A novel group I intron-encoded endonuclease specific for the anticodon region of tRNAfMet genes
Abstract
Open reading frames (ORFs) are frequently inserted into group I self-splicing introns. These ORFs encode either maturases that are required for splicing of the intron or DNA endonucleases that promote intron mobility. A self-splicing intron in the tRNAfMet gene of Synechocystis PCC 6803, which has been proposed to have moved laterally within the cyanobacteria, contains an ORF that is unrelated to known intron-encoded endonucleases or maturases. Here, using an in vitro transcription–translation system, we show that this intronic ORF encodes a double-strand DNA endonuclease, I-Ssp6803I. I-Ssp6803I cleaves each strand of the intronless tRNAfMet gene adjacent to the anticodon triplet leaving 3 bp 3′ extensions and has no activity at intron–exon boundaries. Using an in vitro cleavage assay and scanning deletion mutants of the intronless target site, the minimal recognition site was determined to be a partially palindromic 20 bp region encompassing the entire anticodon stem and loop of the tRNAfMet gene. I-Ssp6803I represents a novel intron-encoded DNA endonuclease and is the first example of a chromosomally encoded group I intron endonuclease in bacteria.
Introduction
Self-splicing group I introns have a broad phylogenetic distribution, interrupting a wide variety of mitochondrial and plastid genes, nuclear rRNA genes, bacterial tRNA genes and protein coding genes of eukaryotic and bacterial viruses (Lambowitz and Belfort, 1993). Although some of these introns have been relatively stable for a substantial fraction of evolutionary time (e.g. an intron in the gene encoding a tRNALeu in both cyanobacteria and chloroplasts may have occupied that position before the establishment of endosymbiosis; Kuhsel et al., 1990; Xu et al., 1990; Paquin et al., 1997), others, including those in additional bacterial tRNA genes, have been suggested to be mobile on the basis of phylogenetic data (Biniszkiewicz et al., 1994; Paquin et al., 1997; 1999; Rudi and Jakobsen, 1997; 1999).
Movement of an intron to the same position of a cognate intronless gene is called homing, whereas insertion of the intron into a new genomic site is referred to as intron transposition. Two processes have been offered to explain intron mobility. Introns could be transferred by reverse splicing into mature RNA, followed by reverse transcription and insertion into chromosomal DNA by homologous recombination (Sharp, 1985). Although the first step, reverse splicing, has been demonstrated experimentally in vitro and in vivo (Roman and Woodson, 1995; 1998), reverse transcription and chromosomal integration of the products of this reaction have not been demonstrated, and it is uncertain whether natural movement of introns occurs by this mechanism.
Rather, in many cases, group I intron mobility has been attributed to the action of site-specific DNA endonucleases whose coding sequences are themselves inserted into the introns at positions (usually terminal loops of the RNA structure) where they do not interfere with splicing (reviewed by Dujon et al., 1989; Lambowitz and Belfort, 1993; Jurica and Stoddard, 1999). These endonucleases (referred to as homing endonucleases) share the property of recognizing and cleaving cognate uninterrupted (recipient) genes near the intron insertion site. Using the intron-containing (donor) gene as a template, the cleaved DNA is repaired, resulting in copying of the intron into the recipient gene. Generally, intron insertion disrupts the endonuclease recognition sequence, rendering the intron-containing recipient gene resistant to further cleavage. Homing endonucleases were originally grouped into four families based on conserved amino acid sequence motifs: LAGLIDADG, GIY-YIG, HNH and His–Cys box. However, structural comparisons between HNH and His–Cys box endonucleases revealed conservation in active-site topology and has led to a proposed reclassification of these endonucleases into a single family (Kuhlmann et al., 1999).
Although phylogenetic evidence indicates horizontal (cross-species) inheritance of introns in bacterial tRNA genes, the only open reading frame (ORF) that has been encountered so far is in the intron inserted in tRNAfMet genes of two closely related cyanobacterial isolates, Synechocystis PCC 6803 and PCC 6906 (Paquin et al., 1997). The ORF that interrupts the tRNAfMet gene of Synechocystis PCC 6803 comprises 150 codons (Biniszkiewicz et al., 1994) and, although most of the ORF does not participate in the conserved intron secondary structure, its 5′ and 3′ ends comprise part of the P1 stem and all of the P2 stem–loop structural elements of the intron respectively (Fig. 1). Unfortunately, no indication for a function of the ORF could be obtained by sequence comparisons; the only match using blastp with standard parameters (Altschul et al., 1997) is to another gene of unknown function, phage T7 gene 5.3 (Biniszkiewicz et al., 1994). The absence of a recognizable ribosome binding sequence (RBS) and the involvement of the start codon in a region of potential secondary structure (Fig. 1) both indicate that the ORF may be inefficiently translated.
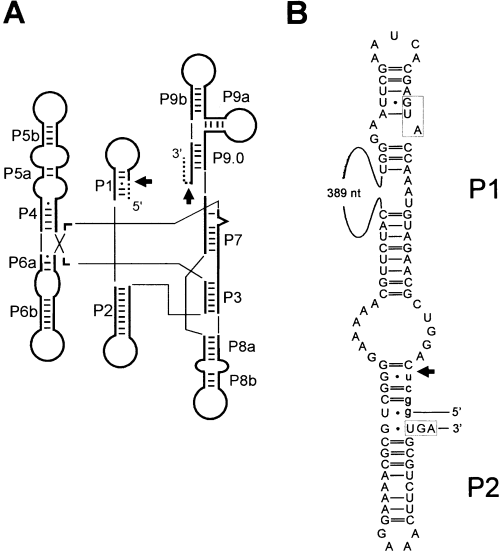
Secondary structure of the P1 and P2 region of the Synechocystis PCC 6803 tRNAfMet intron.
A. Schematic representation of the consensus secondary structure of eubacterial tRNA group I introns, drawn according to Cech et al. (1994). Phylogenetically conserved stems (P1 to P9), Watson–Crick basepairs (bars) and G-U pairs (dots) are shown according to Michel and Westhof (1990). Exons (dashed lines), the intron (thick lines) and splice sites (arrows) are indicated. Thin lines are used to join helical domains of a typical group I intron.
B. Secondary structure of the P1 and P2 stems of the Synechocystis PCC 6803 tRNAfMet intron. Intron sequence (upper case) and exon sequences (lower case) are shown. The initiation and termination codons of the ORF are boxed. The 5′ splice site is indicated by an arrow. A 389 nucleotide region encoding most of the ORF has been omitted.
Attempts to clone this ORF in the context of a bacterial promoter and RBS have been consistently negative. Stable transformants always contain point mutations or gross rearrangements of the coding sequence, indicating that a small amount of the gene product is toxic to Escherichia coli (our unpublished experiments). To circumvent this problem, we transcribed and translated the ORF in vitro using cell-free extracts. We report here that the product of transcription and translation of the ORF is a novel double-strand DNA endonuclease that cleaves the uninterrupted tRNAfMet gene at the site of intron insertion.
Results
The Synechocystis PCC 6803 tRNAfMet intron ORF encodes a DNA endonuclease, I-Ssp6803I
To test whether the Synechocystis tRNAfMet intron ORF is a DNA endonuclease, we synthesized the protein in vitro. We used the amplification product from primers SORF5′-2 and SORF3′-2 as a template for in vitro T7 RNA polymerase transcription and subsequent translation with either an E. coli S-30 extract or a rabbit reticulocyte lysate. Incubation of a 432 bp PvuII fragment from pSFM21, containing the putative endonuclease target site, with the in vitro-synthesized protein resulted in the appearance of cleavage products (Fig. 2A, lane 1). No cleavage products were observed when this substrate DNA was incubated with a mock transcription/translation reaction or incubated alone (Fig. 2A, lanes 2 and 3 respectively). The equivalent PvuII fragment from the vector (without the target site inserted) was not cleaved (Fig. 2A, lanes 4–6). This endonuclease has been named I-Ssp6803I according to accepted guidelines (Belfort and Roberts, 1997).
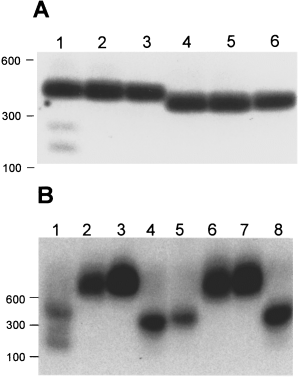
I-Ssp6803I exhibits endonuclease activity. Reaction products were separated by electrophoresis through a 1% agarose gel, blotted and probed with pSFM21, which contains the I-Ssp6803I target site, labelled with 32P by random priming. Positions of molecular size markers are indicated on the left.
A. Gel-purified PvuII fragments from pSFM21 (lanes 1–3) and pBSM13+, which contains vector sequence only (lanes 4–6), were incubated with and without in vitro-synthesized I-Ssp6803I. Lanes 1 and 4 contain in vitro-synthesized I-Ssp6803I; lanes 2 and 5 contain the product of a mock transcription–translation reaction; and lanes 3 and 6 contain substrate incubated under the same reaction conditions excluding protein.
B. Gel-purified PvuII fragments from pSFM21 (lanes 1 and 5), pSB, which contains the 5′ exon–intron junction (lanes 2 and 6), pSX, which contains the 3′ exon–intron junction (lanes 3 and 7), and pBSM13+ (lanes 4 and 8) were incubated with in vitro-synthesized I-Ssp6803I (lanes 1–4) or a mock transcription–translation reaction (lanes 5–8).
Generally, once homing has occurred, the recognition site is disrupted by the intron and is resistant to cleavage. The exceptions to this rule are the intron-encoded endonucleases I-HmuI and I-HmuII from the Bacillus subtilis bacteriophages SP01 and SP82 respectively (Goodrich-Blair and Shub, 1996). We examined the activity of I-Ssp6803I on DNA substrates containing the intron–exon junctions. In vitro-synthesized I-Ssp6803I was incubated with gel-purified restriction fragments corresponding to both the 5′ and the 3′ exon–intron boundaries of the wild-type tRNAfMet gene (Fig. 2B). Neither exon–intron boundary was cleaved by I-Ssp6803I (Fig. 2B, lanes 2 and 3), although a parallel reaction using the intronless substrate was cleaved extensively (Fig. 2B, lane 1).
I-Ssp6803I cleaves at the intron insertion site
Most homing endonucleases characterized to date cleave within a few basepairs of their intron insertion sites (Belfort and Roberts, 1997). Notable exceptions are the homing endonucleases encoded in the three group I introns of T-even bacteriophages, I-TevI, I-TevII and I-TevIII (Bell-Pedersen et al., 1990; Eddy and Gold, 1991), and I-HmuII, the intron-encoded endonuclease in the DNA polymerase gene of phage SP82 (Goodrich-Blair and Shub, 1996). The T-even homing endonucleases cleave at distances of 13–25 bp from their intron insertion sites, and I-HmuII cleaves 52 bp from its intron insertion site.
To determine where I-Ssp6803I cleaves the intronless substrate, individually 5′ end-labelled polymerase chain reaction (PCR) products (in which only one of the two amplification primers was radiolabelled with 32P) were incubated with in vitro-synthesized I-Ssp6803I. Cleavage products were separated by electrophoresis on a denaturing polyacrylamide gel next to sequencing ladders generated with the same 5′ end-labelled primers used in the PCR. Figures 3A and B show the products of the cleavage reaction, which correspond to cleavage immediately 3′ to the anticodon triplet on both strands (Fig. 3C). We confirmed the identity of these cleavage sites in a similar experiment, except that we mixed each sequencing reaction with the products of the appropriate endonuclease cleavage reaction before electrophoresis, observing the dideoxy termination product that displayed exact co-migration with the cleavage product (data not shown).
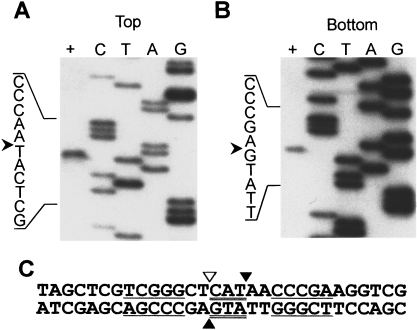
I-Ssp6803I cleavage site mapping. A 208 bp region of pSFM21 was amplified (using universal −20 and reverse primers) for use as the target DNA. Each strand was individually 5′ end-labelled with 32P in different PCRs. Sequencing ladders were generated using the corresponding labelled primer and pSFM21 DNA. Sequencing reactions are denoted by the ddNTP used in the reaction. The sequence of the target DNA immediately flanking the cut site (represented by an arrowhead) is indicated on the left. Target DNA incubated with in vitro-synthesized I-Ssp6803I is indicated by the (+) lane.
A. Top strand cleavage.
B. Bottom strand cleavage.
C. The I-Ssp6803I cleavage site.
Regions involved in basepairing of the anticodon stem of the tRNA are underlined. The anticodon triplet is double underlined. Cleavage sites are indicated by filled triangles. The intron insertion site is indicated by an open triangle.
Determination of the minimal I-Ssp6803I recognition site
Homing endonucleases recognize long, generally asymmetric stretches of DNA (from 12 bp to 40 bp) spanning the intron insertion site (Belfort and Roberts, 1997). As I-Ssp6803I cleaves the intronless target at the site of intron insertion, we focused our investigation on a 43 bp region surrounding this position. We used a PCR-based mutagenesis strategy to introduce site-directed 1 or 2 bp deletions into the region (Fig. 4A). Deletions, in addition to changing the basepair at the site of deletion, shift the remainder of the recognition sequence relative to the DNA helix axis. We presumed that deletion of basepairs within the recognition site would greatly reduce, or eliminate, cleavage of the substrate by I-Ssp6803I. Figure 4B shows that plasmids containing deletions extending from positions −7 to +(13 and 14) were not cleaved by in vitro-synthesized I-Ssp6803I. Interestingly, whereas deletion of both basepairs at positions +13 and +14 rendered the substrate resistant to cleavage, deletion of a single basepair at either position did not abolish cleavage. Deletions outside this region did not eliminate cleavage of the substrates, although some variants were cleaved to a lesser extent (Fig. 4B, −10, −9, −8, +13 and +14). The results indicate that the sequence necessary for cleavage by I-Ssp6803I comprises at least 19 bp (Fig. 4C) and contains the entire anticodon stem–loop region of tRNAfMet.
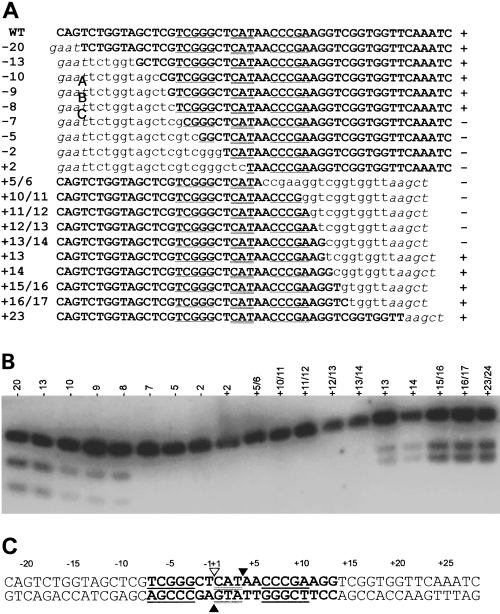
Mapping of the minimal I-Ssp6803I recognition site.
A. Recognition site sequences. Position of deletion relative to the intron insertion site is noted on the left. The sequence of the sense strand of the tRNA gene is shown. Wild-type sequence (upper case and bold), regions encoding the anticodon stem (underlined) and the anticodon triplet (double underlined) are indicated. The portion of each sequence that is out of register as a result of deletion is indicated in lower case. Vector sequence is designated in italicized lower case. The results of in vitro cleavage by I-Ssp6803I (see Fig. 4B) are indicated to the right of the sequence; cleaved (+) and uncleaved (–).
B. Cleavage assay of recognition site mutants. Target DNAs are designated by the position of the bases deleted relative to the intron insertion site.
C. Summary of recognition site mapping. Symbols are as in Fig. 3C. The region of the target site in which deletions abolished cleavage is indicated in bold.
Discussion
Group I introns have been found in many bacteriophage genomes in a wide variety of genes. Most of these introns contain ORFs that encode proteins (belonging to two different homing endonuclease families), some of which have been shown to be required for intron mobility. In contrast, the only chromosomally encoded group I introns found in bacteria are in tRNA genes and, with the exception of sporadic occurrences in the proteobacteria, these are confined to tRNALeu and tRNAfMet genes in cyanobacteria (Reinhold-Hurek and Shub, 1992; Paquin et al., 1997; 1999; Rudi and Jakobsen, 1999). Phylogenetic evidence indicates that the intron inserted immediately 5′ of the anticodon triplet of tRNAfMet genes of widely divergent cyanobacteria has been transferred laterally (Paquin et al., 1997). Most of the bacterial introns do not contain an ORF, the only exceptions being two closely related Synechocystis isolates (PCC 6803 and PCC 6906) that have an ORF in their tRNAfMet introns (Biniszkiewicz et al., 1994; Paquin et al., 1997). As these ORFs do not resemble any proteins of known function in sequence databases, the role of homing endonucleases in the mobility of bacterial introns has remained entirely speculative. Interestingly, an element inserted in the 23S rRNA of Simkania negevensis ZT structurally resembles a group I intron with a LAGLIDADG ORF. However, neither splicing of the intron-like element nor endonuclease activity of the ORF has been demonstrated (Everett et al., 1999).
This report demonstrates that the protein encoded in the tRNAfMet intron from Synechocystis PCC 6803 displays a specific double-strand DNA endonuclease activity (I-Ssp6803I) typical of intron-encoded homing endonucleases. The inability to cleave intron–exon junctions is a hallmark of true homing endonucleases and, under conditions in which the intron insertion site of the Synechocystis tRNAfMet is cleaved extensively, intron–exon boundary sequences are resistant to cleavage (Fig. 2). Also, as in most other group I intron endonucleases, I-Ssp6803I cuts target DNA on both strands leaving 3′ single-strand extensions. However, I-Ssp6803I is unique in that the extensions are three nucleotides in length, rather than the 2 or 4 nucleotide extensions that are left by most other homing endonucleases. Homing endonucleases typically cut their targets close to the intron insertion site, and I-Ssp6803I cleaves one DNA strand precisely at the position of intron insertion. The size of the recognition site for cleavage (≈ 20 bp) is typical of homing endonucleases, but is remarkable among these enzymes only because of the relatively small size of I-Ssp6803I (at 150 amino acids, it is the smallest of the homing endonucleases). Interestingly, the target site encompasses the anticodon stem and loop region of the tRNAfMet gene. This partially palindromic recognition site suggests that a dimer or other multimer with twofold symmetry may be the active form of the nuclease. I-Ssp6803I is the first example of a homing endonuclease encoded by a group I intron in a eubacterial chromosomal gene.
The most intriguing property of I-Ssp6803I is its apparent uniqueness. All other group I intron endonucleases belong to one of three families of proteins that are characterized by signature amino acid sequence motifs. I-Ssp6803I is remarkable in having no relatives with a known function. In fact, a search of the databases with blastp (Altschul et al., 1997) using default parameters reveals only one candidate of 118 amino acids (e-value = 4.2, 27% identity over 99 aligned amino acids), a freestanding ORF (gene 5.3) in bacteriophage T7 (Fig. 5). It is of interest to note that no additional ORFs sharing similarity to I-Ssp6803I have been discovered since the similarity between these two ORFs was first described (Biniszkiewicz et al., 1994). Although nothing is known of the function of bacteriophage T7 gene 5.3, and there is only modest similarity between the two proteins, there is some support for the notion that gene 5.3 may also encode an endonuclease. Like I-Ssp6803I, the product of gene 5.3 is resistant to cloning and overexpression in E. coli, suggesting that the products of these genes are toxic to E. coli (Studier, 1991; R. P. Bonocora and D. A. Shub, unpublished).
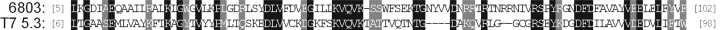
Amino acid sequence alignment between I-Ssp6803I and gene 5.3 from bacteriophage T7. The number of residues before and after the displayed sequence is indicated in brackets. Identical amino acids are indicated by white lettering on a black background. Conserved amino acids are indicated by white lettering on a grey background.
The high toxicity of I-Ssp6803I (and other homing endonucleases) to E. coli is consistent with the fact that, although group I introns have been successful in colonizing bacteriophage genomes, they are relatively rare in the eubacteria. The high sequence similarity of anticodon stem–loops of tRNAfMet genes makes it likely that most bacterial genomes will be sensitive to I-Ssp6803I. In fact, the E. coli tRNAfMet gene is cleaved by I-Ssp6803I (data not shown) and, as there are four of these genes per chromosome, even a single molecule of I-Ssp6803I could be lethal to E. coli. When inserted into a cell that has only a single copy of the recipient gene, an intron-containing tRNA gene would be immune to cleavage by the homing endonuclease, but rare cleavage events at ectopic sites could still be lethal. These considerations may help to explain why homing endonucleases are so rarely encountered in bacteria, which are haploid organisms. It is interesting to note in this context that, as is the case with replicating bacteriophages, there are several copies of the Synechocystis genome per cell, allowing efficient repair of rare double-strand breaks using uncleaved homologous genes as templates (Labarre et al., 1989; Edgell et al., 2000).
Even under these conditions, it seems that the homing endonuclease is only tolerated when expressed at very low levels. The presumptive AUG start codon for I-Ssp6803I is located in a region of potential RNA secondary structure (Fig. 1), and it is not preceded at an appropriate distance by a good match to the RBS region of complementarity to 16S rRNA. Although little is known of the parameters that are required for efficient translation in Synechocystis, it is possible that high expression of I-Ssp6803I might be deleterious even in its natural cellular environment. Interestingly, the RBSs of the homing endonuclease genes in phage T4 are also sequestered in RNA secondary structures that regulate their expression (Gott et al., 1988).
A peculiarity of their RNA secondary structures suggests that other cyanobacterial tRNAfMet introns once possessed homing endonuclease genes that were subsequently lost. The P1 stems of the tRNAfMet introns differ from the canonical P1. Most P1 stems, including those from introns in other bacterial tRNA genes, are simple helices with small terminal loops (Michel and Westhof, 1990; Paquin et al., 1997; 1999), yet all cyanobacterial tRNAfMet P1 structures comprise long interrupted helices with large terminal loops (Paquin et al., 1997). It may be that endonuclease genes similar to I-Ssp6803I inhabited these stems when the introns were acquired but, as a result of the high burden of maintaining the endonucleases, they were subsequently lost. In this case, it is possible that the extended P1 helices are remnants, or molecular ‘scars’, resulting from the excision of the endonuclease ORFs.
Endonuclease invasion appears to have occurred multiple independent times in Synechocystis. In addition to the intron and endonuclease in the tRNAfMet gene, dnaB (encoding a DNA helicase; Pietrokovski, 1996), dnaX (encoding the τ-subunit of DNA polymerase III; Liu and Hu, 1997), dnaE (encoding the catalytic subunit α of DNA polymerase III; Wu et al., 1998) and gyrB (encoding DNA gyrase; Dalgaard et al., 1997) all contain inteins. Although none of these inteins have been shown to be mobile, three of them in dnaB, dnaX and gyrB possess motifs indicative of homing endonucleases. The dnaE intein lacks homing endonuclease motifs, but is split into two genes located 745 kb apart and encoded on opposite strands. This split intein promotes ligation of the exteins in a trans-splicing event. Interestingly, the site at which this intein is split corresponds to the predicted endonuclease insertion site (Wu et al., 1998).
Biochemical and structural analysis of the novel I-Ssp6803I protein is sure to reveal interesting insights into DNA binding and catalysis. It will also be of great interest to determine the evolutionary origin of I-Ssp6803I and how it became associated with a group I intron. Assuming that the successful lateral spread of the tRNAfMet intron among the cyanobacteria results from the presence (however fleetingly) of I-Ssp6803I in those introns, it will also be of interest to determine why the intron is restricted to the cyanobacteria and has not spread more widely into eubacterial tRNAfMet genes. Moreover, I-Ssp6803I is potentially a very useful reagent for genomic mapping studies, as it has a long target sequence, and many initiator tRNAMet genes are likely to be substrates for the enzyme.
Experimental procedures
Polymerase chain reaction
PCR was performed routinely using 10 mM Tris-HCl (pH 8.8), 50 mM KCl, 0.08% Nonidet P40, 1.5 mM MgCl2, 20 pmol of each primer, 200 µM dNTPs, ≈ 100 ng of template DNA and 2.5 units of Taq DNA polymerase. Number of cycles, times and annealing temperatures varied for different applications and are stated where appropriate.
Plasmids
Intronless target plasmid. The intronless target was created by annealing the partially complementary oligonucleotides SYNFMET5′ (5′-CAGTCTGGTAGCTCGTCGGGCTCATAA CCCGAAGGTC) and SYNFMET3′ (5′-GATTTGAACCACCGACCTTCGGGTTATGAGC), corresponding to positions 13–49 and the complement of positions 32–62 of the mature tRNA, respectively, filling in the overhangs using the Klenow fragment of E. coli DNA polymerase and ligating into the HincII site of pBSM13+ (Stratagene). The ligation mix was transformed into E. coli JM109. The resulting plasmid, pSFM21, contains a 50 bp insert corresponding to positions 13–62 of the mature Synechocystis PCC6803 tRNAfMet.
Recognition site plasmids. Plasmids used to determine the I-Ssp6803I recognition site boundaries contain variants of the wild-type intronless sequence differing by 1 or 2 bp deleted from the putative recognition site. Plasmids containing altered target sites with deletions between positions −20 and +2 (with respect to the intron insertion site) were created by amplification of the target site using variants of oligonucleotide E (5′-GAATTCTGGTAGCTCGTCGGGCTCATAAC CCGAAGG), which corresponds to positions 16–47 of the mature tRNA, and the universal reverse primer (5′-AACAGCTATGACCATG). Plasmids containing altered target sites with deletions between positions +5 and +23 were created by amplification of the target site using variants of oligonucleotide H (5′-AAGCTTAACCACCGACCTTCGGGTTATGA GCCCGAC), which corresponds to the complement of positions 56–27 of the mature tRNA, and the universal −20 primer (5′-GTAAAACGACGGCCAGT). Reactions used pSFM21 template DNA and were cycled 20 times at 95°C for 30 s, 47°C for 30 s and 72°C for 30 s with a final extension at 72°C for 10 min. Amplification products were cleaved with EcoRI and HindIII, ligated into EcoRI–HindIII-digested pBSM13+ and transformed into E. coli JM109. Cloned inserts were identified by electrophoretic screening of PCR products from single colonies using universal −20 and reverse primers (Taylor, 1991) and were confirmed by sequencing (for variant target site sequences, see Fig. 4A). Note that plasmids created from cloning of products amplified with primer E and the universal reverse primer have a deletion in the polylinker region of pBSM13+ between the EcoRI and HincII sites, whereas plasmids created from cloning of products amplified with primer H and the universal −20 primer have a deletion of the polylinker between the HindIII and HincII sites.
Splice junction plasmids. Plasmids containing the 5′ splice junction and the 3′ splice junction were used to test for cleavage of intron-containing DNA. Plasmid pSB, containing the 5′ splice junction, was created by amplifying a 339 bp fragment from genomic DNA with primers SB2 (5′-CCCGATCAGTTAAGATCTTCGAAGCGACGC) and SB1 (5′-CCCACGATAAGGAGATCTAACAATGTTTC), which correspond to positions 141–170 and the complement of positions 444–470 respectively (accession no. U10482; Biniszkiewicz et al., 1994). PCR products were digested with BglII and ligated into BamHI-digested pBSM13+. Plasmid pSX, containing the 3′ splice junction, was created by amplifying a 355 bp fragment from genomic DNA with primers SX5prime; (5′-GCGTGATTATCTCTAGAATTCGGGGAAGCC) and SX3prime; (5′-CTGAATAGTTTCTAGAGCATCGTCT), which correspond to positions 669–698 and the complement of positions 1000–1024 respectively. PCR products were digested with XbaI and ligated into XbaI-digested pBSM13+. Both constructs were transformed into JM109 and confirmed by sequencing. Plasmid pSX has a deletion of a single AT basepair from a run of nine AT pairs (at positions 899–907) downstream of the tRNA coding sequence.
In vitro transcription and translation
In vitro transcription and translation was carried out using phage T7 RNA polymerase and either an E. coli S-30 extract (Promega) or a rabbit reticulocyte lysate (MBI Fermentas) according to the manufacturer's specifications. The template DNA used to programme the expression systems was created by PCR amplification from Synechocystis PCC 6803 genomic DNA as above, except that the reactions were cycled 30 times at 92°C for 1 min, 36.3°C for 1 min and 72°C for 2 min using primers SORF5′-2 (5′-TAATACGACTCACTATACGGGCTCAGGTAAGGAGATGTAAACCATG; 29 nucleotides at the 3′ end of the oligonucleotide anneal to positions 196–224), which incorporates a T7 promoter and RBS upstream of the start codon, and the downstream primer SORF3′-2 (5′-TTGGCTCGGGATGATCATTTGAAAGGC; complement of positions 696–722).
I-Ssp6803I cleavage reactions
DNA from plasmids pSFM21, pBSM13+, pSB and pSX DNA was prepared using QIAprep-spin miniprep kit (Qiagen) and digested to completion with PvuII, heat inactivated, extracted with phenol, followed by chloroform–isoamyl alcohol (CIA) and precipitated with ethanol. For use as substrates in the cleavage reaction, fragments containing the target site(s) were purified from agarose gels using the QIAquick gel extraction kit (Qiagen). Target DNA was incubated in cleavage reaction buffer [50 mM Tris-HCl, 10 mM MgCl2, 0.4 µg ml−1 poly-(dI–dC)] with 2 µl of rabbit reticulocyte lysate for 30 min at 30°C. The reaction products were separated by electrophoresis on a 1.2% agarose gel and vacuum blotted onto a positively charged nylon membrane (Hybond-N+ Amersham). Hybridizations contained 5× SSC, 5× Denhardt's, 1% SDS and 100 µg ml−1 salmon sperm DNA and were probed overnight at 60°C with 107 c.p.m. of radiolabelled NdeI-digested pSFM21. Probes were radiolabelled with [α-32P]-dATP using random hexamer primers (Feinberg and Vogelstein, 1983). Blots were washed once in 0.2× SSC−0.1% SDS at room temperature and 65°C for 5 min and once in 0.2× SSC−0.1% SDS at 65°C for 20 min.
Mapping of the cleavage site
Target DNA was amplified from pSFM21 using universal −20 and reverse primers. Each primer was radiolabelled on the 5′ end using T4 polynucleotide kinase. Each labelled primer was used in separate PCRs with a non-isotopically labelled primer partner. The reactions were cycled 20 times at 95°C for 30 s, 45°C for 30 s and 72°C for 30 s. The product was gel purified on an 8% native polyacrylamide gel and run over an Elutip D column (Schleicher and Schuell) according to the manufacture's recommendations. Cleavage reactions were performed as above except that only 1 µl of E. coli S-30 (Promega) was used. Reactions were stopped on ice, followed by phenol and CIA extraction and ethanol precipitation. Reaction products were separated by electrophoresis on a 8% polyacrylamide−7 M urea gel next to sequencing reactions of pSFM21 DNA generated from the same 5′ end-labelled primers.
Recognition site mapping
Target plasmids were digested with PvuII, resulting in a fragment of ≈ 0.43 kb containing the target site and a 2.8 kb fragment containing only vector sequence. Cleavage reactions were performed as above except that 1 µl of rabbit reticulocyte lysate was used. Reaction products were separated by gel electrophoresis on a 1.2% agarose gel and transferred to a positively charged nylon membrane. Hybridization and wash conditions were the same as stated above except that the probe used was a random primer-labelled, gel-purified 432 bp PvuII fragment from pSFM21.
Acknowledgements
We would like to thank Archana Belle, David R. Edgell and Markus Landthaler for advice and critical reading of the manuscript. This work was supported by research grant GM37746 from the National Institutes of Health.




