Regulation of RpoS by a novel small RNA: the characterization of RprA
Abstract
Translational regulation of the stationary phase sigma factor RpoS is mediated by the formation of a double-stranded RNA stem–loop structure in the upstream region of the rpoS messenger RNA, occluding the translation initiation site. The interaction of the rpoS mRNA with a small RNA, DsrA, disrupts the double-strand pairing and allows high levels of translation initiation. We screened a multicopy library of Escherichia coli DNA fragments for novel activators of RpoS translation when DsrA is absent. Clones carrying rprA (RpoS regulator RNA) increased the translation of RpoS. The rprA gene encodes a 106 nucleotide regulatory RNA. As with DsrA, RprA is predicted to form three stem–loops and is highly conserved in Salmonella and Klebsiella species. Thus, at least two small RNAs, DsrA and RprA, participate in the positive regulation of RpoS translation. Unlike DsrA, RprA does not have an extensive region of complementarity to the RpoS leader, leaving its mechanism of action unclear. RprA is non-essential. Mutations in the gene interfere with the induction of RpoS after osmotic shock when DsrA is absent, demonstrating a physiological role for RprA. The existence of two very different small RNA regulators of RpoS translation suggests that such additional regulatory RNAs are likely to exist, both for regulation of RpoS and for regulation of other important cellular components.
Introduction
The stationary phase sigma factor RpoS plays a critical role in gene expression in Gram-negative bacteria, in which it regulates genes that are required for entry into, survival in and exit from stationary phase (Hengge-Aronis, 1999). RpoS also regulates the response of bacterial organisms to stress conditions (Fischer et al., 1998; Hengge-Aronis, 1999; Jorgensen et al., 1999). In vivo, RpoS is involved in the pathogenicity of Salmonella typhimurium (Swords et al., 1997), the survival of Legionella pneumophila within a protozoan host (Hales and Shuman, 1999) as well as the virulence and stress tolerance of the plant pathogen Erwinia carotovora (Andersson et al., 1999). Much of the regulation of RpoS-dependent genes is dependent on changes in the levels of RpoS. Not surprisingly, the regulation of this critical protein is complex. In Escherichia coli, there is a fivefold increase in the activity of the rpoS promoter as cells enter stationary phase (Lange and Hengge-Aronis, 1994). However, most of the regulation occurs at the post-transcriptional level, involving both regulation of translation and regulated degradation of RpoS (Lange and Hengge-Aronis, 1994; Schweder et al., 1996; Hengge-Aronis, 1999). The mRNA coding region for RpoS is preceded by a long leader region that is required for translational regulation (Lange et al., 1995). This region is capable of forming a hairpin stem–loop structure that blocks the ribosome binding site and prevents translation. Several point mutations that weaken the pairings within the double-stranded stem structure lead to the constitutive expression of RpoS (Brown and Elliott, 1997). The Hfq protein (also called HF-I) is required to activate the translation of rpoS, presumably by helping to disrupt the stem–loop structure under appropriate conditions (Brown and Elliott, 1996). Translation is also increased by the expression of a small RNA, DsrA (Sledjeski et al., 1996). DsrA interacts with one strand of the rpoS mRNA, interfering with the formation of the stem structure and allowing high-level translation (Sledjeski et al., 1996; Majdalani et al., 1998). The work described here was undertaken to determine whether small RNAs other than DsrA might participate in the translational regulation of RpoS. An E. coli library was screened for clones that would activate an RpoS–lacZ translational fusion in a dsrA− strain background. Although this screen for activities did not specifically require a small RNA activator, we report the isolation and characterization of one set of eight overlapping fragments that yielded a novel small RNA, RprA (RpoS regulator).
Results
Isolation of novel RpoS regulators as multicopy suppressors of dsrA mutations
Translation of RpoS and of an RpoS–lacZ translational fusion is stimulated by the presence of the small RNA DsrA (Sledjeski et al., 1996; Majdalani et al., 1998). Although expression of an RpoS–lacZ fusion is very low in the absence of DsrA, we observed some activity from the fusion on MacConkey lactose plates, particularly at higher temperatures (37°C and 42°C). We were interested in determining whether the cause of the residual expression of RpoS at elevated temperatures in the absence of DsrA resulted from translation that is independent of factors other than Hfq or the action of trans-acting factors other than DsrA. To isolate potential new regulators of RpoS, a pBR322-based E. coli library (Ulbrandt et al., 1997) was introduced into a ΔdsrA, RpoS–lacZ strain (NM22554; Table 1) by electroporation and screened on MacConkey lactose ampicillin plates for Lac+ colonies. On these plates, the starting strain is white (Lac−); red (Lac+) colonies were picked for further study. About 25 000 colonies were screened; 12 active plasmids were isolated, and the sequence of the inserts was determined (Table 2A). We recovered one isolate at each of regions 40, 177 and 252 (classes 1, 3 and 4 respectively) of the 400 region E. coli genome. Two identical clones mapped in region 290 (class 5), whereas the other eight were overlapping and mapped in region 154 (class 2) of the chromosome (min 37–38). The clone from region 177 (class 3) carries dsrA. In theory, the increase in RpoS–lacZ accumulation could reflect increased transcription or translation or decreased degradation of the fusion protein, which is subject to the same regulated degradation by the ClpXP protease as RpoS itself (Schweder et al., 1996). In order to distinguish between decreased degradation and increased synthesis, we tested the phenotypes of the dsrA-suppressing plasmids in dsrA− RpoS–lacZ cells carrying a clpP or a clpX mutation, either of which blocks degradation (Table 2A, last column). For all the plasmid classes other than class 1, the two effects were additive. Thus, although RpoS–lacZ expression was increased by a clpX mutation, the suppressing plasmid raised it further. Therefore, we conclude that these other classes of plasmids increase synthesis of RpoS–lacZ rather than slowing degradation. Table 2B shows the results of assays for the class 2 plasmid pSAC1. The stimulation of RpoS–lacZ activity by pSAC1 was as high in the presence or absence of ClpP (compare vector and pSAC1 in Table 2B, columns 3 and 4). Even in a dsrA+ host, pSAC1 significantly stimulated RpoS–lac activity in both clpP+ and clpP− hosts (Table 2B, columns 1 and 2). In contrast, pSAC2, the plasmid in class 1, did not have a stimulatory effect in a clp mutant host (Table 2A). It was shown by sequencing that pSAC2 encodes ClpP and a ClpX protein truncated at its C-terminus. Therefore, it seems likely that the ClpX peptide expressed from this plasmid interferes with the function of wild-type ClpX and increases RpoS–lacZ expression by inhibiting degradation.
| Strain | Description or relevant genotype | Source or reference |
|---|---|---|
| MC1061 | Standard strain for electroporation | New England Biolabs |
| NM22506 | Δara, leu+imm21 RpoS::lacZ | From SG22500; this study |
| NM22507 | imm 21 RpoS::lacZ translational fusion to amino acid 250 (nt 750) of RpoS; dsrA+…zed3069::ΔTn10;Δara714 | From NM22506; this study |
| NM22508 | imm 21 RpoS::lacZ translational fusion to amino acid 250 (nt 750) of RpoS; ΔdsrA5…zed3069::ΔTn10;Δara714 | From NM22506; this study |
| NM22554 | hfq2::kan imm21 RpoS::lacZ; ΔdsrA5…zed3069::ΔTn10 | From NM22508, this study; Tsui et al. (1994) |
| KSJ6 | imm 21 RpoS::lacZ translational fusion to amino acid 250 (nt 750) of RpoS; ΔdsrA5…zed3069::ΔTn10;Δara714;rprA::kan | From NM22506; this study |
| KSJ7 | imm 21 RpoS::lacZ translational fusion to amino acid 250 (nt 750) of RpoS; ΔdsrA5…zed3069::ΔTn10;Δara714;rprA::kan | From NM22506; this study |
| KSJ13 | imm 21 RpoS::lacZ translational fusion to amino acid 250 (nt 750) of RpoS; dsrA+…zed3069::ΔTn10;Δara714, rprA::kan | From NM22506; this study |
| KSJ15 | imm 21 RpoS::lacZ translational fusion to amino acid 250 (nt 750) of RpoS; ΔdsrA5…zed3069::ΔTn10;Δara714, rprA::kan | From NM22506; this study |
| KSJ16 | clpP1::cat imm21 RpoS::lacZ; dsrA+…zed3069::ΔTn10;Δara714 | From NM22507; this study; Maurizi et al. (1990) |
| KSJ17 | clpP1::cat;rprA1::kan imm21 RpoS::lacZ; dsrA+…zed3069::ΔTn10;Δara714 | From NM22507, this study; Maurizi et al. (1990) |
| KSJ18 | clpP1::cat imm21 RpoS::lacZ; ΔdsrA5…zed3069::ΔTn10;Δara714 | From NM22508, this study; Maurizi et al. (1990) |
| KSJ19 | clpP1::cat, rprA1::kan imm21 RpoS::lacZ; ΔdsrA5…zed3069::ΔTn10;Δara714 | From NM22508, this study; Maurizi et al. (1990) |
| SG12073 | himA::ΔTn10 | C600+P1(himA::tet) |
| SG22500 | imm 21 RpoS::lacZ translational fusion to amino acid 250 (nt 750) of RpoS | Sledjeski et al. (1996) |
| Class | No. of isolates | Genome segment | Gene(s) on fragment | Additive effect of clpX− |
|---|---|---|---|---|
| 1 | 1 | 40 | clpP, clpX | – |
| 2 | 8 | 154 | ydiK, ydiL | + |
| 3 | 1 | 177 | dsrA | + |
| 4 | 1 | 252 | barA | + |
| 5 | 1 | 290 | yrbM | + |
| Table 2B. Multicopy RprA effects on RpoS–lacZ. | ||||
| dsrA +, clpP+(NM22507) | dsrA +, clpP−(KSJ16) | dsrA −, clpP+(NM22508) | dsrA −, clpP−(KSJ18) | |
| Vector | 2.4 | 2.6 | 0.46 | 0.62 |
| pSAC1 | 4.5 | 5.8 | 2.8 | 3.4 |
- Cells transformed with the vector, pHDB1 or pSAC1 were assayed for expression of RpoS–lac during growth in LB broth at 32°C. The numbers are the average of at least three points and give the specific activity during exponential growth. Although no strong effect of clpP− was observed here, on lactose MacConkey agar plates, a dsrA− host is Lac−, whereas a dsrA−clpP− strain is moderately Lac+.
We chose the eight overlapping clones in class 2 for further characterization and focused on pSAC1, which carries the shortest insert (1.7 kb) among the overlapping clones. To determine whether this plasmid affects the transcription or the translation of the RpoS–lacZ fusion, we tested the activity of pSAC1 on an RpoS–lacZ transcriptional fusion. The β-galactosidase activity was 7.7 units with both the vector and pSAC1. This suggests that the pSAC1 product does not affect transcription, but rather affects the translation of the RpoS–lacZ translational fusion used in the original screening host.
Identification of a novel RNA activator
The active fragment within pSAC1 was defined by a series of deletions (Fig. 1). These identified a 420 bp fragment between the AgeI and BstXI sites as essential for activity. Computer analysis of this region using pcsearch identified several potential promoters and several potential stem–loops but no significant open reading frames (ORFs). In particular, we noted the presence of a long inverted repeat followed by a stretch of Ts, a potential ρ-independent terminator. Somewhat upstream is a second long inverted repeat (Fig. 2). These two structured regions were reminiscent of the DsrA structure and suggested that the region might encode a small RNA.
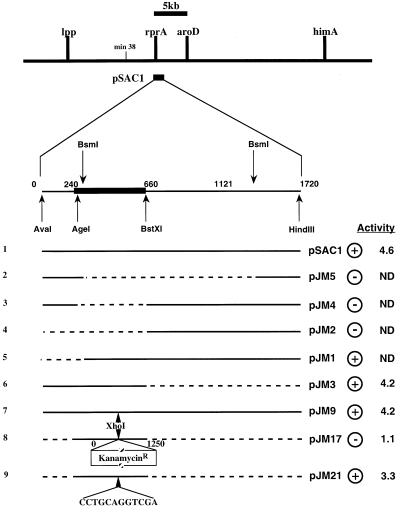
Mapping the active fragment carried by the pSAC1 plasmid. The 1.7 kb fragment in plasmid pSAC1 (line 1) was subjected to a series of deletions using restriction enzymes as indicated (lines 2–6; plasmids pJM5, pJM4, pJM2, pJM1 and pJM3 respectively). Solid lines represent the DNA fragment carried by the plasmid; dashed lines represent DNA fragments that have been deleted. The plasmid was also mutagenized to introduce an XhoI restriction site into pSAC1 by changing 6 nucleotides (at positions 69–74 in Fig. 2) in the original sequence (line 7; pJM9). That site was used to introduce a KanR cassette, and PCR was used to amplify and clone the AgeI–BstXI fragment with KanR (line 8; pJM17). This cassette, when deleted by treatment with SbfI, results in the addition of 12 nucleotides (CCTGCAGGTCGA) to the XhoI-modified sequence (line 9, pJM21). Expression of the RpoS–lac fusion was initially estimated on MacConkey lactose indicator plates (+ and – in the next to last column). Critical plasmids were assayed in liquid culture, as described in Experimental procedures; the specific activity is given in the final column. ND, not done.
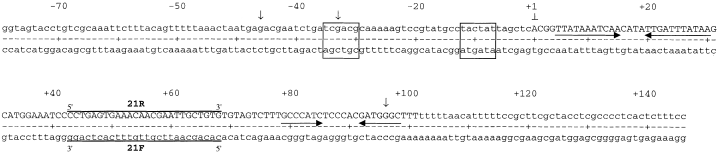
Analysis of the DNA sequence encoding RprA. Three activity-abolishing NTG mutations are indicated by downward arrows; the predicted −35 and −10 of the promoter are represented by boxed sequences. Horizontal arrows represent predicted inverted repeats; solid lines above or below the sequence represent the sequences of the biotinylated probes or primers used in Northern blots or primer extension experiments. The numbering system is based on the experimentally determined +1 of transcription of rprA.
A subclone carrying the first 660 nucleotides (nt) of the pSAC1 insert, pJM3 (Fig. 1, line 6), was mutagenized with nitrosoguanidine (NTG) and screened for loss of the ability to stimulate RpoS–lacZ expression. Three G:C → A:T transition mutations were isolated as shown by the downward arrows in Fig. 2. One of these, NTG-2 (nt −33), changes the sequence of a predicted promoter −35 region, reducing the match to consensus; this change would be consistent with a loss of promoter activity. The NTG-1 (nt −46) mutation is just upstream of this −35 region and might also disrupt promoter activity. Another, NTG-3 (nt +96), disrupts the long inverted repeat we had identified as a potential ρ-independent transcription-termination signal. These results were consistent with the suppressing activity resulting from a transcript initiated at the promoter mutated in NTG-1 and NTG-2 and terminated in the region defined by NTG-3. If so, this would encode an RNA of about 106 nt in length with no obvious ORFs within it.
We created an XhoI site (nt 73–78) within this region (see Experimental procedures) that still retained full activity. However, insertion of a KanR cassette at this site (pJM14; Fig. 1, line 8) totally abolished activity. An SbfI cleavage and ligation to remove the cassette restores activity despite the addition of 12 nt to the original sequence (Fig. 1, line 9).
Total RNA was isolated from cells carrying either the vector (pHBD3) or the pSAC1 plasmid. A 5′ biotinylated probe (21F probe) was designed as indicated in Fig. 2. In cells carrying vector alone, we detected a weak hybridization signal that migrated just below the 100 nt RNA marker, presumably from the chromosomally encoded RNA (Fig. 3A, lane 1). The chromosomally encoded RNA hybridization signal is easier to detect upon longer exposure (Fig. 3A, lanes 7 and 8), and that signal is completely eliminated in two null mutant isolates described below (Fig. 3A, lanes 9 and 10). A strong hybridization signal was obtained when RNA from cells carrying the pSAC1 plasmid was probed (Fig. 3A, lane 2). Furthermore, cells carrying a plasmid with any of the NTG mutations showed a significant reduction in RNA levels (Fig. 3A, lanes 3–5) that corresponds to their loss of activity on the RpoS–lacZ fusion. As NTG-3 is within the termination stem–loop and not the promoter, the reduction in RNA levels must result from a destabilization of the RNA. We did not detect high-molecular-weight signals that could have resulted from transcriptional readthrough (data not shown). As expected, only a weak hybridization signal was detected in RNA extracted from cells carrying the pSAC1 plasmid with the KanR cassette insertion (data not shown). However, two signals were recovered after the KanR cassette was deleted: a weak one of wild-type size (chromosomally encoded RNA) and a strong signal from the plasmid-encoded RNA (Fig. 3A, lane 6). The size is larger than the wild-type RNA signal because of the 12 nt that remain after the deletion of kan (Fig. 1, line 9). No signal was detected with a probe (21R probe; Fig. 2) for the other strand (data not shown). As expected, under the conditions in which RprA itself is expressed at high levels, RpoS protein levels are also increased (Fig. 3B, lane 2). Therefore, the effect of RprA is not limited to the RpoS–lacZ fusion. This increase in RpoS expression also results in the increased expression of dsrB–lacZ, an RpoS-dependent fusion (D. Hernandez, N. Majdalani and S. Gottesman, in preparation; Sledjeski et al., 1996).
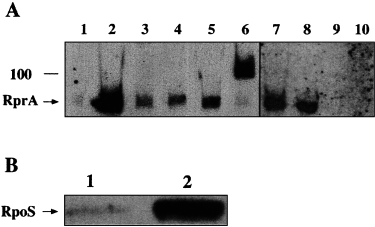
A. Northern blot analysis of RprA. In lanes 1–6, total RNA was extracted from strain NM22508 carrying various plasmids. About 3 µg of RNA was loaded per lane of gel and electroblotted onto positively charged nylon membranes. The membranes were probed with 21F probe, a synthetic DNA probe biotinylated at its 5′ end. Lane 1, vector control (pHDB3); lane 2, wild-type multicopy RprA (pSAC1); lane 3, multicopy NTG-1 (G−46A) RprA (pJM6); lane 4 multicopy NTG-3 (G+96A) RprA (pJM7); lane 5, multicopy NTG-2 (G−33A) RprA (pJM8); lane 6, multicopy RprA–ΔKanR after the SbfI deletion of Kan (pJM20). To detect chromosomally encoded RprA better, exposure times had to be increased (lanes 7–10) from 2 to 3 min up to 15–20 min. Thus, a Northern blot was performed on total RNA extracted from two isolates of NM22508 (lanes 7 and 8) or on RNA from two independently isolated null mutants, KSJ6 and KSJ7, derived from NM22508 (rprA::kan; lanes 9, 10) (see Experimental procedures).
B. Western blot of RpoS. Cells carrying either the vector plasmid (lane 1) or pSAC1 (lane 2) were grown in LB Amp at 32°C to an OD600 of about 0.4 units. A 1 ml aliquot was taken and precipitated with TCA and washed with acetone. Samples were loaded on a Novex NuPAGE 10% gel, migrated, transferred and probed with anti-RpoS antibodies as described elsewhere (Zhou and Gottesman, 1998).
The 5′ end of the small RNA made in cells containing pSAC1 was determined by primer extension, using a primer that is identical in sequence to the 21F probe but lacking the 5′ biotinylation (21F) (Sledjeski and Gottesman, 1995). This extension yielded a single radiolabelled product that is about 68–69 nt long, consistent with either C or A (nt −1 or +1 in Fig. 2) as the starting 5′ nucleotide. To determine the 5′ end unambiguously, we synthesized the RNA using an in vitro transcription system and [γ-32P]-ATP (Zhou and Jin, 1998). The product of this reaction was radiolabelled and migrates at about 100 nt (the 3′ end was not determined). This establishes that the 5′ end of the RNA is the A nucleotide indicated as +1 in Fig. 2 and that the promoter has some activity in a purified in vitro system (data not shown).
The RNA size estimated from the 100 nt RNA marker in Fig. 3 would suggest that RprA is slightly less than 100 nt long. However, our size estimate based on the start point determined experimentally to the termination after the stretch of Ts is 106 nt (Fig. 2). At this time, we do not know the basis for this discrepancy.
We cloned the gene for this novel RNA (nucleotides +1 to 179; this ends 33 nt beyond the +146 marked in Fig. 2) in a derivative of pBAD24 (Guzman et al., 1995), pNM12 (Majdalani et al., 1998). Expression of the RNA is inducible and under the control of the araBAD promoter. This plasmid, pNM100, was used to transform a ΔdsrA strain that carries the RpoS–lacZ translational fusion (NM22508). Induction of transcription in this plasmid was necessary and sufficient to stimulate the expression of the RpoS–lacZ translational fusion (Fig. 4). We have called this new RNA RprA (RpoS regulator A) and the gene encoding it rprA.
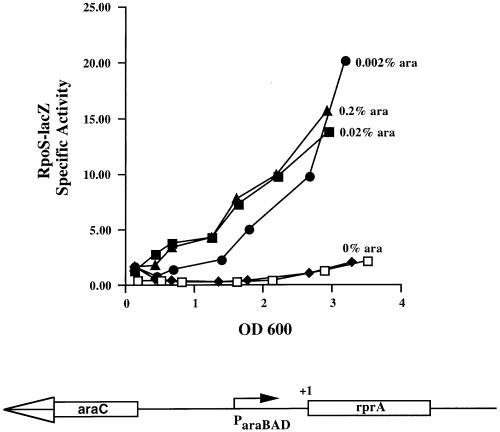
Map and activity of the pBAD–rprA construct. A schematic map of the pBAD–rprA plasmid is shown at the bottom of the figure (not to scale). To assay the effect of RprA on the RpoS–lacZ fusion, the vector (pNM12) or the pBAD–rprA (pNM100) plasmid were introduced into the dsrA−, RpoS–lacZ host NM22508. The open symbol (□) represents cells carrying vector in the presence of 0.2% ara as a control. The filled symbols represent cells carrying the pBAD–rprA plasmid and grown without or with various concentration of arabinose as indicated on the graph.
Determining the half-life of RprA
The half-life of RprA was determined in both the presence and the absence of DsrA, using a dsrA+, rprA+ strain (KSJ16) and a dsrA−, rprA+ (KSJ18) strain. Cells were grown to an OD600 of 0.7. Rifampicin (300 µg ml−1) was added to prevent further RNA synthesis, and samples were taken at 0, 2, 4, 6, 8, 10 and 20 min to determine stability (Fig. 5). Similar results were obtained with the dsrA− strain KSJ18 (data not shown). These data indicate that SsrA, used as a control, is stable throughout the time course (Chauhan and Apirion, 1989). On the other hand, the signal for RprA showed a 75% decrease within 10 min and reached a stable level of 15% within 20 min. The half-life of the chromosomally encoded RprA RNA was determined to be 7–8 min. This value is somewhat longer than that of most mRNAs (average half-life of 2–3 min; Belasco, 1993) but is shorter than that of other small RNAs, such as OxyS (≈12 min) (Altuvia et al., 1997), Spot 42 (≈ 20 min) (Sahagan, 1977; Sahagan and Dahlberg, 1979) and DsrA (> 25 min) (Majdalani et al., 1998).
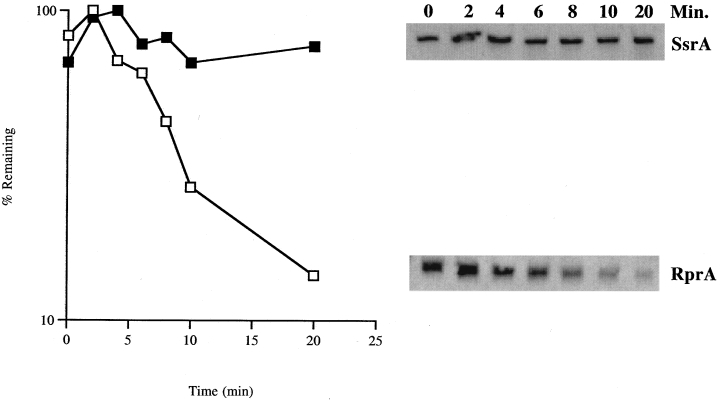
Stability of RprA. The stability of RprA was assessed in a Northern blot of RNA samples from a rifampicin chase experiment. A dsrA+, clpP− host (KSJ16) was grown to an OD600 of 0.7. Rifampicin was added to a final concentration of 300 µg ml−1, and a 1 ml aliquot was taken immediately as t0. Aliquots (1 ml) were then taken at 10, 20, 30 and 60 min after the Rif addition. Total RNA was extracted, and 3 µg was used to run on a gel, blot and probe as described elsewhere. Quantification was performed from a radiographic film using Stratagene's eagleeye. The stable RNA, SsrA, was used as a control.
Constructing a chromosomal null mutation of rprA
To construct a null mutation of rprA, we transferred the KanR insert of pJM14 (Fig. 1, line 8) into the chromosomal copy of rprA. The 3 kb fragment of pJM14 was amplified and cloned into the SmaI site of plasmid pKO3 (Link et al., 1997) to create pKSJ1. This plasmid was used to transform an RpoS–lacZ, ΔdsrA strain (NM22508) and carry out a precise genomic rearrangement (see Experimental procedures) (Link et al., 1997). Several KanR CmS clones were isolated and purified. The correct insertion was confirmed by polymerase chain reaction (PCR; see Experimental procedures).
The genome map of E. coli indicates that rprA is near aroD and himA (5 kb and 25 kb downstream respectively; Fig. 1). The calculated frequencies of linkage over such map distances are 87% and 70% respectively. The rprA::kan allele was introduced by P1 transduction into strain AB2848 (aroD−), and KanR transductants were scored for the AroD− phenotype. The linkage of the two alleles was 85–90%, consistent with the expected 5 kb map distance. The rprA::kan allele was also introduced into a himA::tet strain (SG12073), and a resulting TetR, KanR transductant was saved. A P1 lysate grown on this strain was used to transduce MG1655, selecting for either kanamycin resistance or tetracycline resistance and scoring for the other marker. In each case, the frequency of linkage was between 60% and 80%, which is in line with the expected frequency. More importantly, initial selection for Kan or Tet before scoring the other marker yielded the same linkage frequencies. This indicates that the rprA gene is neither essential nor strongly advantageous for growth under these conditions.
Phenotype of an RprA null mutant
An rprA mutant does not show any obvious physiological defects such as changes in growth rate in rich media. However, RpoS translation is known to be affected by growth on minimal media and by osmotic shock (Lange and Hengge-Aronis, 1994). We tested the expression of the RpoS–lacZ translational fusion under these stress conditions in the presence and absence of DsrA and RprA.
Four isogenic strains (dsrA±, rprA±, NM22507, KSJ13, NM22508, KSJ15) carrying the RpoS–lacZ translational fusion were grown at 32°C in M63 minimal medium, and β-galactosidase activity was assayed as described (Experimental procedures). As expected, dsrA+ cells had significantly more activity than dsrA−, but the presence or absence of RprA did not show any additional effects (data not shown). Therefore, DsrA but not RprA is important for minimal medium expression of RpoS.
Because expression of the RpoS–lacZ fusion is very low in dsrA− hosts and to avoid complications from the regulated degradation of the RpoS–lacZ fusion, we tested for the osmotic shock response in hosts mutant for clpP, in which RpoS and RpoS–lacZ are significantly more stable (Schweder et al., 1996). An isogenic set of clpP− (RpoS–lacZ) strains [dsrA+, rprA+ (KSJ16); dsrA+, rprA− (KSJ17); dsrA−, rprA+ (KSJ18) dsrA−, rprA− (KSJ19)] were constructed. Cells were grown in LB at 32°C to an OD600 of 0.2 and then subjected to a 0.4 M NaCl or 0.464 M sucrose osmotic shock. As shown in Fig. 6A, dsrA+ cells show a rapid increase in β-galactosidase activity consistent with previous observations (Lange and Hengge-Aronis, 1994). The presence or absence of RprA did not affect this expression. In the absence of DsrA (Fig. 6B), the overall levels of RpoS–lacZ are much lower; rprA+ cells respond significantly better to an osmotic shock than cells lacking both DsrA and RprA. We note, however, some residual osmotic induction even in the absence of both DsrA and RprA. In addition, we see a small but reproducible difference in the basal levels of expression of RpoS–lacZ between the rprA+ and rprA− hosts in the absence of DsrA and ClpP, suggesting a role for RprA even in the absence of osmotic shock.
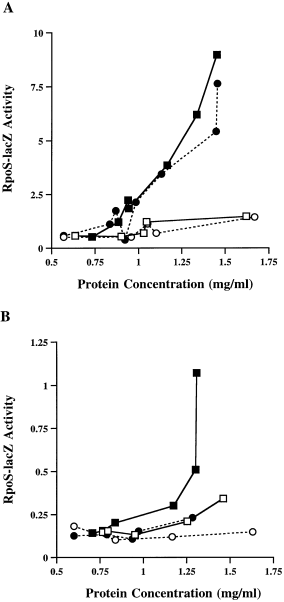
Effects of an rprA null mutation on RpoS–lacZ under osmotic shock conditions. A set of clpP− strains carrying an RpoS–lacZ fusion with dsrA (rprA+ KSJ16, rprA− KSJ17; A) or without dsrA (rprA+ KSJ18, rprA− KSJ19; B) was grown to an OD600 of ≈ 0.3. Cultures were divided; one half was subjected to a sucrose osmotic shock (0.464 M final concentration), while the other half was retained as a control. Squares represent rprA+ strains; circles represent rprA− strains. Open symbols represent control cultures; filled symbols represent cultures subjected to the osmotic shock. Similar results were obtained using an NaCl osmotic shock (data not shown). Cells subjected to osmotic shock plasmolyse, whereas untreated cells are slightly diluted (see Experimental procedures). This skews the OD reading at the time of shock induction or dilution even though the number of cells is the same. To correct for this phenomenon, graphs are plotted as activity (machine units; see Experimental procedures) versus the protein concentration.
Basis for osmotic shock regulation
In theory, osmotic shock could act by stimulating the expression of dsrA and rprA and/or by allowing a more efficient use of these RNAs. In order to investigate these possibilities, we chose the more active DsrA and asked whether the DsrA-dependent osmotic shock response was seen when DsrA was synthesized from the pBAD promoter rather than from its own promoter. ΔdsrA cells carrying the RpoS–lacZ fusion and either the vector control (pNM12) or the pBAD–dsrA plasmid (pNM13) were grown and subjected to an osmotic shock. If synthesis of DsrA is induced by osmotic shock, and this is the sole basis for increased RpoS expression, we would have expected changing the promoter to change the response to osmotic shock. Expression of the RpoS–lacZ fusion was very low in cells carrying the vector (squares) or the pBAD–dsrA plasmid without arabinose induction (Fig. 7, open circles, solid line). Some increase was seen when these cells were given an osmotic shock (Fig. 7, filled squares, filled circles with solid line). The RpoS–lacZ activity rose when cells carrying pBAD–dsrA were grown with 0.02% arabinose (without osmotic shock; Fig. 7, open circles, dotted line). However, a further increase in RpoS–lacZ activity was observed when cells carrying the pBAD–dsrA plasmid were subjected to an osmotic shock in the presence of the inducer (Fig. 7, filled circles, dotted line). The slope of these lines gives an estimate of the increase in expression as a result of osmotic shock. Cells with only the vector show a 3.8-fold increase in activity after shock. Cells with induced pBAD–DsrA also increase 3.8-fold after osmotic shock, although the actual values are almost fivefold higher both before and after shock. This suggests that DsrA is not itself necessary for sensing osmotic shock. However, the sensing mechanism results in an enhanced sensitivity of RpoS translation (and possibly the RpoS leader structure itself) to whatever small RNAs or other factors are present in the cell. Synthesis of these small RNAs does not need to be induced by the shock. The very similar induction values with and without DsrA also suggest that the pBAD promoter does not itself respond to osmotic shock. As a control for an effect of osmotic shock on pBAD, cells carrying a pBAD–lacZ plasmid were subjected to a similar osmotic shock in the absence or presence of arabinose. Under both conditions, the levels of β-galactosidase activity remained the same before and after the osmotic shock (data not shown). None of these experiments rules out an additional effect of osmotic shock in inducing DsrA and RprA RNAs from their natural promoters. To address this issue for the dsrA promoter, cells carrying a dsrA–lacZ transcriptional fusion were assayed with and without osmotic shock treatment; no increase in expression of the fusion after osmotic shock was detected (data not shown).
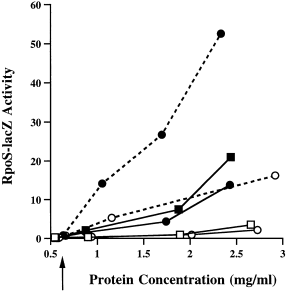
Osmotic shock induction is independent of the dsrA promoter. A dsrA− strain (NM22508) carrying the RpoS–lacZ fusion was transformed with either the vector (pNM12; squares) or pBAD–dsrA (pNM13; circles). Cells were grown without ara (solid lines) or with 0.02% ara (dashed lines) and subjected to an osmotic shock at the point indicated by the arrow. Open symbols indicate cells grown without osmotic shock; filled symbols indicate those grown with the sucrose osmotic shock. To correct for the plasmolysis effect of the osmotic shock, data were plotted as described in the legend to Fig. 6.
Discussion
We began this work to investigate whether residual translation of RpoS in a dsrA mutant might result from additional small regulatory RNAs. In fact, a novel small RNA, RprA, was detected from a random screen of clones capable of stimulating RpoS–lacZ translation. Increased transcription of this RNA from the araBAD promoter increases RpoS–lacZ synthesis in a dsrA− host. In addition, we were able to detect a physiological role for the single-copy gene for this new RNA in stimulating the osmotic shock-dependent increase in RpoS in the absence of DsrA. Thus, RpoS translation is positively regulated by at least two small RNAs, and possibly more. An additional small RNA that acts as a negative regulator of RpoS, OxyS, has also been described (Zhang et al., 1998).
DsrA and RprA share a number of common characteristics. Both are reasonably small molecules (85 nt for DsrA, 106 nt for RprA) that are predicted to form multiple stable stem–loops. In addition, both show a dependence on HFQ, a protein that has been shown to be necessary for RpoS translation (Muffler et al., 1996). DsrA action is impaired in the hfq mutant (Sledjeski et al., 2001); we find that RprA stimulation of RpoS translation is also abolished by hfq inactivation (N. Majdalani, unpublished results).
Another characteristic shared by both these small RNAs, and possibly a characteristic of many small RNAs, is the high degree of conservation in closely related species. For rprA and dsrA, this is significantly higher than conservation within the coding regions for nearby ORFs. The high conservation between E. coli, Klebsiella and Salmonella for dsrA has been noted previously (Majdalani et al., 1998); within the body of DsrA, 85% of positions are identical in all three species, whereas in a comparable 60 bp stretch of the DNA from the nearby rcsA genes from these same species, the most conserved region seen among all three species was 78%, with the norm showing 55–65% conservation. rprA, which lies at min 38 of the E. coli genome, is bordered by a number of uncharacterized ORFs. When the DNA for the most conserved 120 bp region of the ORF immediately upstream of rprA, b1688, is compared for E. coli, Klebsiella and Salmonella, there is a 67% conservation between all three species, whereas within the rprA gene, conservation in all three species is 94% (Fig. 8). In fact, the only variation seen within the rprA gene is either in the loop of the putative 5′ stem–loop or compensatory changes in the terminator hairpin. We suggest that a region of about 100 nt with high conservation between closely related species may serve as one of the signs of such regulatory RNAs, in which structure and sequence, rather than coding capacity, have been conserved. The additional presence of secondary structure may provide a second level of evidence for such RNAs.

Sequence conservation for the small RNAs. The DNA sequences of the RprA RNA region and of the upstream gene b1688 ORF from E. coli, S. typhimurium and K. pneumoniae were aligned. Identity is indicated by a dot. Sequences for Klebsiella and Salmonella were obtained from a blast search of the E. coli sequences with the unfinished microbial genome database at the Institute for Genomic Research website at http://www.tigr.org. The underlines in the rprA sequence represent the −35 and −10 boxes. Arrows above the sequence indicate the inverted repeats predicted to fold into stem structures.
Despite these similarities, there are also striking differences between DsrA and RprA. DsrA has extensive complementarity to the leader RNA of RpoS, and we have shown that direct RNA–RNA pairing between DsrA and the RpoS leader is necessary for efficient activation of translation by DsrA (Majdalani et al., 1998). RprA, on the other hand, does not carry this extensive complementarity. We noted a number of short regions of possible complementarity, but mutations in these regions of rprA have not affected activity significantly (Fig. 1, line 7; data not shown). Thus, the mode of action of RprA remains a subject of further investigation; if it pairs with the RpoS mRNA, it may be in other regions or via pairing interactions that we have not thus far predicted by our sequence inspection. It is worth noting that a role for sequences within the coding region of rpoS has recently been implicated in translational regulation by DksA (Webb et al., 1999); we do not know whether this region is necessary for regulation by RprA. Alternatively, RprA may stimulate RpoS translation only indirectly, via another RpoS regulator. It is clear, however, from the results of our studies of the multicopy RprA under the pBAD promoter, as well as from the contribution of the single-copy gene to osmotic regulation, that RprA activity on RpoS–lacZ translation is significantly lower than that for DsrA, possibly reflecting the lack of extensive complementarity. Also, RprA is significantly less stable than DsrA, further limiting activity.
Why would the cell have multiple small RNA regulators of RpoS? Two advantages are apparent. It seems possible that, by independently regulating the synthesis of each RNA, different signals can lead to increased amounts of RpoS. Thus, dsrA synthesis is increased at low temperatures, and we have shown that high amounts of RpoS during exponential growth at low temperatures are dependent upon DsrA (Sledjeski et al., 1996). Recent work has demonstrated that it is the promoter of dsrA that is primarily responsible for this temperature regulation (F. Repoila and S. Gottesman, in preparation.). The promoter of rprA, however, does not appear to show the same response to temperature. We have found recently that the rprA promoter is positively regulated by RcsB, the response regulator used for capsule synthesis (Gottesman, 1995). Mutations that activate RcsB upregulated RpoS in an RprA-dependent fashion (D. Hernandez, N. Majdalani, and S. Gottesman, in preparation). Thus, under conditions that activate RcsB, RprA may become a primary regulator of RpoS. A second advantage may lie in the multiple activities of small RNAs. We know that DsrA not only stimulates RpoS translation, it downregulates H-NS (Sledjeski and Gottesman, 1995; Lease et al., 1998), and the regions of dsrA necessary for RpoS stimulation are independent of those regulating H-NS (Majdalani et al., 1998). This co-regulation of H-NS and RpoS may be important to the cell under the conditions in which DsrA is made. Although we were unable to detect any effect of RprA on H-NS (data not shown), there may well be other targets for RprA and, again, the co-regulation of RpoS with these targets may be important for the cell under particular environmental conditions.
Five classes of plasmids were identified as able to increase RpoS–Lac expression during these experiments (Table 2). Class 2 encoded RprA. Of the other plasmids isolated in this screen, only one (class 1) increased the expression of the RpoS–LacZ fusion by interfering with degradation, and the presence of a truncated ClpX gene on that plasmid suggests a dominant negative interference with ClpX function as an explanation. Another plasmid (class 3) carries the gene encoding DsrA, expected to increase RpoS translation (Sledjeski et al., 1996). Although the genes responsible for the increase in RpoS–LacZ synthesis have not been identified for class 4 and class 5 plasmids, class 4 includes the region encoding barA, a gene recently implicated in positively controlling RpoS transcription (Mukhopadhyay et al., 2000). Thus, this search has identified three known regulators of RpoS, one novel small RNA regulator and one plasmid operating in an as yet unknown manner.
Several small RNAs have now been implicated in gene regulation in E. coli. Many of these genes act as antisense elements that inhibit the translation of their target (Wassarman et al., 1999; Lease and Belfort, 2000). However, other mechanisms have been identified as well. SsrA adds a specific tag to unterminated transcripts that target the gene product to elimination by degradation (Keiler et al., 1996). OxyS seems to titrate an essential factor away from the target transcript, thereby causing the inhibition of RpoS translation (Zhang et al., 1998). The 6S RNA modulates the activity of RNA polymerase (Wassarman and Storz, 2000). DsrA is the first small RNA implicated in the stimulation of translation by disrupting an antisense cis element present within the leader region of the RpoS mRNA (Majdalani et al., 1998; Wassarman et al., 1999). The additional regulation by RprA suggests that the participation of a positively acting small RNA is not a peculiarity of DsrA, and the involvement of small RNAs may be the rule rather than the exception for genes subject to translational regulation.
Experimental procedures
Strains and genetic techniques
Strains are listed in Table 1. The himA::tet donor was obtained from R. Weisberg (NICHD); the derivative we used was a transductant from the original host, SG12073. The aroD− strain AB2848 was obtained from the E. coli Genetic Stock Center at Yale University (EC-GSC). Strain NM22506 was derived from SG22500 by P1 transduction (Miller, 1972) to introduce Δara;leu::tet from KS272 (Guzman et al., 1995) followed by another P1 transduction to introduce Δara;leu+, selecting for Leu+ on M63 minimal medium. The ΔdsrA and dsrA+ derivatives were constructed by P1 transduction from DDS719 and DDS720 (Sledjeski et al., 1996), respectively, using a linked mini-Tn10 marker to select for TetR, screened by loss or gain of RpoS–lacZ activity, respectively, and confirmed by PCR (Sledjeski et al., 1996). NM22554 is a derivative, by P1 transduction, of NM22506 carrying ΔdsrA and hfq2::kan, a Kan insertion near the 3′ end of hfq that is phenotypically Hfq+ (Tsui et al., 1994).
Transformations were performed by either electroporation using a GenePulsar II (Bio-Rad) or the transformation and storage solution method (TSS) (Chung et al., 1989).
β-Galactosidase assays
The β-galactosidase (β-gal) activity of the lacZ fusion was assayed on a SpectraMax 250 (Molecular Devices) microtitre plate reader as described previously (Majdalani et al., 1998). Specific activities are represented as the Vmax divided by the OD600, and these units are about 25 times lower than standard Miller units.
For β-gal assays after osmotic shock, dilutions of overnight cultures were allowed to grow to an OD600 of 0.3–0.4 before the cultures were divided. Sucrose (0.464 M final) was added to half of the cultures to induce osmotic shock; prewarmed media was added to the other half. At this point, shocked cells plasmolyse, and their OD600 reading becomes skewed even though the number of cells is the same. To correct for this phenomenon, the total protein concentration was determined for each time point collected, and the β-gal activities (Vmax numbers from SpectraMax assays) rather than specific activities were plotted against the total protein concentration rather than OD. Therefore, the slopes of the lines (6, 7) reflect the rate of RpoS–lacZ expression.
Protein assays were performed using a Lowry-based DC protein assay from Bio-Rad. Briefly, for each time point to be measured, a 0.5 ml aliquot of cell culture was taken; cells were pelleted and resuspended in 100–200 µl of 50 mM Tris, 2% SDS buffer. Samples were vortexed for 5 min and boiled at 95°C for 3 min. Depending on OD, 10–100 µl of these samples was used for the assay according to the manufacturer's specifications.
Chemicals, enzymes and reagents
Rifampicin, ONPG and nitrosoguanidine (NTG) were purchased from Sigma Chemicals. Sucrose was purchased from ICN. The [γ-32P]-ATP radionuclides were purchased from Amersham Pharmacia Biotech. Primers and biotinylated probes were synthesized by Sigma-Genosys.
Plasmid construction and DNA manipulations
Construction of the series of deletions from plasmid pSAC1 was performed using double digestion with the enzymes indicated in the legend to Fig. 1. In situations where the ends were not compatible cohesive ends, the resulting 5′ overhangs were end filled using T4 polymerase and nucleotides, while the 3′ overhangs were blunted using the T4 polymerase without nucleotides (Sambrook et al., 1989; Ausubel et al., 1992). Blunt ends were then ligated.
To construct the pBAD–rprA plasmid, PCR was used to generate a promoterless DNA fragment with the forward primer (AAGTCCGTATGCCTACTATGGCCACACGGTTATAAATC) carrying an MscI site and the reverse primer (ACGTACGTGAATTCGAAGAGAGTTCACAGTATC) containing an EcoRI site. This PCR fragment was then cloned into the same sites in pNM12, a derivative of pBAD24 (Guzman et al., 1995) that contains an MscI site at the −7 to −2 region upstream of the +1 transcription start site (Majdalani et al., 1998). The resulting plasmid expresses rprA under the control of the araBAD promoter.
To construct plasmid pKSJ1, PCR was used to amplify the DNA fragment from pJM14 carrying the 1.7 kb clone from pSAC1 but with a Kan insertion in rprA. This 3 kb fragment was then blunt end cloned into the unique SmaI site of plasmid pKO3 (Link et al., 1997).
Sequencing was performed using a Gibco BRL double-stranded DNA cycle sequencing kit, while site-directed mutagenesis was carried out using a Stratagene QuickChange mutagenesis kit according to the respective manufacturers' specifications. Other methods for DNA manipulations were as described by Maniatis et al. (1982), Sambrook et al. (1989) and Ausubel et al. (1992).
The accession number for the rprA sequence is AF326576.
RNA half-life determination
Briefly, 10 ml cultures were inoculated from an overnight culture and allowed to grow at 32°C to an OD600 of ≈ 0.7 in LB media. Rifampicin stock solution in dimethyl formamide was added to a final concentration of 300 µg ml−1. Total RNA was extracted from 1 ml aliquots of culture taken 0, 2, 4, 6, 8, 10 and 20 min after the addition of rifampicin. A Northern blot analysis was performed using the RprA probe, a 5′ end biotinylated DNA oligonucleotide probe (GGGGATTTCCATGCTTATAAATCAATATGT). Quantification of signal was done from a film exposure using Stratagene's EagleEye II machine and its eagleeye sight analysis software.
Northern blot analysis
Northern blot analyses were performed as described elsewhere (Majdalani et al., 1998) with the following modifications. An 8% SequaGel (National Diagnostics) urea–acrylamide sequencing gel was used. RNA (3 µg) was loaded in each lane of the 16 × 16 cm gels after a 1 h preheating of the gel to 50°C. Gels were electrophoresed at 400–420 V for 80 min in 1× TBE in DEPC-treated water. Electrotransfer onto NytranN positively charged nylon membranes (Schleicher and Schuell,) was set up and run overnight in 0.5× TBE as described previously (Majdalani et al., 1998). After UV cross-linking, the prehybridization and hybridization steps were carried out using Ambion's UltraHyb solution. The 5′ biotinylated DNA probes were used at 100 ng ml−1 concentrations, and the signal was developed with Ambion's BrightStar Biodetect non-isotopic kit but with additional washes required by the UltraHyb manufacturer's protocol.
In vitro transcription
The standard reaction contained final concentrations of 50 mM KCl, 40 mM Tris-HCl (pH 8.0), 10 mM MgCl2, 1 mM dithiothreitol (DTT) and 100 µg ml−1 BSA. For each reaction, 0.2 µg of DNA was added along with 50 ng of RNA polymerase holoenzyme, 2 µl of [γ-32P]-ATP and 2 µl of 10× NTP mix 1 (0.05 mM ATP, 0.2 mM CTP, 0.2 mM GTP, 2 mM UTP). The reaction was incubated at 37°C for 15 min. Two microlitres of 10× NTP mix 2 (2 mM ATP, CTP, GTP, UTP) was then added, and the reactions were incubated at 37°C for an additional 15 min before adding the stop solution (Zhou and Jin, 1998). Samples of 10–15 µl were loaded per lane on a 6% sequencing gel.
Library screening
Transformations were performed by electroporation into NM22554 and plated on MacConkey lactose plates containing 50 µg ml−1 ampicillin (MacLac Amp) to give about 2000 colonies per plate and incubated at 32°C. We screened about 25 000 colonies and isolated 23 red colonies. Plasmid DNA was purified using Promega's Wizard Plus Minipreps kit, and insert junctions were sequenced using primers pBRlib.for (CCTGACGTCTAAGAAACCATTATTATC) and pBRlib.rev (AACGACAGGAGCACGATCATGCG). The UW-GCG blast search program (http://www.ncbi.nlm.nih.gov/BLAST version 2.0) was used to map these sequences in the E. coli genome.
Construction of an rprA knock-out strain
A derivative of plasmid pKO3 carrying rprA::kan (pKSJ1) was used to transform strain NM22508. The parent plasmid has temperature-sensitive replication and carries cat, encoding resistance to chloramphenicol, and sucrase (sacB), causing sucrose sensitivity in E. coli (Link et al., 1997). Integration of the plasmid by homology with the bacterial insert was selected for at 43°C on LB-chloramphenicol (Cm) (25 µg µl−1) plates. Two colonies were picked, serially diluted and plated at 30°C on LB−5% sucrose (LBS) or LB–kanamycin (Kn) (25 µg µl−1)−5% sucrose (LBKS) to select for the loss of the plasmid vector sequences. These clones were then screened on LB, LB-Kn and LB-Cm. Stable KanR CmS isolates were identified, and two independent isolates, KSJ6 and KSJ7, were used as donors for P1 transduction into other strains.
The correct insertion into rprA was initially confirmed by PCR. Using primers (38F and 38R) flanking the rprA region, genomic DNA from two clones was shown to have an insert of the size expected for the kanamycin cassette. A second PCR reaction using one of the flanking primers and one primer internal to Kan (KanF or KanR) confirmed the position of the cassette (data not shown). The insertions were further confirmed in our linkage mapping as described in the Results.
DNA alignments and computer analysis
DNA alignments between E. coli sequences and those of Klebsiella pneumoniae and Salmonella typhimurium were carried out using preliminary sequence data obtained from the Institute for Genomic Research website at http://www.tigr.org. The Washington University Consortium sequencing project obtained the data for the Klebsiella and Salmonella sequences. The E. coli sequences were used in a blast search, and the alignment was expanded manually from the blast alignment using the contig for the unfinished genome fragment.
Potential promoters were identified using pcsearch (Mulligan and McClure, 1986). Open reading frames were defined using the gcg program (Wisconsin Package, version 10.0).
Acknowledgements
We thank George Church for providing plasmid pKO3. We particularly thank Ding Jin, Xiangdong Wang and Julio Cabrera for their help with primer extensions and in vitro expression of rprA, and David Hernandez for unpublished data. We would also like to thank Francis Repoila and Yan Ning Zhou for critical discussions and comments, and Gisela Storz and Karen Wassarman for comments on the manuscript.




