Expression of the INO2 regulatory gene of Saccharomyces cerevisiae is controlled by positive and negative promoter elements and an upstream open reading frame
Abstract
The INO2 gene encodes a transcriptional activator of the phospholipid biosynthetic genes of Saccharomyces cerevisiae. Complete derepression of phospholipid biosynthetic gene expression in response to inositol/choline deprivation requires both INO2 and INO4. Ino2p dimerizes with Ino4p to bind the upstream activating sequence (UAS)INO element found in the promoters of the target genes. We have demonstrated previously that transcription from the INO2 promoter is autoregulated 12-fold in a manner identical to that of the target genes. Here, we show that this regulation occurs at the levels of transcription and translation. Transcription accounts for fourfold regulation, whereas translation accounts for an additional threefold regulation. Regulation of transcription requires a UASINO element. Additional promoter elements include an upstream essential sequence (UES) located upstream of the UASINO element and a negative regulatory element in the vicinity of the UASINO element. Regulation of translation is dependent on an upstream open reading frame (uORF) in the INO2 leader. These data support the model that regulatory gene promoters may display unusual organizations and may be subject to multiple levels of regulation. We have shown previously that the UME6 gene positively regulates INO2 expression. Here, we limit the UME6-responsive region of the INO2 promoter to nucleotides −217 to −56.
Introduction
In Saccharomyces cerevisiae, structural gene expression can be regulated by controlling the transcription or translation of a regulatory gene (Griggs and Johnston, 1991; Hinnebusch, 1997; Vilela et al., 1998). However, very little is known about promoter elements that control the transcription of regulatory genes. Our understanding of the regulation of translation is limited to a few examples that require an upstream open reading frame (uORF; Hinnebusch, 1997; Vilela et al., 1998). The INO2 gene is expressed from the weakest characterized yeast promoter, and the region immediately preceding the Ino2p initiator codon contains an out-of-frame overlapping uORF. In an effort to understand the mechanisms controlling the expression of regulatory genes better, we determined the role of several cis-acting elements and the uORF in regulating INO2 gene expression.
The INO2 and INO4 genes were previously identified among a group of inositol auxotrophs (Culbertson and Henry, 1975). Cells harbouring an ino2Δ mutant allele exhibit strange growth patterns resulting in cells that are larger than wild type, have aberrant shapes, exhibit nuclear segregation defects and have defects in bud formation (Hammond et al., 1993). This pleiotropy suggests that the INO2 gene may play a role in the regulation of other cellular processes.
The INO2 and INO4 genes are required for complete derepression of the phospholipid biosynthetic genes in response to inositol and choline deprivation (reviewed by Greenberg and Lopes, 1996). This response requires an interaction between the Ino2p:Ino4p heterodimer and the upstream activating sequence (UAS)INO element (5′-CATGTGAAAT-3′) (Lopes et al., 1991; Ambroziak and Henry, 1994; Nikoloff and Henry, 1994; Koipally et al., 1996). Within this arrangement, Ino2p provides the transactivation domain (Schwank et al., 1995), whereas dimerization with Ino4p is a prerequisite for UASINO binding. Substitutions in the first 6 bp of the UASINO element severely reduce or completely eliminate its function (Bachhawat et al., 1995). This is expected because Ino2p and Ino4p are members of the basic helix–loop–helix (bHLH) family (Hoshizaki et al., 1990; Nikoloff and Henry, 1991; Nikoloff et al., 1992) that bind the consensus sequence (Blackwell and Weintraub, 1990) represented in the first 6 bp of the UASINO element (5′-CANNTG-3′).
The promoters of the INO2 and INO4 genes each contain copies of the UASINO element (Schüller et al., 1992; Ashburner and Lopes, 1995a). Analysis of an INO2–cat reporter gene reveals a 10- to 12-fold response to inositol and choline (Ashburner and Lopes, 1995a). This regulation requires wild-type alleles of both the INO2 gene and the INO4 genes (Ashburner and Lopes, 1995a). Expression of an INO4–cat construct, however, is constitutive under all growth conditions and is not dependent on the presence of either the INO4 or the INO2 genes (Ashburner and Lopes, 1995a; Robinson and Lopes, 2000).
To determine whether regulation of the INO2 gene is required for regulation of its target genes, the expression of the INO2 gene has been placed under the control of the galactoseinducible GAL1 promoter (Ashburner and Lopes, 1995b). These experiments show that the level of transcription of the INO1 and CHO1 target genes is dependent on the expression levels of the INO2 gene. Consequently, target gene derepression is tightly correlated with INO2 transcription.
A number of other genes function to repress phospholipid biosynthesis. These include the OPI1, SIN3, RPD3 and UME6 genes (reviewed by Greenberg and Lopes, 1996). The products of the SIN3 and RPD3 genes are required for UME6-mediated transcriptional repression. Ume6p binds a URS1 element and attracts a Sin3p–Rpd3 complex, which deacetylates histones resulting in repression (Kadosh and Struhl, 1997). Interestingly, although UME6 is required for repression of the INO1 phospholipid biosynthetic gene, it is also required for derepression of the other phospholipid biosynthetic genes (CHO1, CHO2 and OPI3) and the INO2 regulatory gene (Jackson and Lopes, 1996). We have proposed that either Ume6p functions as a transcriptional activator of INO2 expression or, more likely, Ume6p functions to repress a transcriptional repressor of INO2 transcription (Jackson and Lopes, 1996; Elkhaimi et al., 2000).
Here, we address three questions pertaining to the regulation of INO2 gene expression. What are the cis-acting promoter elements required for INO2 transcription? What is the role of a potential uORF in regulating INO2 gene expression? Lastly, which region of the INO2 promoter is responsive to the Ume6p global regulator?
Results
Transcriptional regulation of the INO2 gene in response to inositol and choline
Previous studies have demonstrated that the expression of an INO2–cat reporter gene is induced 10- to 12-fold in response to inositol and choline deprivation (Ashburner and Lopes, 1995a). To determine whether this response occurs at the level of transcript abundance, the INO2 transcript was quantified using Northern blot hybridization. Wild-type (BRS1001), ino2Δ (BRS2001), ino4Δ (BRS2004) and opi1Δ (BRS2005) strains were grown under completely repressing conditions (I+C+), partially derepressing conditions (I+10C+) or completely derepressing conditions (I–C–). Partially derepressing conditions were necessary because ino2Δ and ino4Δ strains do not grow in the absence of inositol (Culbertson and Henry, 1975). Total RNA was hybridized with cRNA probes specific for both the INO2 gene and the TCM1 gene (loading control) (Fig. 1A).
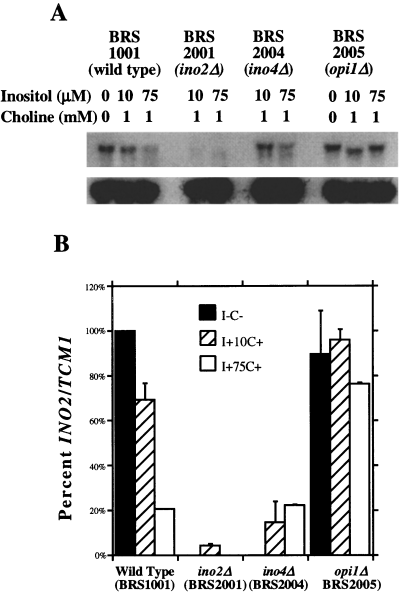
Quantitative analysis of INO2 mRNA.
A. A representative Northern blot hybridization showing INO2 transcript levels. TCM1, a constitutively expressed ribosomal protein gene, is used to normalize for loading variations. The relevant genotypes of the strains used are shown in parenthesis.
B. Bar values represent the ratio of INO2 to TCM1 c.p.m. quantified using the Betascope 603 blot analyser (Beta-gen). The values are the average of at least three separate trials and are normalized to the wild-type I–C– (100%). Error bars represent the standard error of the mean value.
Quantification of Northern blot hybridization revealed a four- to fivefold regulation of INO2 gene expression in the wild-type strain (Fig. 1B). The INO2 transcript was absent in the ino2Δ strain (the small amount of signal shown in Fig. 1B is caused by elevated background in that region of one of the blots). As predicted for an inositol/choline-responsive gene, the INO2 transcript was present at repressed amounts in the ino4Δ strain and at constitutive derepressed amounts in the opi1Δ strain.
Identification of cis-acting elements in the INO2 promoter
Most yeast promoters contain either a TATA element or some type of essential sequence required for the initiation of transcription, as well as a UAS element (or URS element) required for the regulation of transcription (reviewed by Guarente, 1984). A polymerase chain reaction (PCR)-based strategy was used to create a series of 5′, 3′ and internal deletions of the INO2 promoter in order to identify its cis-acting elements. The resultant PCR products were fused upstream of the cat reporter gene in plasmid pBM2015 (Griggs and Johnston, 1993). This plasmid contains both the cat reporter gene and a URA3 selectable marker, all flanked by GAL4 homologous sequences. The GAL4 sequences target integration at the GAL4 locus. Each transformant was grown in both completely repressing (I+C+) and derepressing (I–C–) conditions and assayed for CAT activity. Removal of sequences from −506 to −217 did not affect the expression or regulation of the cat reporter gene (Fig. 2). However, removal of an additional 10 bp (to −208) completely eliminated expression of the reporter gene (Fig. 2). Furthermore, a construct lacking sequences −215 to −208 yielded background levels of CAT activity. Inspection of this 8 bp sequence revealed a TATA-like sequence (5′-TAAAAAAT-3′).
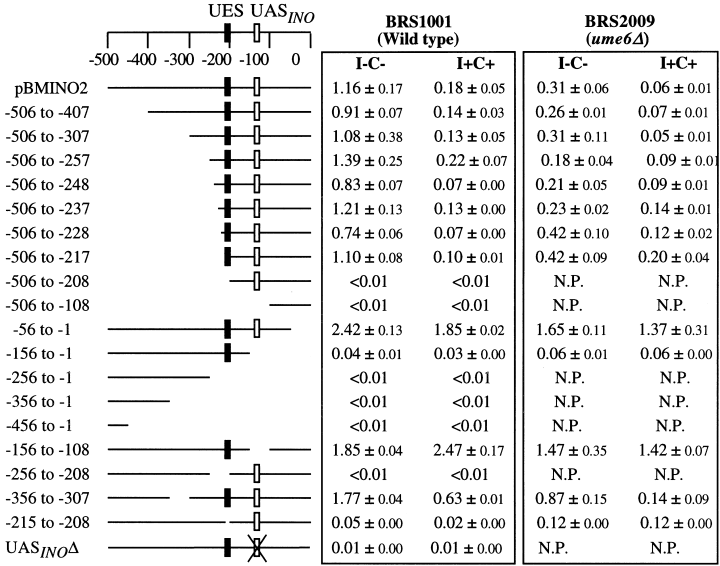
Expression of INO2–cat fusions in a wild-type (BRS1001) and a ume6Δ (BRS2009) strain. Transformants containing the various promoter deletions were grown in uracil minus synthetic media containing either 75 µM inositol and 1 mM choline (I+C+) or lacking inositol and choline (I–C–). Yeast extracts were prepared and assayed for CAT activity. The values are the average of at least three separate trials. The location of a putative UES element (filled box) and a potential UASINO element (open box) are shown. NP, not performed.
The deletion study also revealed that removal of 49 bp surrounding a putative UASINO element (−150 to −100) resulted in elevated levels of expression. It was surprising that deletion of the putative UASINO element yielded elevated expression rather than abolishing expression. A previously published report shows that point mutations of the UASINO element of the INO2 promoter eliminate promoter activity (Schwank et al., 1997). To address this inconsistency, we created specific substitution mutations that abolish the UASINO element. These mutations changed the first 6 bp of the UASINO element (Fig. 3) and were chosen because they constitute the core bHLH binding site (Bachhawat et al., 1995). The mutant promoter (UASINOΔ) was transformed into a wild-type strain (BRS1001) and assayed for CAT activity. Consistent with the published report (Schwank et al., 1997), the UASINO point mutations completely eliminated CAT activity (Fig. 2). The different results obtained for the UASINO point mutations and the 49 bp deletion removing the UASINO element will be discussed below.

Summary of mutations created in the INO2 5′ flanking sequences.
A. Listing of the INO2 UASINO element and a mutant allele containing a SalI polymorphism. The consensus UASINO element is shown for reference.
B. Listing of the wild-type INO2 and uORF translation products and an uORF AUG mutant containing a SnaBI polymorphism. The resulting translation fusions to cat and lacZ are shown.
The INO2 mRNA leader sequence contains an out-of-frame uORF that partially overlaps the Ino2p coding sequence (Fig. 3). The fact that transcription of the INO2 gene is regulated four- to fivefold (Fig. 1) while expression of an INO2–cat reporter gene is regulated 10- to 12-fold (Ashburner and Lopes, 1995a) in response to inositol suggested a post-transcriptional mechanism of regulation. In further support of this, a 56 bp deletion (−56 to −1) that removes the non-overlapping portion of the uORF resulted in elevated expression of the cat reporter gene (Fig. 2). However, some inositol/choline-mediated regulation was still observed. This regulation is probably transcriptional and driven by the UASINO element.
Deletions lacking either the UASINO or the uORF yield constitutive expression in ino2Δ and ino4Δ activator-deficient strains
The inositol–choline response of INO2 gene expression requires the UASINO element in the INO2 promoter and the uORF (Fig. 2), as well as the Ino2p and Ino4p transcriptional activators (Ashburner and Lopes, 1995a). To dissect the mechanisms regulating INO2 expression further, we examined the role of the Ino2p and Ino4p activator proteins in the context of the promoter deletions removing the UASINO and uORF regions. The two constructs containing the deletions fused upstream of the cat reporter gene (Fig. 2) were transformed into an ino2Δ strain (BRS2001) and an ino4Δ strain (BRS2004). The ino2Δ and ino4Δ strains were grown in completely repressing (I+C+) and partially derepressing (I+10C+) media as described earlier. In a wild-type strain, we observed that the 49 bp deletion construct (−156 to −108) lacking the UASINO element yielded unregulated, elevated expression of the INO2–cat reporter under both growth conditions (Table 1A). Moreover, the absence of one or other of the activator proteins did not affect the unregulated expression of the INO2–cat reporter. Again, this result will be addressed below. However, although we observed some regulation (≈1.5-fold) with the 56 bp deletion construct (−56 to −1) lacking the uORF in the wild-type strain, this regulation was eliminated in the ino2Δ and ino4Δ strains (Table 1B). This result again suggests that the modest regulation observed in the wild-type strain is at the transcriptional level and UASINO dependent.
| Strain | I–C– | I+10C+ | I+C+ | Fold regulation |
|---|---|---|---|---|
| A. An INO2–cat construct (−156 to −108) lacking the UASINO element | ||||
| BRS1001 | 1.53 ± 0.45a | NPb | 1.91 ± 0.44 | 0.80 ± 0.09 |
| (Wild type) | ||||
| BRS2001 | 1.81 ± 0.05 | 1.82 ± 0.13 | 0.99 ± 0.09 | |
| (ino2Δ) | ||||
| BRS2004 | 1.15 ± 0.16 | 0.77 ± 0.13 | 1.49 ± 0.06 | |
| (ino4Δ) | ||||
| B. An INO2-cat construct (−56 to −1) lacking the uORF | ||||
| BRS1001 | 1.77 ± 0.03 | NP | 1.19 ± 0.16 | 1.49 ± 0.23 |
| (Wild type) | ||||
| BRS2001 | 2.18 ± 0.07 | 2.17 ± 0.04 | 1.00 ± 0.02 | |
| (ino2Δ) | ||||
| BRS2004 | 1.93 ± 0.20 | 1.61 ± 0.18 | 1.20 ± 0.09 | |
| (ino4Δ) | ||||
- a . All values reported represent the mean of at least three independent assays ± the standard error of the mean.
- b . NP, not performed.
Mutating the uAUG start codon disrupts wild-type regulation of the INO2 gene
To understand the role of the uORF in the regulation of the INO2 gene, we examined the effect of mutating the uAUG start codon on the expression of the INO2–cat gene. A specific substitution mutation of the uAUG initiator codon was created (Fig. 3) using a PCR-based approach (Higuchi et al., 1988). The uAUG mutant construct was transformed into a wild-type strain (BRS1001) and assayed for CAT activity under both repressing (I+C+) and derepressing (I–C–) conditions. CAT expression in the uAUG mutant construct was elevated relative to CAT expression seen with the wild-type INO2–cat construct under both growth conditions (Table 2). Interestingly, a greater relative increase was seen in cells grown under repressing conditions. This greater relative increase resulted in a substantial reduction in regulation (12.8-fold compared with 3.7-fold).
| Construct | Strain | I–C– | I+C+ | Fold regulation |
|---|---|---|---|---|
| Wild-type uORF | BRS1001 | 1.02 ± 0.07a | 0.08 ± 0.02 | 12.75 ± 1.72 |
| INO2-cat | (wild type) | |||
| Mutant uORF | BRS1001 | 1.72 ± 0.14 | 0.47 ± 0.08 | 3.66 ± 0.42 |
| INO2-cat | (wild type) | |||
| Wild-type uORF | BRS2009 | 0.63 ± 0.11 | 0.08 ± 0.00 | 7.88 ± 1.23 |
| INO2-cat | (ume6Δ) | |||
| Mutant uORF | BRS2009 | 0.49 ± 0.11 | 0.15 ± 0.03 | 3.27 ± 0.35 |
| INO2-cat | (ume6Δ) |
- a. All values reported represent the mean of at least three independent assays ± the standard error of the mean.
To examine the transcriptional efficiency of an INO2 gene that lacked the uAUG start codon, the site-specific uAUG mutation detailed above was created in the context of the INO2 gene. YCp50-based plasmids were constructed that contained a full-length wild-type copy of the INO2 gene (YCpINO2), as well as a full-length copy of the INO2 gene containing the uAUG mutation (YCpAUG). Both constructs were transformed into an ino2Δ yeast strain (BRS2001), and transformants were grown under both repressing (I+C+) and derepressing (I–C–) conditions. The YCpAUG plasmid was able to complement the ino2 phenotype, indicating that, in cells containing the mutant uAUG start codon, the Ino2p was functional (data not shown). Total RNA was extracted from both wild-type and mutant cells and hybridized with a riboprobe specific for the INO2 gene as well as with a riboprobe specific for the TCM1 gene. Quantification revealed that there was no difference in transcriptional regulation between the wild-type uAUG construct (1.77 fold) and the mutant uAUG construct (1.72-fold) (Fig. 4A).
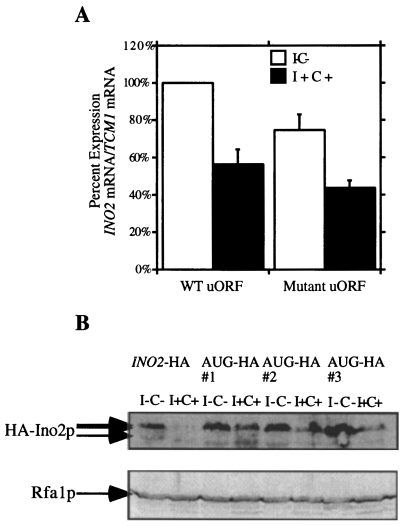
Effect of a mutant uORF AUG codon on transcription and translation of the INO2 gene.
A. Quantification of Northern blot hybridization from an ino2Δ strain transformed with YCp50 derivatives expressing the INO2 gene in the presence of a wild-type uORF or the uORF uAUG mutant. Each strain was grown in complete synthetic media either lacking (open bars) or supplemented (solid bars) with 75 µM inositol and 1 mM choline. Values were obtained by densitometric scanning. The values are the average of at least three separate trials and are normalized to the wild-type I–C– (100%). Error bars represent the standard error of the mean value.
B. A representative Western blot showing HA-Ino2p levels. The first two lanes contain control proteins isolated from a strain containing HA-Ino2p in the presence of a wild-type uORF. The next six lanes contain proteins isolated from three different transformants expressing the HA-tagged Ino2p in the presence of the uORF AUG mutant. The same blot reprobed with anti-Rfa1p to control for loading variations is shown at the bottom.
Western blot analysis was used to examine the post-transcriptional consequences of the uAUG mutation. Three copies of an HA tag (14 amino acids) were inserted in frame into the INO2 coding sequence in YCp50-INO2 (YCpINO2-HA) and YCpAUG (YCpAUG-HA). Extracts were prepared from mid-log phase cultures and assayed for HA-tagged Ino2p by immunoblotting. The results of this immunoblotting demonstrate that cells containing the YCpAUG-HA construct overexpress the HA tag relative to cells containing the YCpINO2-HA construct under both growth conditions (Fig. 4B). The effect of the uAUG mutation is more pronounced under repressing conditions. This result is in agreement with the cat reporter gene results reported above.
The uORF is competent for translation in vivo
The above results show that there is an effect on the regulation of the INO2 gene when the uAUG codon is disrupted. These experiments, however, did not address whether or not the short uORF can be translated. An in frame fusion of the entire uORF to the lacZ reporter gene (in plasmid YEp357R) was generated to examine the translatability of the uORF. A mutant uAUG-containing construct was created similarly. The YEp357R plasmid lacks an AUG start codon for the lacZ gene (Myers et al., 1986). Hence, the translational efficiency of the uORF can be analysed by measuring β-galactosidase activity. The plasmids were transformed into a wild-type yeast strain (BRS1001), and transformants were grown under both repressing (I+C+) and derepressing (I–C–) conditions. Transformants containing the wild-type uAUG start codon were positive for β-galactosidase activity, as assayed using both a plate assay (data not shown) and a quantitative liquid assay (Table 3). In contrast, none of the transformants containing the mutated uAUG start codon expressed any β-galactosidase activity (Table 3). Moreover, the liquid assays revealed that expression of the uORF–lacZ fusion is regulated approximately twofold by inositol and choline. This reflects regulation at the level of transcription, as the INO2 promoter in these constructs drives lacZ expression.
| Plasmid | I–C– | I+C+ |
|---|---|---|
| uORF–lacZ | 1.16 ± 0.17a | 0.50 ± 0.06 |
| Mutant uORF–lacZ | BDb | BD |
- a . All values reported represent the mean of at least three independent assays ± the standard error of the mean.
- b . BD, below detection.
Promoter deletions in a ume6Δ strain
As discussed in the Introduction, the effect of a ume6Δ mutation on INO2 expression is surprising. Although Ume6p generally functions as a repressor protein, and a disruption in Ume6p function generally results in the increased expression of target genes, a deletion of the UME6 gene resulted in a decrease in expression of the INO2–cat reporter under both repressing and derepressing conditions (Jackson and Lopes, 1996). In an attempt to understand this result, an isogenic ume6Δ strain (BRS2009) was transformed with each of the promoter deletion–cat constructs described above (Fig. 2). The deletion constructs behaved as reported previously, i.e. decreased expression under both repressing and derepressing conditions for each of the deletion constructs tested limiting the region of Ume6p function from −217 to −56 of the INO2 promoter (Fig. 2). More precise mapping is complicated by the fact that constructs removing sequences within the −217 to −56 region abolished expression of the cat reporter gene.
To investigate the role of the uAUG start codon in UME6-mediated INO2 regulation, the mutant uAUG–cat reporter construct was also assayed for CAT activity in the ume6Δ strain (BRS2009) under both repressing and derepressing conditions. Similar to the results seen with the wild-type INO2–cat construct, the mutant uAUG construct exhibited reduced levels of CAT activity under both repressing and derepressing conditions (Table 2). The amount of regulation seen, however, remained constant.
Discussion
In this study, we demonstrate that precise control of a highly regulated transcriptional regulator, such as INO2, is dependent on several independent mechanisms. These mechanisms, in concert, are responsible for the precise levels of activator protein in the cell. Quantification of mRNA levels reveal an approximately fourfold level of regulation of INO2 mRNA in wild-type cells. This is in contrast to the approximately 12-fold level of regulation seen with the INO2–cat reporter fusion studies. We take this to mean that translational regulation plays a role in the total regulatory response of the INO2 gene to inositol and choline.
The mRNA leader sequence of the INO2 gene contains an uORF, which, if translated, would encode a 19-amino-acid polypeptide. A 56 bp deletion, which removes the uORF, resulted in elevated expression of a cat reporter construct. We propose that the removal of the uORF disrupted INO2 translational control. A substitution mutation that eliminated the uAUG codon was inserted into a variety of constructs to enable analysis at transcriptional as well as post-transcriptional levels. The INO2–cat reporter indicated that perturbation of the uAUG start codon affected cat expression, as the mutant construct produced more CAT activity under both repressing and derepressing conditions. Western blot analysis revealed that the mutant uAUG-containing construct overproduces Ino2p under both repressing and derepressing conditions of inositol. These results suggest that INO2 expression is regulated at the level of translation.
The INO2 uORF is an overlapping, out-of-frame uORF. As a result of this overlapping configuration, a mechanism of ribosomal reinitiation at the INO2 ORF is unlikely, because ribosomes that complete translation of the uORF are already downstream of the ORF initiation codon. A stalling, reinitiation-type mechanism seen in the Gcn4p regulation system may be possible. However, in such a mechanism, translation of the uORF is unnecessary (reviewed by Hinnebusch, 1997). Although we have not proved that the uORF is translated, we have demonstrated the capacity of the uAUG to function in translation initiation. The peptide product of the uORF may play a role in the control of initiation at the downstream ORF. If this is the case, some type of internal ribosome entry site (IRES) may be necessary to allow the ribosomes to bind the message downstream of the uAUG. An IRES has not yet been identified in the INO2 mRNA.
One common feature of all promoters is a region in which the transcriptional preinitiation complex assembles. In most promoters in S. cerevisiae, this sequence is a TATA element (reviewed by Guarente, 1984). This element has the consensus sequence TATAAA, although some divergence has been reported. A few promoters in yeast, such as the GAL4, HIS3, HIS4 and PGK promoters (Ogden et al., 1986; Mahadevan and Struhl, 1990; Pellman et al., 1990; Griggs and Johnston, 1991), do not contain a TATA element, but do contain a promoter region required for transcription. In the case of GAL4, this region is referred to as the UES. In higher eukaryotes, it is generally accepted that promoters lacking a canonical TATA element are found in genes that are not highly regulated, such as housekeeping genes. Genes that do not contain a TATA element are still dependent on the TATA binding protein, and the mechanism of transcription initiation appears to be quite similar to that of promoters that contain a TATA element.
Examination of the INO2 promoter sequence did not reveal any region resembling a consensus TATA element. The deletion experiments reported here identify an 8 bp region of the INO2 promoter that is required for INO2 expression. This 8 bp region has the following sequence: TAAAAAAT. This AT-rich sequence may represent a divergent TATA element, which may account for the low level of transcription seen in the INO2 gene. The sequence differs from the consensus TATA element at the third position (TATAAA versus TAAAAA). Earlier studies that examined the role of specific nucleotides in TATA function revealed that changing the third position from the consensus sequence to an A residue resulted in the weakest amount of transcriptional activation seen when compared with the other two possible base changes (Wobbe and Struhl, 1990). This nucleotide change may function in the INO2 promoter to keep regulated transcription levels extremely low. There are currently no reports in the literature of TATA elements that contain this substitution at the third position.
One piece of evidence, supportive of the argument that the INO2 UES sequence is indeed a divergent TATA element, is the ability of this element to function in conjunction with the UASINO element. The UES element found in the GAL4 promoter (which is not a TATA element) functions only in conjunction with the UASGAL4 activator sequence (Griggs and Johnston, 1993). The activation sequence UASINO found in the INO2 promoter is also found in the promoters of a variety of INO2 target genes, all of which contain a canonical TATA element, suggesting that this UES may indeed represent a TATA element. In addition, primer extension experiments have mapped the major INO2 transcription start site to position −181, which is immediately downstream of the potential TATA site (data not shown).
Another interesting feature of the INO2 promoter is the spatial arrangement of regulatory elements. Typically, UAS elements in yeast are found upstream of the TATA element. In the INO2 promoter, the UAS element is found downstream of the putative TATA element. This is one of the few reported examples of a UAS element that is located downstream of the TATA element. Other examples include the PYK1 (Purvis et al., 1987), PGK1 (Mellor et al., 1987) and SRP1 (Fantino et al., 1992) genes, all of which have regulatory elements (downstream activation sites) located in their coding sequences. Consistent with our results, the UASGCN4 can function when placed downstream of a TATA box (Brandl et al., 1992). However, this is not a general property of UAS elements, as the CYC1 UAS element will not function when placed downstream of the TATA box (Guarente and Hoar, 1984).
A 49 bp deletion, which removed the UASINO element in the INO2 promoter, resulted in constitutive, elevated expression of the INO2–cat reporter construct. This expression was independent of Ino2p and Ino4p. This result was surprising considering the positive regulatory function normally played by the UASINO. More predictably, a specific mutation of the UASINO element resulted in a loss of CAT activity. This result confirmed the work of another group that had created a similar INO2 promoter UASINO mutation and fused it to the lacZ gene (Schwank et al., 1997). Although repression sequences have not been identified in the INO2 promoter, our data suggest that they may exist in the vicinity of the UASINO element. An identical situation has been reported for the INO1 gene (Swift and McGraw, 1995). In that case, a model was proposed in which the deletions removed chromatin-mediated, negative regulatory elements, resulting in Ino2p:Ino4p-independent constitutive expression of INO1 (Swift and McGraw, 1995). The existence of similar elements in the INO2 promoter would explain the results obtained in this study. We have compared the pertinent regions of the INO1 and INO2 promoters and found no obvious sequence conservation. We have also been unable to identify any established yeast regulatory sequences surrounding the INO2 UASINO element. Clearly, more experiments will have to be done to address this problem.
The results presented here describe multiple levels of control of gene expression, acting in concert to control the synthesis of a transcriptional activator that must be tightly regulated. The cell has used transcriptional and translational mechanisms to achieve the level of regulation required. Our studies suggest that further investigation into weakly expressed, highly regulated genes is important and likely to reveal additional levels of control.
Experimental procedures
Strains and growth conditions
The S. cerevisiae strains used in this study were BRS1001 (MATa, ade2, his3, leu2, can1, trp1, ura3), BRS2001 (MATa, ade2, his3, leu2, can1, trp1, ura3, ino2::TRP1), BRS2004 (MATa, ade2, his3, leu2, can1, trp1, ura3, ino4::TRP1), BRS2005 (MATa, ade2, his3, leu2, can1, trp1, ura3, opi1::LEU2) and BRS2009 (MATa, ade2, his3, leu2, can1, trp1, ura3, ume6::LEU2). Yeast cultures were grown at 30°C in complete synthetic media (Kelly and Greenberg, 1990) containing 2% glucose (w/v) supplemented with 75 µM inositol and 1 mM choline (I+C+) or 10 µM inositol and 1 mM choline (I+10C+) or lacking inositol and choline (I–C–). Where noted, amino acids were omitted for plasmid maintenance. The Xgal medium has been described previously (Hudak et al., 1994).
Plasmid and strain constructions
A nested set of INO2 promoter 5′ and 3′ deletion constructs was generated using PCR. PCR was conducted using ORF-proximal primers containing a BamHI site and ORF-distal primers containing a BglII site (Table 4). Primers INO2-Bgl and INO2-Bam (Table 4) have been used previously to amplify 506 bp of the INO2 promoter (Ashburner and Lopes, 1995a). The other primers used each contained 15 nucleotides of INO2 promoter sequence flanked by these same restriction sites. The end-points for each primer are indicated in Fig. 2. The template DNA was one of the original INO2-containing clones, YESB (M. Nikoloff and S. Henry, unpublished data). PCR fragments were cloned into pGEM-T (Promega) and sequenced. The appropriate fragments were excised by digestion with BglII and BamHI and inserted into the BamHI site of pBM2015 (Griggs and Johnston, 1993) to create a fusion with the cat gene (Fig. 3B). Each pBM2015 derivative was digested with SstI and KpnI and transformed into the appropriate yeast strain using a standard procedure (Chen et al., 1992). The SstI–KpnI fragments contained the INO2 promoter–cat fusion gene and a URA3 selectable marker, flanked by GAL4 sequences. The GAL4 sequences serve to target integration of the reporter gene to the GAL4 locus in single copy and orientation (Griggs and Johnston, 1991). Accurate integration was confirmed by Southern blot hybridization for all transformants used in this study. Internal deletion constructs were created by combining various 5′ and 3′ deletion PCR fragments in pBM2015.
| Designation | Sequence |
|---|---|
| INO2-Bgl | 5′-GGGGAGATCTGGATCTGAGTTACTT-3′ |
| INO2-Bam | 5′-GGGGGGATCCCTCCTTTGCTGTTCC-3′ |
| INO2-3′ | 5′-GATCATTGCACCGTT-3′ |
| DAE-UAS | 5′-GGGGGGATCCCTCCTTTGCTGTT-3′ |
| DAE1 | 5′-GGATCCCTCCTTTGCTGTTCTACGTATTT-3′ |
| DAE2 | 5′-CTCCTTTGCTGTTCTACGTATTT-3′ |
| DAE3 | 5′-AAATACGTAGAACAGCAAAGGAG-3′ |
- a . Because a large number of primers were used to create the promoter deletion mutants, they are not listed here. However, each of the primers contained 15 bp of INO2 promoter-complementary DNA sequence flanked by either a BglII site (promoter distal) or a BamHI site (promoter proximal).
An INO2–cat construct containing point mutations in the UASINO element (Fig. 3A) was created using primer DAE-UAS (Table 4) and the GeneEditor in vitro site-directed mutagenesis system. The template for the mutagenesis was pBMINO2, which contains 506 bp of the INO2 promoter fused to the cat gene in pBM2015 (Fig. 2). The substitution mutation created a SalI polymorphism in place of the UASINO element (Fig. 3).
A substitution mutation of the INO2 uORF AUG was created using a PCR-based strategy (Higuchi et al., 1988) (Fig. 3B). Complementary primers (DAE2 and DAE3) contained the desired substitution mutation, which created a SnaBI site in place of the uORF AUG codon. A PCR using DAE2 and INO2-Bgl generated a product containing the INO2 promoter and the mutant uORF AUG. A separate PCR using DAE3 and a primer that annealed 403 bp downstream of the INO2 ORF (INO2-3′; Table 4) generated a product containing the INO2 ORF and the mutant uORF AUG. The two PCR products were annealed (overlapping mutant uORF region) and extended with Taq polymerase. A PCR using the two external primers (INO2-Bgl and INO2-3′) was used to produce the INO2 ORF with flanking promoter and 3′ untranslated region (UTR) and the mutated uORF AUG codon. This PCR product was inserted into pGEM-T to create pTAAUGg. The entire INO2 ORF and flanking sequences were excised by digestion with SalI and SphI (both present in the pGEM-T polylinker) and cloned into YCp50 digested with the same enzymes. A wild-type version was constructed using the same strategy. For this, YESB was used as the template DNA for PCR using primers INO2-Bgl and INO2-3′. A cat reporter construct containing the mutant uORF AUG codon was created by PCR using the INO2-Bgl and DAE1 (identical to DAE2 with BamHI site) primers and the strategy described above for the deletion studies, but using pTAAUGg as the template DNA.
In frame fusions of the uORF to lacZ containing either the wild-type uORF or the uORF with the mutant AUG were created (Fig. 3B). PCR was performed using either the YESB (wild type) or the pTAAUGg (mutant uORF) plasmids as template DNA. In the YESB reactions, primers INO2-Bgl and INO2-Bam were used; in the pTAAUGg reactions, primers INO2-Bgl and DAE1 were used. Each of the PCR products was cloned into pGEM-T. The appropriate fragments were excised by digestion with SphI (pGEM-T polylinker) and BamHI (downstream primer) and inserted into YEp357R (Myers et al., 1986) digested with the same enzymes to create in frame fusions of the uORF with lacZ.
An HA-tagged version of the wild-type INO2 gene and the mutant uORF INO2 gene were created in YCp50. A PCR was carried out using plasmid pMN103-HA provided by Susan Henry (Carnegie Mellon University, Pittsburgh, PA, USA). This plasmid contains the INO2 ORF and flanking DNA with a 120 bp insert into a BglII site of the INO2 ORF (residue 14 from the amino-terminus). The 120 bp insert contains three copies of the HA tag. The INO2 ORF and flanking DNA were amplified using INO2-BglII and INO2-3′, and the PCR product was cloned into pGEM-T to create pTAINO2g-HA. The HA-tagged INO2 gene was excised from pTAINO2g-HA by digestion with SalI and SphI and inserted into YCp50 digested with the same enzymes to create YCpINO2-HA. The mutant uORF version was created by inserting a 120 bp BglII fragment from pSM492 (Berkower et al., 1994), containing the triple HA tag, into pTAAUGg (described above) digested with BglII to create pTAAUGg-HA. The HA-tagged mutant uORF was excised by digestion with SalI and SphI and cloned into YCp50 as described above to create YCpAUG-HA.
Enzyme assays
CAT assays were performed as described previously using 5 ml cultures grown to 50–60 Klett units in appropriate media (Seed and Sheen, 1988). Units of CAT activity were defined as c.p.m. measured in the organic phase and expressed as a percentage of the total counts min−1 (percentage conversion) divided by the amount of protein assayed (µg) and the time of incubation (h). β-Galactosidase assays were performed as described previously (Lopes et al., 1991). Units of β-galactosidase activity were defined as OD420 min−1 mg−1 total protein.
RNA analyses
RNA was isolated from yeast by a glass bead disruption and hot phenol extraction procedure (Elion and Warner, 1984). RNA probes (cRNA) for Northern (RNA) hybridizations were synthesized with the Gemini II Core system (Promega) from plasmids linearized with a restriction enzyme and transcribed with an RNA polymerase as follows (shown as plasmid, restriction enzyme, RNA polymerase) for the indicated parenthesized probe: pTA-INO2, SalI, T7 (INO2) (Ashburner and Lopes, 1995b); pAB309Δ, EcoRI, SP6 (TCM1) (Hudak et al., 1994). Northern hybridizations were performed as described previously (Hirsch and Henry, 1986), the results were visualized by autoradiography, and gene-specific c.p.m. were quantified using the Betascope 603 blot analyser (Beta-gen).
Western analysis
Preparation of whole-cell extracts from S. cerevisiae was performed as described previously (Lopes and Henry, 1991), and 50 µg of yeast extract was fractionated by SDS–PAGE. Western blot analyses to detect the HA-tagged Ino2p were performed using anti-HA antibody (Boehringer Mannheim) at a final dilution of 1:250 as described previously (Robinson and Lopes, 2000) and visualized using a chemiluminescent substrate, CDP-Star (Tropix). Blots were subsequently probed with anti-Rfa1p (Brush and Thomas, 2000) (generously provided by Dr George Brush, Wayne State University) at a final dilution of 1:2500. The detection system used to visualize the Rfa1p was colorimetric (Robinson and Lopes, 2000). Rfa1p served as a standard for loading variations.
Acknowledgements
We thank Dr Gary Englemann (Loyola University of Chicago) for providing laboratory space and helpful discussions. We thank Dr Susan Henry (Carnegie Mellon University) for providing the HA-tagged INO2 clone, and Dr Susan Michaelis (Johns Hopkins University) for providing the HA tag-containing plasmid. We also thank Mohan Kaadige, Mary Elizabeth Gardocki and Lisa Lepeak for critical reading of the manuscript. This work was supported by a grant (RPG-97-002-01) from the American Cancer Society to J.M.L.


