC and CpaE depend on PodJ for localization, stabilization of PodJS in the mmpA mutant did not lead to stabilization of these downstream proteins.
Monitoring PodJ levels during the course of the cell cycle led us to make the following prediction regarding PodJ localization (Fig. 2D). PodJ typically localizes to one pole at a time, as PodJS is cleared from the nascent stalked pole before PodJL is newly synthesized and directed to the opposite pole. Since PodJS degradation is inhibited in the mmpA mutant, PodJS may remain at the stalked pole as newly synthesized PodJL localizes to the opposite pole, leading to bipolar localization of PodJ (Fig. 2D).
To test this hypothesis, we used fluorescence microscopy to examine strains in which the chromosomal podJ allele is replaced by a yfp–podJ fusion (Fig. 3). In the wild-type background, YFP-PodJ appeared as a single polar focus, as previously described (Viollier et al., 2002a). As predicted, mmpA mutant cells exhibited bipolar foci (in 25% of the cells examined; see TableS1). Complementation of the mmpA deletion with a plasmid expressing mmpA yielded cells with a single polar focus, whereas the mutant strain with the vector alone continued to exhibit bipolar localization. These results suggest that PodJS persists at the flagellated pole of the swarmer cell as it differentiates into the stalked pole in an mmpA mutant, providing further support for the protease's role in PodJS degradation. The mmpA deletion strain appears to carry out an otherwise normal swarmer-to-stalked cell transition. Closer examination of PodJ function in ΔmmpA strains revealed no effect on pili formation, as assessed by sensitivity to phage ΦCbK, and rosette formation and chemotaxis were both normal, indicating proper biogenesis of the holdfast and chemotactic machinery (data not shown).

Localization of PodJ. Live cells expressing yfp-podJ from the endogenous podJ locus (Viollier et al., 2002a) were placed on agarose pads and examined by microscopy. Left panels show differential interference contrast (DIC) images, and right panels show corresponding fluorescence images. Relevant genotypes are indicated on the right. Arrows point to cells with bipolar localization of YFP-PodJ in strains without MmpA; bipolar foci were observed in approximately 19–25% of the population in mixed cultures, which contain cells at all stages of the cell cycle. Cells were grown in minimal media and, when necessary, induced for at least 3 h in the presence of xylose. Strains used: LS3780, LS4038, LS4038/pJC210, LS4038/pMR10.
To demonstrate that the presence of MmpA affects the stability of PodJS, we compared the half-life of PodJ in wild-type versus mmpA cells by pulse-chase analysis. Cultures of Caulobacter cells were pulsed for 10 min with l-[35S]-methionine and then chased with unlabelled l-methionine. Samples were removed at 30 min intervals after the chase and subjected to immunoprecipitation with antibodies against the cytoplasmic domain of PodJ. In both wild-type and mmpA cultures, PodJL was the only isoform present initially, and its level decreased over time, disappearing completely 120 min after the chase (Fig. 4A), in accordance with previously published results (Hinz et al., 2003). In the wild-type culture, PodJS level rose as PodJL level fell, reaching the maximum at around 90 min after the chase before starting its steady decline (Fig. 4A). In the mmpA mutant, PodJS level reached a higher maximum at a later time, 120 min after the chase, but then also started to decrease (Fig. 4A).
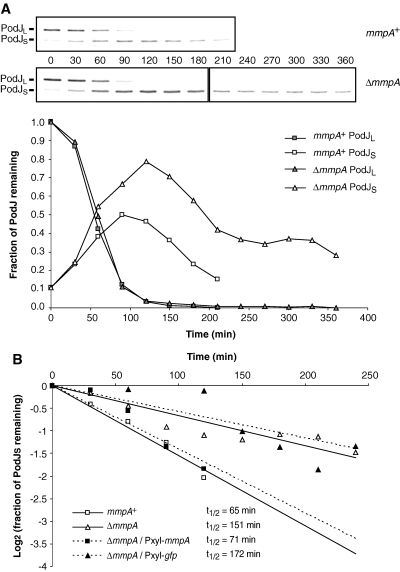
Pulse-chase analysis of PodJ stability. A. Immunoprecipitation of [35S]-labelled PodJ. Wild-type (NA1000) or ΔmmpA (LS3706) cells grown in M2G media were pulsed with l-[35S]-methionine for 10 min and chased with excess unlabelled methionine. Samples were taken at 30 min intervals following the chase and subjected to immunoprecipitation with antibodies against the cytoplasmic domain of PodJ. The positions of PodJL and PodJS bands are as indicated. The intensities of these bands were quantified and normalized to the intensities of the PodJL bands at the start of the chase. The plot of relative band intensities over time shows conversion of PodJL to PodJS and subsequent degradation of PodJS. Representative data sets are shown. B. Half-life of PodJS. PodJS stability in various strains were analysed as in (A), with band intensities normalized to the level achieved by PodJS 120 min after the chase, when conversion of PodJL to PodJS appeared complete. Fraction of PodJS remaining as a function of time was plotted on a logarithmic scale (base 2). Half-lives were determined by fitting to the exponential decay equation. Strains used: NA1000, LS3706, LS4059, LS4068.
The half-life of PodJS was calculated by plotting the fraction of PodJS remaining over time, starting at 120 min after the chase, when conversion of PodJL to PodJS appeared complete. As shown in Fig. 4B, the half-life of PodJS in the mmpA mutant was approximately double that in wild-type cells. PodJS half-life in the mutant was shortened to wild-type duration when the deletion was complemented by inducing mmpA expression from the chromosomal Pxyl promoter, but no reduction occurred when gfp instead of mmpA was under Pxyl control (Fig. 4B). These pulse-chase results indicate that MmpA facilitates the degradation of PodJS, since PodJS reached a higher level and was more stable in the mmpA mutant. However, because PodJS is still degraded in the mmpA mutant, albeit at a slower pace, other proteases may be involved as backup when MmpA is absent, perhaps accounting for the lack of an obvious phenotype at the swarmer-to-stalked cell transition.
MmpA releases PodJS from the membrane into the cytoplasm
Analysis of the PodJ degradation pattern (Viollier et al., 2002a; Hinz et al., 2003) and analogy to proteolytic pathways involving other members of S2P metalloprotease family (Duncan et al., 1998; An et al., 1999; Rudner et al., 1999; Alba et al., 2002; Kanehara et al., 2002; Schobel et al., 2004) have led us to propose a model of how PodJ is sequentially proteolyzed (Fig. 5A). First, a periplasmic protease removes the C-terminal domain of PodJ, converting PodJL to PodJS, which remains membrane-bound. Second, MmpA cleaves PodJS within or near its transmembrane segment, releasing it into the cytoplasm. Third, cytoplasmic proteases degrade the now susceptible N-terminal domain of PodJ. This model most closely resembles the RseA proteolysis cascade that activates σE-dependent expression in E. coli when the bacterium responds to extracytoplasmic stress (Fig. 5B) (Raivio and Silhavy, 2001; Alba and Gross, 2004). In that pathway, RseA is an anti-sigma factor with a central transmembrane segment whose N-terminal, cytoplasmic domain sequesters σE and represses its essential activity (De Las Penas et al., 1997; Missiakas et al., 1997; Ades et al., 1999; Campbell et al., 2003). When periplasmic protease DegS is activated by cell envelope stresses, it makes the first cleavage that removes the C-terminal domain of RseA (Ades et al., 1999; Alba et al., 2001; Walsh et al., 2003; Wilken et al., 2004). The truncated RseA fragment, RseA(ΔP), remains membrane-bound and continues to inhibit σE until the membrane metalloprotease YaeL makes the second cleavage; the N-terminal domain is then released into the cytoplasm for complete degradation, possibly by the ClpXP protease, relieving repression of σE (Alba et al., 2002; Kanehara et al., 2002; Flynn et al., 2003).
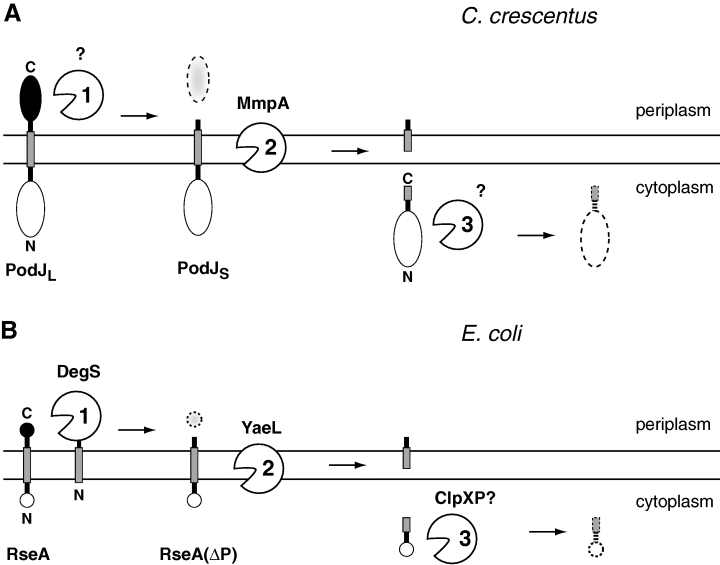
Models of how PodJ (A) and RseA (B) are sequentially proteolyzed in C. crescentus and E. coli respectively. See text for further details.
According to our model, absence of MmpA should lead to accumulation of membrane-bound PodJS. To test this hypothesis, we compared the membrane association of PodJ isoforms in wild-type versus mmpA cells. Cell extracts were separated into cytoplasmic (C) and membrane (M) fractions and probed for the presence of PodJ by immunoblot analysis (Fig. 6A, top panel). As shown previously (Viollier et al., 2002a), in wild-type cells PodJL was found exclusively in the membrane fraction, whereas PodJS was distributed about evenly between the cytoplasmic and membrane fractions. In contrast, in the mmpA mutant, while PodJL remained in the membrane fraction, PodJS accumulated predominantly in the membrane fraction as well. The same fractions were probed for the presence of CtrA, a cytoplasmic protein, and FliF, an integral membrane protein; these proteins appeared in the appropriate fractions (Fig. 6A, bottom panel). Hence, MmpA facilitates the release of its substrate PodJS from the membrane, probably by cleaving near or within its transmembrane segment.
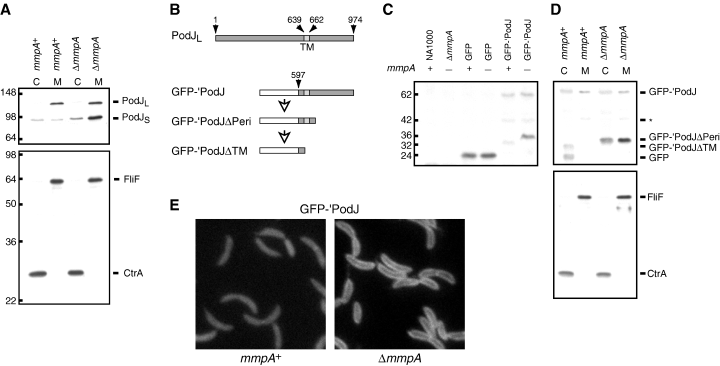
MmpA releases PodJS from the cell membrane. A. Immunoblots of wild-type (NA1000) and ΔmmpA (LS3706) cell extracts separated into cytoplasmic (C) and membrane (M) fractions. The blots were probed with antibodies to the cytoplasmic domain of PodJ (top panel) or with both CtrA and FliF antibodies (bottom panel). Protein positions are indicated on the right, while molecular weight markers are indicated on the left. B. Schematic representation of full-length PodJ (PodJL) and the GFP-′PodJ fusion. GFP-′PodJΔPeri and GFP-′PodJΔTM are putative degradation intermediates of GFP-′PodJ. Numbered arrows point to relevant amino acid residues. Residues 639 and 662 mark the boundaries of the transmembrane segment (TM). GFP is fused to amino acids 597–974 of PodJ in the GFP-′PodJ construct. C. Immunoblot of wild-type and ΔmmpA cells expressing GFP or GFP-′PodJ, probed with antibodies to GFP. The presence or absence of chromosomal mmpA and the protein, if any, being expressed in each strain are indicated above each lane. NA1000 and ΔmmpA indicate wild-type and ΔmmpA strains, respectively, that do not carry expression constructs. Truncation of full-length GFP-′PodJ (62 kDa) generates GFP-′PodJΔPeri (36 kDa), which is processed into GFP-′PodJΔTM (32 kDa) in the presence of MmpA. GFP-′PodJΔPeri is only observed in the absence of MmpA. The 42 kDa band probably represents another degradation intermediate. Approximate molecular weights in kDa are indicated on the left. Cell cultures were grown in rich media containing xylose for 3–4 h until OD600 reached 0.3–0.4. Strains used: NA1000, LS3706, LS4058, LS4059, LS4060, LS4061. D. Fractionation of wild-type and ΔmmpA cells expressing GFP-′PodJ. Immunoblots of cell extracts separated into cytoplasmic (C) and membrane (M) fractions were probed with GFP antibodies (top panel) or with both FliF and CtrA antibodies (bottom panel). The identities of the protein bands are indicated to the right. In the mmpA+ background, the fractionation procedure led to the appearance of a fragment resembling GFP in size. Cultures of LS4060 and LS4061 cells were grown in rich media containing xylose for 3–4 h until OD600 reached 0.7 and then harvested for fractionation. E. Fluorescence images of wild-type (left) and ΔmmpA (right) cells expressing GFP-′PodJ. LS4060 and LS4061 cells were grown in minimal media containing xylose.
To gain insight into the PodJ degradation pathway, we made a construct, GFP-′PodJ, in which GFP replaces most of the cytoplasmic domain of PodJ, but the transmembrane and periplasmic domains of PodJ are retained (Fig. 6B): we fused the last 378 codons of podJ to the 3′ end of gfp and placed the fusion under Pxyl control on the chromosome. Immunoblot analysis using polyclonal antibodies against GFP was performed on cells expressing this construct (Fig. 6C). In the wild-type background, we observed full-length GFP-′PodJ as a faint band of around 62 kDa, consistent with the predicted size of the GFP-′PodJ construct. In addition, we saw two bands of lower molecular weights, around 42 and 32 kDa, that most likely represent degradation products of GFP-′PodJ. In the mmpA mutant, we also observed the 62 and 42 kDa bands, but the lowest band was shifted from 32 kDa up to around 36 kDa, and its intensity was increased. In control strains expressing GFP alone, GFP appeared as a band of around 24 kDa. We interpret these results in the following manner (Fig. 6B): full-length GFP-′PodJ is processed and most of its periplasmic domain removed, resulting in a membrane-bound form of about 36 kDa (GFP-′PodJΔPeri). In wild-type cells, this intermediate is immediately cleaved by MmpA, resulting in a shorter, cytoplasmic fragment of 32 kDa (GFP-′PodJΔTM) that is prone to degradation. In the mmpA mutant, GFP-′PodJΔPeri is not cleaved and therefore accumulates. We do not know whether the 42 kDa band (asterisk in Fig. 6) represents a true intermediate of PodJ processing, or is only a degradation artefact generated with the GFP-′PodJ construct. We would be unable to detect an intermediate between PodJL and PodJS if the difference in molecular mass between the intermediate and one of the two PodJ isoforms is relatively small.
To confirm our proposed degradation pathway, we performed immunoblot analysis on cytoplasmic (C) and membrane (M) fractions from wild-type and ΔmmpA cells expressing GFP-′PodJ (Fig. 6D). Probing with anti-GFP antibodies revealed that the GFP-′PodJΔTM fragment, which is observed in wild-type cells, resided in the cytoplasmic fraction. On the other hand, GFP-′PodJΔPeri, which is observed in ΔmmpA cells, resided predominantly in the membrane fraction. As controls, probing the same fractions with anti-CtrA and anti-FliF antibodies indicated that CtrA and FliF were located in the appropriate fractions. These observations agreed with our interpretation of how GFP-′PodJ is degraded. However, we did observe unexpected band patterns in the anti-GFP blot, possibly artefactual consequences of the fractionation procedure. First, full-length GFP-′PodJ appeared in both the cytoplasmic and membrane fractions. The portion in the cytoplasmic fraction may have resulted from inefficient insertion of the fusion protein into the membrane. Second, a fragment similar in size to GFP appeared in the cytoplasmic fraction of wild-type cells. Accumulation of this fragment may derive from non-specific cleavage or slower degradation of GFP-′PodJΔTM during fractionation. Third, a significant portion of GFP-′PodJΔPeri was found in the cytoplasmic fraction. One explanation is that this truncated intermediate can slip into the cytoplasm, but cleavage by MmpA facilitates the release. We do note that the 42 kDa intermediate (* in Fig. 6) appears to reside exclusively in the membrane fraction, suggesting that it is an intermediate between full-length GFP-′PodJ and GFP-′PodJΔTM.
To provide additional evidence that MmpA facilitates the conversion of membrane-bound GFP-′PodJΔPeri to soluble GFP-′PodJΔTM, we examined cells expressing GFP-′PodJ by fluorescence microscopy. The mmpA mutant had a halo-like appearance, with fluorescence around the perimeter of the cell, indicating association of GFP-′PodJΔPeri with the membrane (Fig. 6E, right panel). In the wild-type background, the GFP-′PodJΔPeri fragment was cleaved by MmpA, released into the cytoplasm, and quickly degraded, resulting in diffuse and fainter fluorescence (Fig. 6E, left panel). The GFP-′PodJ fusion shows uniform fluorescence around the cell because the N-terminal domain of PodJ is required for polar localization (P.H. Viollier, unpublished). The last 378 amino acids of PodJ, which includes its transmembrane segment, contain sufficient signal for proteolytic processing, since fusion of GFP to this fragment leads to degradation of GFP, similar to the fate of the cytoplasmic domain of PodJ. Analysis of GFP-′PodJ lends support to our proposal that proteolytic processing of PodJ first involves removal of its periplasmic domain, converting PodJL to PodJS. MmpA then cleaves PodJS near or within its transmembrane segment, releasing the cytoplasmic domain and possibly exposing a signal that targets it for degradation by other proteases (Fig. 5A).
Fusion of MmpA to mRFP1 – an effective, periplasmic fluorescence tag – suggests uniform distribution around the cell
Since the substrate of MmpA, PodJ, is polarly localized, we asked if MmpA is localized to specific regions of the cell as well. We could not use GFP or its derivatives, such as YFP, as a fluorescence tag because both termini of MmpA are predicted to reside in the periplasm (see Fig. 1A), and GFP does not fluoresce when exported out of the cytoplasm via the general secretory (Sec) pathway (Feilmeier et al., 2000). However, we found that mRFP1, a red fluorescent protein derived from DsRed of Discosoma coral (Campbell et al., 2002), does fluoresce in the periplasm (Fig. 7A). We first determined that mRFP1 can label extracytoplasmic protein domains in E. coli by constructing translational fusions to the C-termini of YaeL (Fig. 7A), DsbA and MBP (Fig.S1). YaeL shares the same membrane topology as that predicted for its homologue MmpA (Fig. 1B) (Kanehara et al., 2001; Drew et al., 2002), while DsbA (a disulphide bond oxidoreductase) and MBP (maltose binding protein) are both soluble periplasmic proteins (Collier et al., 1988; Kumamoto and Gannon, 1988; Liu et al., 1989; Schierle et al., 2003).

Localization of MmpA using mRFP1 as a periplasmic fluorescence tag and determination of MmpA expression levels. A. Fluorescence micrographs of E. coli cells expressing protein fusions to mRFP1 (left panels) or YFP (right panels). As indicated on the left, fusions were made to the C-termini of YaeL or the signal sequence of TorA (TorAss). Insets show corresponding DIC images of the cells. Levels of brightness in the fluorescence images were scaled similarly. Live cells from overnight or exponential cultures grown in rich media at 30°C were dropped directly onto slides coated with a thin layer of agarose for examination. Strains used: JOE309/pJC240, DH10B/pJC246, JOE309/pJC250, JOE309/pJC251. B. DIC (left) and fluorescence (right) images of live Caulobacter cells expressing MmpA-mRFP1 from the Pxyl promoter on the chromosome. LS4063 cells were grown in rich media containing xylose. C. Expression levels of MmpA-mRFP1 in Caulobacter. Cytoplasmic and membrane fractions of cell lysates were subjected to immunoblot analysis with anti-mRFP1 antibodies. Lane 1 contains sample from wild-type (NA1000) cells. Lane 2 contains sample from ΔmmpA cells carrying Pxyl-mmpA (LS4063) grown in the presence of glucose to repress expression, whereas lane 3 contains sample from the same strain grown with xylose to induce expression. Lane 4 contains sample from a strain (LS4064) in which the endogenous mmpA locus has been replaced with the mmpA–mrfp1 fusion. Position of full-length MmpA-mRFP1 is indicated. Molecular weight markers in kDa are shown on the left. Cytoplasmic fractions contain contents from both the cytoplasm and the periplasm. D. MmpA levels during the Caulobacter cell cycle. Samples were collected from a synchronous culture of wild-type (NA1000) cells every 20 min and subjected to immunoblotting with anti-MmpA. The upper band represents MmpA, while the lower band (*) is non- specific. Increases in band intensities over the course of the cell cycle are attributed to cell growth and accumulation in biomass. Molecular weight in kDa is indicated on the left.
YaeL was fused to mRFP1 or to YFP, placed under the control of a LacI-regulated promoter on a relatively high-copy plasmid, and expressed in E. coli without induction. Both the yaeL–mrfp1 and yaeL–yfp fusions complemented an yaeL deletion (discussed below), indicating that the fusion proteins are assembled into the cytoplasmic membrane correctly and that their C-terminal mRFP1 and YFP are exported into the periplasm. Live cells expressing the different fusions were examined by fluorescence microscopy. Those expressing the mRFP1 fusion exhibited fluorescent haloes, or brighter perimeters, indicating export into the periplasm (7, 1). In contrast, those expressing the YFP fusion showed only dim and diffuse fluorescence (7, 2), no brighter than the background fluorescence of cells lacking YFP. Expression of unfused cytoplasmic mRFP1 or YFP resulted in bright, uniform fluorescence throughout the cell (Fig.S1 and data not shown). For comparison, we fused the signal sequence of TorA (TorAss) to mRFP1 and YFP to direct export via the twin-arginine transport (Tat) pathway. These fusions were also expressed from a plasmid without induction. In a strain expressing TorAss-YFP, most cells showed bright, uniform fluorescence, but some cells (about 10%) exhibited the same fluorescent haloes as those described in previous studies (7, 4) (Santini et al., 2001; Thomas et al., 2001). We attributed the uniform fluorescence to overproduction of the fusion protein and inefficient export by the Tat system. Finally, cells expressing TorAss-mRFP1 (7, 3) showed complete fluorescent haloes comparable to those observed in cells expressing mRFP1 fused to YaeL. Differences in fluorescence patterns between cells expressing TorAss-YFP and TorAss-mRFP1 may be a consequence of differences in protein folding properties and export efficiencies. Results similar to those observed with the YaeL fusions were obtained with fusions of DsbA and MBP to mRFP1 or to GFP (Fig.S1). Therefore, periplasmic mRFP1 can form an active chromophore whether export occurs via the Sec or Tat pathway.
Having determined that mRFP1 can fluoresce in the periplasm, we fused it to the C-terminus of MmpA to localize the protease in Caulobacter. An mmpA–mrfp1 fusion was constructed and placed under Pxyl control on the chromosome. Cells expressing the fusion showed no distinct fluorescent foci, but rather exhibited diffuse fluorescence, whether in wild-type background (data not shown) or ΔmmpA background (Fig. 7B). No fluorescence was observed when expression was repressed by growth in glucose, similar to that seen in cells without the fusion construct (data not shown). We also examined a strain in which the endogenous chromosomal mmpA+ allele was replaced with the mmpA-mrfp1 construct. The fluorescent signal was dimmer than that in cells expressing MmpA-mRFP1 from the Pxyl promoter, but it was evenly distributed throughout the cell as well (data not shown). In strains expressing MmpA-mRFP1 as the only form of the protease, immunoblot analysis indicated that PodJS was degraded normally, suggesting that the fusion protein is functional (Fig.S2).
To examine expression levels of mmpA and mmpA-mrfp1, we raised polyclonal antibodies against the periplasmic domain, between the second and third transmembrane segments, of MmpA (Fig. 1A). Immunoblotting with anti-MmpA in mmpA+ strains revealed a band of approximately 36 kDa that is absent in ΔmmpA strains (Fig. 1C, bottom panel). Constitutive mmpA expression from a plasmid-borne Pxyl promoter led to increased intensity of this band compared to expression from the endogenous chromosomal promoter (Fig. 1C); notably, overproduction of MmpA did not appear to reduce PodJL and PodJS levels below those in wild-type cells (Fig. 1C). When immunoblot analysis was performed on the membrane fractions of strains expressing mmpA-mrfp1, full-length MmpA-mRFP1 appeared as a band of around 63 kDa (Fig.S2). Although breakdown products could be observed, a significant portion remained as full-length protein. Immunoblotting with anti-mRFP1 antibodies indicated that, while a considerable fraction of MmpA-mRFP1 stayed in the cytoplasm when the fusion was overexpressed from the chromosomal Pxyl promoter, the majority of full-length MmpA-mRFP1 was inserted into the membrane (Fig. 7C). When the fusion was expressed from the endogenous mmpA locus, full-length, membrane-bound MmpA-mRFP1 appeared to be the predominant form (Fig. 7C). No significant degradation product containing mRFP1 was detected, whether MmpA-mRFP1 was expressed from the Pxyl promoter or the mmpA locus. Thus, the observed mRFP1 fluorescence signals probably represent the uniform distribution of MmpA throughout the cell. Although MmpA is a membrane protein, we did not observe fluorescent haloes with MmpA-mRFP1, similar to ones seen in E. coli cells with other mRFP1 fusions. We attribute this difference to the low intensity of the fluorescence signal and the long exposure time required for visualization of MmpA-mRFP1.
Because all cell types were fluorescent in the strain expressing mmpA-mrfp1 from the endogenous chromosomal locus, MmpA appears to be present at all stages of the cell cycle. To confirm this, wild-type swarmer cells were isolated, and, as they progressed through the cell cycle, samples were taken for immunoblot analysis with anti-MmpA antibodies. MmpA was indeed present throughout the cell cycle (Fig. 7D). Therefore, the temporally specific degradation of PodJS is not regulated by cyclic variation in MmpA levels.
MmpA and YaeL are functionally interchangeable
To determine if MmpA can functionally replace YaeL in E. coli, we used an E. coli strain in which the ΔyaeL mutation is complemented by a plasmid-borne copy of yaeL under the control of an arabinose-regulated promoter (PBAD) (Alba et al., 2002). Because YaeL is essential for viability (Dartigalongue et al., 2001; Kanehara et al., 2001), the strain formed colonies only when expression was induced in the presence of arabinose. No growth occurred when expression was repressed in the presence of glucose. We introduced into this strain a compatible plasmid that carried yaeL-mrfp1 or yaeL-yfp under the control of an IPTG-regulated promoter. The presence of this additional plasmid allowed colony formation in the presence of glucose (Fig. 8A and data not shown), indicating that both yaeL-mrfp1 and yaeL-yfp were able to complement the chromosomal yaeL deletion. Expression of mmpA-mrfp1 under low-level induction (50 µM IPTG) in this E. coli strain also led to viability (Fig. 8A), suggesting that MmpA is functionally interchangeable with YaeL. Expression of CC3381-mrfp1 or mrfp1 alone did not complement the yaeL deletion (Fig. 8A).

Complementation of ΔyaeL in E. coli and ΔmmpA in C. crescentus. A. Serial dilution of E. coliΔyaeL strains grown on rich media containing arabinose (ARA) or glucose (GLU). The ΔyaeL mutation is complemented by a copy of yaeL under the control of an arabinose-dependent promoter (Alba et al., 2002). Because yaeL is essential for viability, no growth occurs in the absence of arabinose. Expression of yaeL-mrfp1 or mmpA-mrfp1 in this strain background allowed colony formation on glucose plate, whereas expression of mrfp1 alone or CC3381-mrfp1 did not. Strain JAH301 was transformed with pJC249, pJC250, pJC252, or pJC255. Overnight cultures grown in rich media containing arabinose were serially diluted in minimal media without carbon source, spotted onto glucose or arabinose plates containing 50 µM isopropyl-β- d-thiogalactopyranoside (IPTG), and incubated at 37°C. B. Degradation of RseA in the presence of YaeL or MmpA in E. coli. Samples were taken from cultures of ΔrseAΔyaeL degS+ (left) or ΔrseAΔyaeLΔdegS (right) cells expressing HA-tagged RseA under the control of the lac promoter (Kanehara et al., 2003). The cells also co-expressed YaeL (lanes 2 and 5) or MmpA (lanes 3 and 6) that is tagged with 6 histidines and a Myc epitope. Control strains carried the expression vector alone (lanes 1 and 3). The samples were subjected to immunoblotting and probed with antibodies against the HA epitope (top) or the Myc epitope (bottom). Proteins bands are identified on the right: HA-RseA, full-length HA-tagged RseA; HA-RseA(ΔP), HA-tagged RseA in which the periplasmic domain has been truncated; YaeL-His6-Myc, tagged YaeL; MmpA-His6-Myc, tagged MmpA. Molecular weight markers are on the left. Cells were grown and harvested as described (Kanehara et al., 2003). Plasmid pSTD691 was introduced into AD1840 and KK211, and the resulting strains were transformed with pJC58, pJC297, or pJC298 for subsequent analysis. C. Complementation of ΔmmpA in C. crescentus. Samples were taken from cultures of wild-type or ΔmmpA cells expressing, from the chromosomal Pxyl promoter, the same tagged YaeL or MmpA constructs as in (B). Growth in the presence of glucose (G) prevented expression, while growth with xylose (X) induced expression. The samples were subjected to immunoblot analysis, probing with antibodies against PodJ and CtrA (top), MmpA (middle), or the Myc epitope (bottom). Protein positions are labelled on the right. The asterisk (*) in the anti-MmpA blot indicates a non-specific cross-reacting band. Molecular weight markers are shown on the left. Cultures were grown in rich media for 6 h until OD600 reached 0.7–0.8. Strains used: LS4065, LS4066, LS4067, LS4068.
The essential function of YaeL is to participate in the proteolytic inactivation of RseA and thus provide sufficient basal σE activity for viability (Alba et al., 2002; Kanehara et al., 2002). After DegS removes the periplasmic domain of RseA, the remaining fragment, RseA(ΔP), is cleaved by YaeL (Fig. 5B). To confirm that MmpA can replace YaeL, we asked if MmpA can also use RseA as a substrate in E. coli. Immunoblot analysis was performed on ΔrseAΔyaeL strains expressing HA-tagged RseA under the control of the lac promoter on a multicopy plasmid (Kanehara et al., 2003). In the absence of YaeL, cells accumulated the truncated form, RseA(ΔP), in which the periplasmic domain has been degraded by DegS (Fig. 8B, lane 1). When YaeL was co-expressed, this truncated form of RseA did not accumulate, because it is cleaved by YaeL and subsequently degraded (Fig. 8B, lane 2). Similarly, when MmpA was co-expressed, RseA(ΔP) also disappeared, indicating that it is cleaved by the Caulobacter protease (Fig. 8B, lane 3). Since we had tagged both YaeL and MmpA with a Myc epitope, we could probe with anti-Myc antibodies to compare their expression levels, and they were similar (Fig. 8B). Therefore, despite the lack of homology between RseA and PodJ, C. crescentus MmpA can recognize E. coli RseA as a proteolytic substrate.
Intriguingly, the level of full-length RseA is reduced when MmpA is co-expressed (Fig. 8B, lane 3). To determine whether MmpA behaves like YaeL without its PDZ domain and cleaves intact RseA without prior DegS processing (Kanehara et al., 2003; Bohn et al., 2004), we performed the same immunoblot analysis in ΔdegSΔrseAΔyaeL strains. In the absence of YaeL or MmpA, RseA accumulated mostly in its full-length form (Fig. 8B, lane 4). We could also observe a C-terminally truncated fragment of RseA, produced by the action of unknown proteases, as previously reported (Kanehara et al., 2003). This fragment is susceptible to cleavage by YaeL (Fig. 8B, lane 5) and MmpA (Fig. 8B, lane 6). Notably, the level of intact RseA was not significantly reduced in the ΔdegS background when MmpA was co-expressed, indicating that MmpA cleavage is dependent on prior truncation of RseA by DegS. We do not have a clear explanation of why the level of intact RseA differed when MmpA was expressed instead of YaeL in the degS+ background. Perhaps DegS activity in E. coli is repressed by the presence of YaeL, because full-length RseA can be less stable in a degS+ΔyaeL strain compared to a degS+yaeL+ strain under certain conditions (Kanehara et al., 2002). MmpA may not repress DegS because it is a heterologous protein.
Because MmpA can replace YaeL in E. coli, we tested whether YaeL can replace MmpA in C. crescentus. Myc-tagged versions of mmpA and yaeL were placed under the control of the Pxyl promoter on the chromosome. In the mmpA mutant background, growth in the presence of glucose led to accumulation of PodJS (Fig. 8C, top panel). Inducing expression of MmpA by growing cells in the presence of xylose reduced PodJS level back down to the wild-type standard. Expressing YaeL in the mmpA mutant also reduced the PodJS level, but the reduction was not as complete as when MmpA was expressed (Fig. 8C). Immunoblotting with anti-MmpA antibodies indicated that expression of Myc-tagged MmpA from the Pxyl promoter was at a level comparable to that of wild-type MmpA from its endogenous locus (Fig. 8C, middle panel). Immunoblotting with anti-Myc antibodies indicated that YaeL was expressed at a level slightly higher than that of MmpA under Pxyl control (Fig. 8C, bottom panel). Thus, while more YaeL was expressed, it was not as efficient in cleaving PodJS as MmpA. Nevertheless, YaeL does appear to recognize PodJS as a proteolytic substrate.
Discussion
PodJ, a localization factor critical for polar organelle biogenesis, undergoes sequential proteolytic processing during the Caulobacter cell cycle (Viollier et al., 2002a; Hinz et al., 2003). We have determined that MmpA, a member of the S2P membrane metalloprotease family, facilitates the degradation of the short isoform PodJS. Members of this family of zinc metalloproteases cleave within or near the transmembrane domains of their respective substrates under specific cellular conditions (Brown et al., 2000). By analogy to other metalloprotease pathways and analysis of the MmpA substrate in Caulobacter, we propose that PodJ processing involves at least three different proteases (Fig. 5A). The first protease removes the periplasmic domain of PodJ, converting PodJL to membrane-bound PodJS in predivisional cells. Then MmpA, as the second protease, cleaves PodJS within or near its transmembrane segment, releasing it into the cytoplasm during the swarmer-to-stalked transition. Finally, a third protease, possibly an ATP-dependent processive protease, degrades the remaining cytoplasmic domain of PodJ.
Since these proteolytic steps take place during defined stages of the cell cycle, a key question is how they are regulated. Because overexpression of MmpA does not significantly reduce the steady-state level of PodJL (Fig. 1C), one level of control may be exerted during the first cleavage event. In an analogous system in E. coli, the first protease DegS detects the extracytoplasmic signal that initiates the degradation of RseA (Walsh et al., 2003; Alba and Gross, 2004; Wilken et al., 2004). Similarly, the first protease in Caulobacter may act as the sentinel, removing the periplasmic domain of PodJ before subsequent proteolytic steps can occur. The identity of this first protease is not known. We found that various Caulobacter homologues of DegS, alone or together, are not required for the conversion of PodJL to PodJS. This result is not surprising, because members of the MmpA metalloprotease family appear to cooperate with different primary partners, if at all, in different systems. While DegS performs the first cleavage in E. coli, in metazoans site-1 protease (S1P), a subtilisin-like serine protease not homologous to DegS, performs the initial cleavage of substrates ATF6 and SREBP, before S2P makes the intramembrane cut (Sakai et al., 1998; Ye et al., 2000). In E. faecalis, the MmpA homologue Eep participates in the production of sex pheromone cAD1 from a lipoprotein precursor, which appears to be cleaved first by lipoprotein signal peptidase (SPase II) (An et al., 1999; An and Clewell, 2002). Moreover, in B. subtilis, the MmpA homologue SpoIVFB cuts its substrate pro-σK apparently without assistance from a prior cleavage event (Rudner et al., 1999; Yu and Kroos, 2000). Finally, YluC, another MmpA homologue in B. subtilis, participates in the proteolysis of the anti-sigma factor RsiW, but neither of the two DegS homologues tested appears to play a role in RsiW degradation (Schobel et al., 2004).
In the second cleavage step, because YaeL and MmpA can recognize each other's substrates, they may share similar regulatory cues. In E. coli, truncation of RseA by DegS is required before YaeL can act (Alba et al., 2002; Kanehara et al., 2002). Both the PDZ domain of YaeL and a glutamine-rich region in the periplasmic domain of RseA are needed to prevent YaeL-mediated cleavage before DegS processing (Kanehara et al., 2003; Bohn et al., 2004). Similarly, the PDZ domain of MmpA may repress its proteolytic activity until an avoidance signal in the periplasmic domain of PodJ is removed by the first cleavage event. However, the periplasmic domain of PodJ is not rich in glutamine residues. Additional layers of control may exist to ensure that PodJS degradation occurs specifically during the swarmer-to-stalked transition. Other components that interact with the protease or substrate may regulate the process. For example, in B. subtilis SpoIVFA and BofA form a multimeric complex with SpoIVFB to inhibit its proteolytic conversion of pro-σK to σK until the appropriate signal has been received (Rudner and Losick, 2002). Regulatory interactions with other proteins may help explain why subtle differences in substrate degradation occur when YaeL and MmpA are swapped (Fig. 8) and why the PodJS level is not significantly reduced when MmpA is overproduced (Fig. 1C). We cannot rule out the formal possibility that MmpA does not cleave PodJS directly but, instead, acts through another pathway to promote degradation of the localization factor. Further experiments will be needed to confirm that MmpA interacts directly with PodJ. In the mean time, our model is a more efficient explanation.
We postulate that an ATP-dependent processive protease is involved in the third proteolytic event that clears the cell of PodJS. In E. coli, truncated RseA appears to be a substrate of the ClpXP protease (Flynn et al., 2003). PodJS may be degraded by one of four classes of energy-dependent proteases found in bacteria: they are typified by ClpAP/ClpXP, ClpYQ (HslUV), Lon and FtsH (reviewed in Gottesman, 2003). Many substrates of these proteases contain degradation signals at their C-termini, which usually end with non-polar or hydrophobic residues. In Caulobacter in particular, cell cycle-regulated degradation of a number of proteins depends on the presence of such residues in or near their tails (Domian et al., 1997; Keiler et al., 2000; Tsai and Alley, 2001; Potocka et al., 2002; Grunenfelder et al., 2004). Cleavage within or near PodJ′s transmembrane segment may create a new C-terminus (Fig. 5A), revealing a stretch of hydrophobic or non-polar residues that serve as a degradation signal. Evidence for such a signal is the fact that GFP is more unstable when it is fused to residues 597–974 of PodJ, and this instability is dependent on MmpA. Identifying the responsible protease may be complicated by the possibility that PodJS is recognized and removed by several proteases, as have been suggested for proteins with extended hydrophobic tails in Caulobacter (Grunenfelder et al., 2004).
MmpA may have substrates other than PodJS in Caulobacter. In mammalian cells the MmpA homologue S2P is known to cleave at least two substrates, SREBP and ATF6 (Sakai et al., 1998; Ye et al., 2000). That MmpA and YaeL are interchangeable, yet their substrates are not homologous, also suggests a wider range of possible substrates. One explanation for the lack of an obvious phenotype in the mmpA mutant is that a redundant regulatory mechanism exists. Redundancy is suggested by the findings that PodJS is still found in the cytoplasmic fraction (Fig. 6A) and eventually degraded (2, 4) in the ΔmmpA mutant. A physiological effect may only be revealed under specific environmental conditions or in genetic backgrounds with additional mutations. MmpA probably has important functions that remain to be determined, because it is highly conserved across all α-proteobacterial species. Further analysis of this protease, involved in the sequential processing of an important localization factor, should help provide future insights into how proteolysis participates in differential control of protein localization and asymmetry in bacterial cells.
Experimental procedures
Bacterial strains, plasmids and growth conditions
All strains and plasmids used in this study are listed in Tables 1 and 2. Details of strain and plasmid construction are available upon request. Caulobacter crescentus strains were grown at 30°C in peptone-yeast extract (PYE) medium (Poindexter, 1964) or M2 minimal medium (Johnson and Ely, 1977). When appropriate, growth medium was supplemented with 0.2%d-glucose, 0.2%d-xylose, 3% sucrose, nalidixic acid (20 µg ml−1), apramycin (8 µg ml−1), kanamycin (25 or 5 µg ml−1), spectinomycin (25 µg ml−1), streptomycin (5 µg ml−1), and/or oxytetracycline (1 µg ml−1). Synchronization and bacteriophage ΦCr30-mediated generalized transductions were performed as described (Evinger and Agabian, 1977; Ely, 1991). Rosette formation was determined by phase contrast microscopy in CB15 derivatives, chemotaxis was assayed on PYE and M2-glucose (M2G) semisolid media, and the presence of pili was assessed by sensitivity to bacteriophage ΦCbK, as previously described (Wang et al., 1993; Viollier et al., 2002a). Escherichia coli strains were grown at 30 or 37°C in Luria-Bertani (LB) medium (Miller, 1972) or M9 minimal medium (Ausubel et al., 1988), with the appropriate antibiotics: ampicillin (100 µg ml−1), carbenicillin (50 µg ml−1), chloramphenicol (20 µg ml−1), kanamycin (50 µg ml−1), spectinomycin (50 µg ml−1), and/or oxytetracycline (12 µg ml−1). d-Glucose or l-arabinose (0.2%) was added to repress or induce, respectively, the expression of genes under the control of the PBAD promoter (Guzman et al., 1995). Plasmids were mobilized from E. coli to C. crescentus by bacterial conjugation with the help of pRK600 (Finan et al., 1986).
| Strains | Relevant genetic markers, features and/or description | Construction, source or reference |
|---|---|---|
| E. coli strains | ||
| AD1840 | rseA::cat yaeL::kan degS::tetΔpro-lac thi/F′lacIqlacZM15 lacY+pro + | Kanehara et al. (2002) |
| BW25141 | lacI q rrnB T14ΔlacZWJ16ΔphoBR580 hsdR514ΔaraBADAH33ΔrhaBADLD78galU95 endABT333uidA(ΔMluI)::pir+recA1 | Datsenko and Wanner (2000) |
| DH10B | F–mcrAΔ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 endA1 recA1deoRΔ(ara, leu)7697 araD139 galU galK nupG rpsL | Invitrogen |
| JAH143 | ΔmalE444 araD139Δ(ara-leu)7696 galE15 galK16Δ(lac)X74 rpsL (StrR) hsdR 2 mrA mcrB1/F′lacIqkanR | Leeds et al. (2001) |
| JAH301 | KS272 yaeL::kanR/pJAH184 (YaeL depletion strain) | Alba et al. (2002) |
| JOE309 | F–ΔlacU169 relA1 rpsL150 thi mot flb5301 deoC7 ptsF25 rbsR | Chen and Beckwith (2001) |
| KK211 | rseA::cat yaeL::kanΔpro-lac thi/F′lacIqlacZM15 lacY +pro+ | Kanehara et al. (2002) |
| MT616 | MT607/pRK600; helper strain for mobilizing RK2/RP4-derived plasmids | Finan et al. (1986) |
| Rosetta(DE3) pLysS | F–ompT hsdSB(rB– mB–) gal dcmλ(DE3) pLysSRARE; protein overexpression host strain | Novagen |
| C. crescentus strains | ||
| CB15 | Wild type | Poindexter (1964) |
| NA1000 | syn-1000 ; previously called CB15N, a synchronizable derivative of CB15 | Evinger and Agabian, (1977) |
| LS3706 | NA1000 ΔCC1916 (ΔmmpA) | Constructed using pJC201 |
| LS3710 | CB15 ΔCC1916 (ΔmmpA) | Constructed using pJC201 |
| LS3712 | NA1000 ΔCC3381 | Constructed using pJC203 |
| LS3780 | NA1000 yfp-podJ | Viollier et al. (2002a) |
| LS4033 | CB15 ΔCC3381 | Constructed using pJC203 |
| LS4034 | NA1000 ΔmmpAΔCC3381 | Constructed from LS3712 using pJC201 |
| LS4035 | CB15 ΔmmpAΔCC3381 | Constructed from LS4033 using pJC201 |
| LS4038 | NA1000 ΔmmpA yfp-podJ | Same allelic replacement as LS3780, except in LS3706 background |
| LS4041 | NA1000 ΔCC0151 | Constructed using pJC242 |
| LS4042 | CB15 ΔCC0151 | Constructed using pJC242 |
| LS4043 | NA1000 ΔCC0702 | Constructed using pJC212 |
| LS4044 | CB15 ΔCC0702 | Constructed using pJC212 |
| LS4045 | NA1000 ΔCC1282::ΩaacC4 | Constructed using pHPV453 |
| LS4046 | CB15 ΔCC1282::ΩaacC4 | Transduction of Aprar from LS4045 |
| LS4047 | NA1000 ΔCC2758::Ω | Constructed using pHPV439 |
| LS4048 | CB15 ΔCC2758::Ω | Transduction of Specr from LS4047 |
| LS4049 | NA1000 ΔCC0151ΔCC0702ΔCC1282::ΩaacC4ΔCC2758::Ω | Constructed using pJC212, pJC242, and transduction of Aprar and Specr from LS4045 and LS4047 |
| LS4050 | CB15 ΔCC0151ΔCC0702ΔCC1282::ΩaacC4ΔCC2758::Ω | Constructed using pJC212, pJC242, and transduction of Aprar and Specr from LS4045 and LS4047 |
| LS4058 | NA1000 xylX::pXGFP4-C1 | pXGFP4-C1 integrated into NA1000 |
| LS4059 | NA1000 ΔmmpA xylX::pXGFP4-C1 | pXGFP4-C1 integrated into LS3706 |
| LS4060 | NA1000 xylX::pJC295 (GFP-′PodJ) | pJC295 integrated into NA1000 |
| LS4061 | NA1000 ΔmmpA xylX::pJC295 (GFP-′PodJ) | pJC295 integrated into LS3706 |
| LS4062 | NA1000 xylX::pJC283 (MmpA-mRFP1) | pJC283 integrated into NA1000 |
| LS4063 | NA1000 ΔmmpA xylX::pJC283 (MmpA-mRFP1) | pJC283 integrated into LS3706 |
| LS4064 | NA1000 mmpA-mrfp1 | Constructed using pJC288 |
| LS4065 | NA1000 xylX::pJC299 (YaeL-His6-Myc) | pJC299 integrated into NA1000 |
| LS4066 | NA1000 ΔmmpA xylX::pJC299 (YaeL-His6-Myc) | pJC299 integrated into LS3706 |
| LS4067 | NA1000 xylX::pJC300 (MmpA-His6-Myc) | pJC300 integrated into NA1000 |
| LS4068 | NA1000 ΔmmpA xylX::pJC300 (MmpA-His6-Myc) | pJC300 integrated into LS3706 |
| Plasmids | Relevant genetic markers, features, and/or description | Source or reference |
|---|---|---|
| pDSW204 | High-copy-number vector, weakened Ptrc promoter, bla | Weiss et al. (1999) |
| pDSW208 | pDSW204-MCS-gfpmut2 (fusion vector) | Weiss et al. (1999) |
| pHPV439 | pNPTS138-ΔCC2758::Ω | This study |
| pHPV453 | pNPTS138-ΔCC1282::ΩaacC4 | This study |
| pJC58 | pDSW204-MCS-yfp (fusion vector) | Chen and Beckwith (2001) |
| pJC201 | pNPTS138-ΔmmpA | This study |
| pJC203 | pNPTS138-ΔCC3381 | This study |
| pJC210 | Pxyl-mmpA in pMR10 | This study |
| pJC212 | pNPTS138-ΔCC0702 | This study |
| pJC240 | pJC58-yaeL | This study |
| pJC242 | pNPTS138-ΔCC0151 | This study |
| pJC246 | pJC58-torAss | This study |
| pJC249 | pDSW204-MCS-mrfp1 (fusion vector) | This study |
| pJC250 | pJC249-yaeL | This study |
| pJC251 | pJC249-torAss | This study |
| pJC252 | pJC249-CC3381 | This study |
| pJC253 | pJC249-dsbA | This study |
| pJC254 | pJC249-malE | This study |
| pJC255 | pJC249-mmpA | This study |
| pJC256 | pDSW208-dsbA | This study |
| pJC257 | pDSW208-malE | This study |
| pJC283 | mmpA-mrfp1 replaces gfp in pXGFP4-C1 | This study |
| pJC288 | pNPTS138-mmpA-mrfp1 allelic replacement plasmid | This study |
| pJC295 | ‘podJ fragment inserted into pXGFP4-C1 | This study |
| pJC297 | pDSW204-yaeL-His6-myc | This study |
| pJC298 | pDSW204-mmpA-His6-myc | This study |
| pJC299 | yaeL-His 6 -myc replaces gfp in pXGFP4-C1 | This study |
| pJC300 | mmpA-His 6 -myc replaces gfp in pXGFP4-C1 | This study |
| pMR10 | Kanr low-copy-number and broad-host-range vector, descended from RP4/RK2 | C. Mohr and R. Roberts, unpublished |
| pNPTS138 | Used for two-part selection in in-frame deletions, Kanr | M.R.K. Alley, unpublished |
| pRSETb-mRFP1 | Source of mrfp1 | R.Y. Tsien (Campbell et al., 2002) |
| pSTD691 | Plac-ha-rseA, Specr | Kanehara et al. (2003) |
| pXGFP4-C1 | GFP fusion vector for integrating into xylX locus of C. crescentus chromosome, Kanr | M.R.K. Alley, unpublished |
- MCS, multiple cloning sites.
Molecular biology procedures
Standard techniques were used for cloning and analysis of DNA, polymerase chain reaction (PCR), electroporation, and transformation (Ausubel et al., 1988; Sambrook et al., 1989). Isolation of plasmids and DNA fragments were done using commercial kits from Qiagen or Sigma. Enzymes used to manipulate DNA were from New England BioLabs, Fermentas, Invitrogen, Novagen, or Stratagene. Oligonucleotides were obtained from Invitrogen, Qiagen, or the Stanford Protein and Nucleic Acid Biotechnology Facility; the sequences of all oligonucleotides are available upon request. DNA sequencing was performed by Biotech Core, Inc. (Mountain View, CA, USA). Deletions in the C. crescentus chromosome were generated by homologous recombination and allelic replacement, using a two-step selection procedure with the vector pNPTS138, as described previously (Stephens et al., 1996; Hinz et al., 2003); putative deletions were screened and confirmed by PCR.
Antibodies and immunoblots
The periplasmic region of MmpA containing its PDZ domain (residues 151–316) was tagged with hexa-histidine and overexpressed in E. coli strain Rosetta(DE3)pLysS using pET28a (Novagen). The protein was purified by nickel affinity chromatography under standard denaturing conditions (Qiagen) and used to immunize rabbits for the production of polyclonal antibodies (Josman). Antibodies against full-length GFP or mRFP1 were generated in a similar manner. Crude anti-MmpA, anti-GFP, or anti-mRFP1 serum was diluted 1:2000 for immunoblotting. Antibodies against PodJ, CtrA, FliF, PleC, CpaE and McpA were used as previously described (Alley et al., 1992; 1993; Jenal and Shapiro, 1996; Domian et al., 1997; Viollier et al., 2002a,b). Mouse monoclonal antibodies against the HA (Berkeley Antibody Company) or c-Myc (Oncogene Research Products) epitope were diluted to 5 or 0.4 µg ml−1 respectively. Immunoblotting was done using standard procedures (Sambrook et al., 1989). Samples were resuspended or diluted in sodium dodecyl sulphate (SDS) sample buffer (62.5 mM Tris-HCl pH 6.8, 10% glycerol, 2% SDS, 0.005% bromophenol blue, 140 mM 2-mercaptoethanol) and boiled for 4 min. Proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore). Immunodetection was performed with polyclonal antibodies against the protein of interest as the primary antibody, donkey anti-rabbit or anti-mouse immunoglobulin G conjugated to horseradish peroxidase (Jackson ImmunoResearch) as the secondary antibody, chemiluminescence reagent (PerkinElmer) and Kodak Bio-Max MR film. The image on the film was then scanned and processed with Photoshop (Adobe). Relative band intensities were determined using ImageQuant software (Molecular Dynamics). Immunoblotting to determine degradation of HA-RseA in E. coli was done as described (Kanehara et al., 2003).
Cell fractionation
Separation of C. crescentus cell lysates into soluble and membrane fractions was performed as previously described (Sandkvist et al., 1995; Viollier et al., 2002a). Cells were grown in rich media until OD600 reached 0.7–0.8. Ten ml of cell culture was harvested, washed once with 0.2 M Tris-HCl pH 8, and resuspended in 1 ml of 60 mM Tris-HCl pH 8, 0.2 M sucrose, 0.2 mM EDTA and 200 µg ml−1 lysozyme. The resuspension was incubated at room temperature for 10 min, frozen at −80°C, thawed, and sonicated briefly to lyse the cells. Intact cells were removed by centrifugation at 4000 g for 10 min. Membranes were sedimented by centrifugation at 45 000 g for 1 h. The supernatant was collected as the soluble fraction (containing periplasmic and cytoplasmic proteins). The pellet was washed twice with 2 ml of 0.2 M Tris-HCl pH 8; then it was resuspended in 1 ml of SDS sample buffer by gentle agitation at room temperature for 30 min. Equal amounts from each fraction were used for immunoblot analysis.
Pulse-chase and immunoprecipitation
Method used to determine PodJ stability was modified from previously described procedures (Jenal and Shapiro, 1996; Hinz et al., 2003). Cultures were grown in minimal media until OD600 reached 0.3–0.4, and 10 µCi ml−1 Redivue l-[35S]-methionine (Amersham) was added to pulse label the cells for 10 min. As chase, 1 mM unlabelled l-methionine and 0.2 mg ml−1 casamino acids were added. Cells from 1 ml of culture were collected at 30 min intervals after the chase by centrifugation and freezing on dry ice. Cell pellet was resuspended in 50 µl of SDS buffer (10 mM Tris-HCl pH 8, 1% SDS, 1 mM EDTA) and boiled for 2 min to lyse the cells. Cell lysate was then mixed with 0.8 ml of RIPA buffer (50 mM Tris-HCl pH 8, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS), and 20 µl of protein A-agarose (Roche) was incubated with the sample for 15 min on ice to precipitate proteins that bind non-specifically. Equivalent counts of radiolabelled proteins (generally 500 µl of the pre-cleared supernatant) were then used for immunoprecipitation with antiserum to the N-terminal domain of PodJ. Each sample was rocked gently with 1 µl of antiserum at 4°C overnight and then with 20 µl of protein A-agarose at room temperature for 1 h. The immunoprecipitate was collected by centrifugation, washed twice with RIPA buffer, resuspended in 25 µl of SDS sample buffer, and boiled for 4 min. The resulting samples were resolved by SDS-PAGE. The gel was dried and exposed against a Phosphor Screen (Molecular Dynamics) for at least 16 h. Labelled protein bands were scanned and quantified using a PhosphorImager with ImageQuant software (Molecular Dynamics).
Microscopy
Differential interference contrast (DIC) and fluorescence microscopy were performed as described previously (Jensen et al., 2001; Ryan et al., 2002). Cells were placed on pads composed of 1% agarose in water or minimal media for examination. Standard rhodamine (Chroma 41002C), fluorescein (Chroma 96170M), and YFP (Chroma 41028) filter sets were used for mRFP1, GFP and YFP fluorescence respectively. Exposure times were 50 ms for DIC images and 1–5 s for fluorescence images. Images were acquired using Metamorph software (Universal Imaging) and processed with Photoshop (Adobe).
Acknowledgements
We thank Yoshinori Akiyama, Roger Tsien, Damon Huber, Jennifer Leeds, Jon Beckwith, Urs Jenal and M.R.K. Alley for providing antibodies, plasmids and strains. We are indebted to William Burkholder for his time, reagents and advice. Our gratitude also extends to members of the Shapiro and McAdams labs for all levels of assistance, especially to Julia Holtzendorff for help in generating the anti-GFP antibodies.
This work was supported by the National Institutes of Health Grants GM032506 to L.S. and F32 G067472 to J.C.C. Additional funding was provided by the Department of Energy Grant DE-FG03-01ER63219.
Supplementary material
The following material is available from http://www.blackwellpublishing.com/products/journals/suppmat/mmi/mmi4443.sm.htm
Fig. S1. mRFP1 fluoresces in the periplasm.
Fig. S2. Expression levels of and complementation by mmpA-mrfp1.
Table S1. Polar localization of YFP-PodJ.
Note added in proof
Akiyama et al. demonstrated recently that YaeL (now called RseP) has the potential to cut a broad range of membrane protein sequences. That finding helps explain the promiscuous activities of MmpA and YaeL in heterologous systems [Akiyama, Y., Kanehara, K., and Ito, K. (2004) RseP (YaeL), an E. coli RIP protease, cleaves transmembrane sequences. EMBO J22: 4434–4442].




