Replication mutations differentially enhance RecA-dependent and RecA-independent recombination between tandem repeats in Bacillus subtilis
Abstract
We have studied DNA recombination between 513 bp tandem direct repeats present in a kanamycin resistance gene inserted in the Bacillus subtilis chromosome. Tandem repeat deletion was not significantly affected by a recA mutation. However, recombination was stimulated by mutations in genes encoding replication proteins, including the primosomal proteins DnaB, DnaD and the DnaG primase, the putative DNA polymerase III subunits PolC, DnaN and DnaX, as well as the DNA polymerase DnaE. Hyper-recombination was found to be dependent on RecA in the dnaE, dnaN and dnaX mutants, whereas the dnaG and dnaD mutants stimulated recombination independently of RecA. Altogether, these data show that both RecA-dependent and RecA-independent mechanisms contribute to recombination between tandem repeats in B. subtilis and that both types of recombination are stimulated by replication mutations.
Introduction
Tandemly repeated sequences, ranging in length from a few nucleotides to several kilobasepairs, are found in the genomes of many organisms. Recombination between these repeats can lead to the gain (amplification) or loss (deletion) of genetic information. Rearrangements of this type are of particular interest, as they are involved in several human genetic diseases (for a review, see Djian, 1998).
In Escherichia coli, recombination between homologous sequences is dependent on the RecA protein, which catalyses homologous pairing and strand exchange between DNA molecules (for a review, see Bianco et al., 1998). However, recombination between 100 to 800 bp long tandem direct repeats occurs via RecA-dependent and RecA-independent mechanisms in E. coli, and both can be stimulated independently in different genetic contexts (Lovett et al., 1993; Bierne et al., 1997a; Morag et al., 1999). Thus, RecA-dependent deletions are stimulated by mutations in the DnaB and UvrD helicases (Bierne et al., 1997b; Saveson and Lovett, 1997), whereas RecA-independent deletions are stimulated in mutants of the Rep helicase and of the α (DnaE) and ε (DnaQ) subunits of the DNA polymerase III (Bierne et al., 1997a; Saveson and Lovett, 1997).
Two main routes of RecA-dependent recombination have been described in wild-type E. coli cells, the RecBCD pathway, acting at double-strand breaks, and the RecF pathway, acting mainly at single-strand gaps. It is now thought that the main physiological role of these recombination pathways is to reinitiate replication of forks that have been inactivated by collapse or the encounter of a road block (for recent reviews, see Cox et al., 2000; Kowalczykowski, 2000; Michel, 2000). Stimulation of tandem repeat deletion in dnaB mutant cells was shown to be RecBCD dependent and RecFOR independent (Saveson and Lovett, 1999). This suggests that the dnaB mutation blocks replication forks, which are then subjected to breakage, either directly or after reversal (see Fig. 1), followed by double-strand break repair. During this process, misalignment of the repeats by RecA could lead to the loss of one of the repeats (Fig. 1). In contrast, stimulation of recombination in the uvrD mutant was RecFOR dependent and required induction of the SOS response (Bierne et al., 1997b). It is assumed that, in this mutant, recombination via a gap repair mechanism is stimulated. As in double-strand break repair, mispairing of the repeats by RecA during the process could lead to deletion of one of the repeats (Fig. 2).
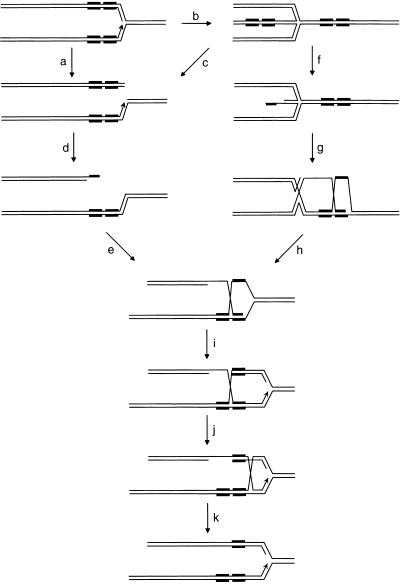
Double-strand break repair model for tandem repeat deletion. DNA molecules are represented as thin lines, and tandem repeats are represented by thick lines (for clarity, tandem repeats are slightly spaced). A blocked replication fork is subject to breakage either directly (a) or after reversal and action of RuvABC (b and c; Michel, 2000) or is simply subject to reversal (b). The free DNA end generated is degraded by the exonuclease action of RecBCD (AddAB in B. subtilis;Chédin et al., 2000) (d and f), and the 3′ end produced invades homologous DNA with the help of RecA (e and g). During this step, mispairing of the repeats by RecA generates the deletion. PriA-dependent primosome assembly on the recombination intermediate allows reinitiation of DNA replication (i) and, after resolution of Holliday junctions by RuvABC (h, j and k), a replication fork carrying a deletion in one arm is produced.
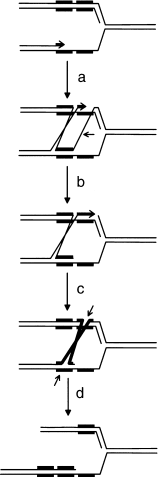
Single-strand gap repair model for tandem repeat deletion. A single-strand gap in the leading strand initiates recombination in the presence of the RecA and RecFOR proteins (a). After nicking by an unknown nuclease (horizontal arrow) and DNA synthesis to fill the gap (b), the Holliday junction is branch migrated (c) and resolved by RuvABC (tilted arrows) to produce a replication fork with a deletion in one arm and a triplication in the other arm (d). A similar model can be drawn with a single-strand gap in the lagging strand.
In contrast, RecA-independent stimulation of tandem repeat recombination in dnaE, dnaQ and rep mutants was proposed to occur by slipped mispairing of the repeats during DNA replication (Lovett et al., 1993; Bierne et al., 1997a; Saveson and Lovett, 1997), a mechanism supported by in vivo and in vitro experiments (d'Alençon et al., 1994; Canceill and Ehrlich, 1996). According to this model, nascent DNA containing a repeat transiently separates from its template during replication and reanneals with the second repeat, thus generating a deletion in the newly synthesized DNA (Fig. 3). Reannealing with the second repeat can occur on the same arm of the fork (replication slippage model) or on the opposite arm (sister chromomatid exchange model; Lovett et al., 1993). Biochemical analysis of plasmid recombination products suggested the existence of both mechanisms (Saveson and Lovett, 1997; Bzymek et al., 1999).
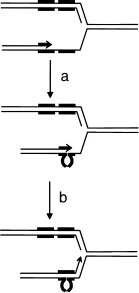
Replication slippage model for tandem repeat deletion. During replication, the nascent DNA strand containing part of one repeat transiently separates from its template and reanneals with the second repeat (a). After reinitiation of replication, a deletion in the neosynthesized strand is generated (b).
In the Gram-positive bacterium Bacillus subtilis, amplification of genes as tandem repeat arrays is used to overproduce proteins of industrial interest (see Young and Ehrlich, 1989 and references therein). Tandem repeat recombination is thus important for both production of the amplified structure and its subsequent stability. However, mechanisms of tandem repeat recombination have not yet been investigated in detail. Nevertheless, it is likely that recombination and replication are strongly linked in B. subtilis, as insertion of an actively replicating plasmid in the vicinity of direct repeats in the B. subtilis chromosome stimulates their recombination (Noirot et al., 1987; Petit et al., 1992; Morel-Deville and Ehrlich, 1996).
Here, we report that recombination between tandem repeats is stimulated by several mutations affecting DNA replication in B. subtilis, including mutations of primosomal proteins (DnaB, DnaD and the DnaG primase), mutations of putative components of the DNA polymerase III holoenzyme (DnaN, DnaX and PolC), as well as a mutation in a DNA polymerase of unknown function (DnaE). Recombination does not require RecA in wild-type cells as well as in the primosome mutants that could be tested (dnaD and dnaG). In contrast, RecA is required in three other mutants (dnaN, dnaX and dnaE). We conclude that both RecA-dependent and RecA-independent recombination between tandem repeats takes place in B. subtilis.
Results
Tandem repeat recombination occurs in the absence of RecA
To measure recombination between tandem repeats in B. subtilis, a kanamycin resistance (KnR) gene carrying an internal 513 bp direct repeat (Kndup513) was introduced in the chromosome near the thyB locus (Fig. 4; for details of construction, see Experimental procedures). The repeats inactivate the KnR gene, and cells are kanamycin sensitive (KnS). Deletion of one of the repeats restores an intact gene and thus confers a KnR phenotype to the cells (Fig. 4). The recombination frequency was therefore estimated by measuring the proportion of KnR cells in several independent growing cultures (see Experimental procedures).

Experimental system and rationale of the deletion assay. The B. subtilis chromosome in the thyB region is shown in wavy lines. White boxes represent pBR322 sequences, and host genes flanking the insertion site of pBR322 sequences are indicated. Arrows stand for KnR and SpcR genes, and the 513-bp-long tandem repeats in the KnR gene are represented by grey arrows. Deletion of one of the repeats gives rise to a functional KnR gene.
Recombinant proportions were very similar in two wild-type strains and were not affected significantly by the presence of a recA mutation (Table 1). We conclude that tandem repeat recombination can occur independently of RecA in B. subtilis. It should be noted that some recA mutants had low plating efficiency on rich medium (not shown). We therefore carried out all experiments with recA strains on minimal medium. The wild-type cells had a slightly higher recombination frequency (≈ threefold) on this medium than on rich medium, for unknown reasons.
| Straina | Relevant genotype | Recombinant proportion (× 104)b | Relative valuec | n |
|---|---|---|---|---|
| A. | ||||
| CBB350 | rec+ | 5.7 (± 2.5) | = 1 | 5 |
| CBB500 | recA::tet | 3.0 (± 2.2) | 0.5 | 5 |
| B. | ||||
| CBB383 | rec+ | 3.9 (± 1.6) | = 1 | 5 |
| CBB506 | recA::tet | 1.9 (± 0.5) | 0.5 | 5 |
- a. Subsections A and B correspond to different genetic backgrounds.
- b. Recombinant proportions were measured as described in Experimental procedures in minimal medium at 37°C. Average and standard deviation of n independent determinations are shown.
- c . Recombinant proportion is expressed relative to that determined for the isogenic wild-type strain, taken as 1.
Effect of replication mutations on tandem repeat recombination
To test the effects of replication defects on recombination between tandem repeats in B. subtilis, the Kndup513 structure was introduced in different B. subtilis mutants affected in various replication functions. The mutations tested can be classified into two distinct families. The first comprises mutations of the so-called ‘primosomal proteins’, namely the replication fork helicase (DnaC), the primase (DnaG) and the proteins involved in their loading onto DNA, i.e. PriA, DnaB, DnaD and DnaI (Wang et al., 1985; Ogasawara et al., 1986a; Bruand et al., 1995a, b; Bruand and Ehrlich, 1995; Sakamoto et al., 1995; P. Polard, personal communication). The second family groups mutations of DNA polymerases. The polA gene encodes the DNA polymerase I, involved in DNA repair and plasmid DNA replication (Gass et al., 1971; Bruand et al., 1993). The polC, dnaN and dnaX genes encode various putative subunits of the DNA polymerase III, the major replicase of the cell (Ogasawara et al., 1986b; Struck et al., 1990; Sanjanwala and Ganesan, 1991). The function of the dnaE gene product is currently unknown (Bruck and O'Donnell, 2000).
All mutations, except priA1 and polA5, are thermosensitive alleles of essential genes. Therefore, the recombinant proportion in these mutants was measured at both the permissive temperature (30°C) and the semi-permissive temperatures (37°C and, when possible, 42°C). priA1 and polA5 cells, which are not thermosensitive, were tested at 37°C only. All measurements were done in rich medium (LB), except for the priA1 mutant, which is sensitive to rich medium (P. Polard, personal communication) and was thus tested in minimal medium.
Recombinant proportions in mutants of primosomal proteins are shown in Table 2. No significant difference was detected between the wild type and the priA1, dnaC14 and dnaI2 strains. In contrast, the dnaG20 mutant showed a 32-fold higher level of recombination relative to the wild-type strain at 37°C, and the dnaB19 and dnaD23 mutants showed a seven- and 117-fold increase, respectively, relative to the wild-type strain at 42°C.
| Straina | Relevantgenotype | Temperature(°C) | Tandem repeat deletionb | ||
|---|---|---|---|---|---|
| n | Recombinant proportion (× 104) | Relative value | |||
| A. | |||||
| CBB350 | wt | 30 | 7 | 1.8 (± 0.4) | = 1 |
| 37 | 6 | 2.2 (± 0.3) | = 1 | ||
| 42 | 6 | 4.0 (± 1.4) | = 1 | ||
| CBB353 | dnaB19 | 30 | 5 | 2.0 (± 0.7) | 1.1 |
| 37 | 7 | 3.5 (± 1.7) | 1.6 | ||
| 42 | 5 | 29 (± 22) | 7.4 | ||
| CBB356 | dnaC14 | 30 | 5 | 2.7 (± 0.7) | 1.5 |
| 37 | 5 | 2.2 (± 0.6) | 1 | ||
| 42 | 7 | 5.8 (± 1.9) | 1.5 | ||
| CBB360 | dnaD23 | 30 | 5 | 1.9 (± 0.6) | 1.1 |
| 37 | 7 | 6.6 (± 4.9) | 3 | ||
| 42 | 8 | 460 (± 360) | 116.6 | ||
| CBB362 | dnaG20 | 30 | 6 | 7.4 (± 1.6) | 4.1 |
| 37 | 6 | 70.5 (± 58) | 31.6 | ||
| CBB374 | dnaI2 | 30 | 6 | 3.8 (± 2.1) | 2.1 |
| 37 | 5 | 4.3 (± 1.3) | 1.9 | ||
| 42 | 7 | 7.5 (± 2.2) | 1.9 | ||
| B. | |||||
| CBB383 | wt | 37 | 5 | 3.9 (± 1.6) | = 1 |
| CBB607 | priA1 | 37 | 5 | 4.8 (± 1.9) | 1.2 |
- a. Subsections A and B correspond to different genetic backgrounds.
- b. As in Table 1, except that experiments were performed at the indicated temperature and in LB (section A) or minimal medium (section B) supplemented with Spc (or Em for the priA mutant).
Table 3 presents the results for DNA polymerase mutants. Whereas the polA5 mutation did not affect the recombinant proportion, all other mutations affecting polymerases stimulated recombination to different extents. The dnaE1, polC133 and dnaX51 mutations stimulated recombination 28-, 18- and 21-fold, relative to the wild-type strain at 37°C respectively. The dnaN5 and dnaX8132 mutants showed 10- and 13-fold stimulations at 42°C respectively.
| Straina | Relevantgenotype | Temperature(°C) | Tandem repeat deletionb | ||
|---|---|---|---|---|---|
| n | Recombinant proportion (× 104) | Relative value | |||
| A. | |||||
| CBB380 | wt | 37 | 5 | 4.6 (± 1.8) | = 1 |
| CBB377 | polA5 | 37 | 5 | 2.1 (± 0.4) | 0.5 |
| B. | |||||
| CBB350 | wt | 30 | 7 | 1.8 (± 0.4) | = 1 |
| 37 | 6 | 2.2 (± 0.3) | = 1 | ||
| 42 | 6 | 4.0 (± 1.4) | = 1 | ||
| CBB368 | dnaN5 | 30 | 6 | 3.1 (± 2.7) | 1.7 |
| 37 | 6 | 7.1 (± 6.9) | 3.2 | ||
| 42 | 7 | 38 (± 20) | 9.6 | ||
| CBB371 | dnaX51 | 30 | 7 | 4.7 (± 4.4) | 2.6 |
| 37 | 5 | 48 (± 27) | 21.4 | ||
| C. | |||||
| CBB383 | wt | 30 | 5 | 2.4 (± 1) | = 1 |
| 37 | 5 | 2.5 (± 0.7) | = 1 | ||
| CBB494 | dnaE1 | 30 | 5 | 15 (± 7.5) | 6.4 |
| 37 | 5 | 71 (± 70) | 28.1 | ||
| D. | |||||
| CBB571 | wt | 30 | 7 | 2.4 (± 0.9) | = 1 |
| 37 | 6 | 4.6 (± 3.9) | = 1 | ||
| 42 | 6 | 4.8 (± 1.4) | = 1 | ||
| CBB569 | dnaX8132 | 30 | 6 | 10.5 (± 4) | 4.4 |
| 37 | 6 | 9.9 (± 7.1) | 2.2 | ||
| 42 | 6 | 62 (± 35) | 13 | ||
| E. | |||||
| CBB577 | wt | 30 | 4 | 2.1 (± 0.4) | = 1 |
| 37 | 6 | 4.2 (± 1.9) | = 1 | ||
| CBB575 | polC133 | 30 | 4 | 6.2 (± 4.1) | 3 |
| 37 | 7 | 75 (± 48) | 17.9 | ||
- a. Subsections A–E correspond to different genetic backgrounds.
- b. As in Table 2.
Altogether, these data show that alterations in DNA replication can result in high rates of recombination between tandem repeats in B. subtilis.
Replication mutants stimulate tandem repeat recombination by at least two different mechanisms
In wild-type cells, recombination between tandem repeats is not significantly affected by a recA mutation (see above). We tested whether stimulation of recombination in replication mutants was RecA dependent or independent. A recA null mutation was transferred into some of the replication mutants that showed an increased deletion frequency. The dnaB19 recA and polC133 recA double mutants, constructed at 30°C, turned out not to be viable at the temperatures at which hyper-recombination had been observed with the single mutants (i.e. 42°C and 37°C respectively; data not shown), and could therefore not be tested.
Inactivation of RecA in the dnaE1 and dnaX51 (at 37°C) and dnaN5 (at 42°C) mutants decreased the recombination frequency to the levels of Dna+ and Dna+recA cells (Table 4). This shows that stimulation of recombination in the dnaE1, dnaX51 and dnaN5 mutants is dependent on RecA. In contrast, the recombination level of the dnaG20 recA (at 37°C) double mutant was similar to that of the dnaG20 single mutant, suggesting that stimulation of recombination in dnaG20 cells is independent of RecA (Table 4).
| Straina | Relevantgenotype | Temperature(°C) | Tandem repeat deletionb | ||
|---|---|---|---|---|---|
| n | Recombinant proportion (× 104) | Relative value | |||
| A. | |||||
| CBB350 | wt | 37 | 5 | 5.7 (± 2.5) | = 1 |
| 42 | 6 | 3.2 (± 1.5) | = 1 | ||
| CBB500 | recA::tet | 37 | 5 | 3.0 (± 2.2) | 0.5 |
| 42 | 7 | 1.1 (± 0.5) | 0.3 | ||
| CBB360 | dnaD23 | 42 | 7 | 28 (± 20) | 8.8 |
| CBB612 | dnaD23 recA::tet | 42 | 7 | 19 (± 16) | 5.9 |
| CBB362 | dnaG20 | 37 | 5 | 46 (± 17) | 8.1 |
| CBB536 | dnaG20 recA::tet | 37 | 5 | 86 (± 79) | 15.1 |
| CBB368 | dnaN5 | 42 | 5 | 42 (± 14) | 13.1 |
| CBB595 | dnaN5 recA::tet | 42 | 5 | 3.3 (± 1.5) | 1.0 |
| CBB371 | dnaX51 | 37 | 5 | 40 (± 12) | 7.0 |
| CBB532 | dnaX51 recA::tet | 37 | 7 | 3.3 (± 0.6) | 0.6 |
| B. | |||||
| CBB383 | wt | 37 | 5 | 3.9 (± 1.6) | 1 |
| CBB506 | recA::tet | 37 | 5 | 1.9 (± 0.5) | 0.5 |
| CBB494 | dnaE1 | 37 | 5 | 29 (± 15) | 7.3 |
| CBB509 | dnaE1 recA::tet | 37 | 6 | 2.9 (± 2.1) | 0.7 |
- a. Subsections A and B correspond to different genetic backgrounds.
- b. As in Table 2, except that experiments were performed in minimal medium.
Interestingly, recombination in the dnaD23 mutant was much lower (≈15-fold) in minimal medium (used for comparison with the dnaD23 recA mutant) than in rich medium (compare Tables 2 and 4). It is possible that the effect of the dnaD23 mutation is more severe in rich than in minimal medium because of the higher number of replication forks. Indeed, the dnaD23 mutant showed a slight plating deficiency (≈ 2.5 fold) on rich medium at 42°C, whereas all other single mutants and the wild-type strain plated with the same efficiency on the two media (differences ranged between 80% and 140%). Importantly, recombination levels in the dnaD23 and dnaD23 recA cells (tested in minimal medium) were similar, indicating that the stimulation of recombination is independent of RecA.
Altogether, these data indicate that the hyper-recombination phenotype of replication mutants can be either RecA dependent or RecA independent, suggesting the existence of at least two different mechanisms of recombination.
Discussion
In this work, we studied recombination between 513 bp tandem direct repeats in the B. subtilis chromosome. Wild-type cells showed a recombinant proportion of around 2 × 10−4, and a recA mutation did not significantly affect this value (Table 1). Recombination was stimulated by mutations in various replication proteins, including the primosomal proteins DnaB, DnaD and the DnaG primase (Table 2), the DNA polymerase III subunits PolC, DnaX and DnaN and the DNA polymerase DnaE (Table 3). No effect was observed in the priA1, polA5, dnaC14 and dnaI2 mutants. RecA dependence of recombination stimulation was examined in some of the mutants that showed a hyper-recombination phenotype. Mutations in the dnaG and dnaD genes stimulated recombination independently of RecA, whereas mutations in the dnaE, dnaX and dnaN genes stimulated recombination in a RecA-dependent manner (Table 4). The effect of recA on stimulation by dnaB and polC mutations could not be determined, as the double mutants were not viable under conditions in which hyper-recombination was observed. These results show that both RecA-dependent and RecA-independent mechanisms can contribute to recombination between tandem repeats in B. subtilis and that each is stimulated by alterations in the DNA replication process.
The B. subtilis dnaX51 and dnaN5 mutations stimulate recombination, which contrasts with data obtained previously in E. coli, in which mutations in the γ–τ- and β-subunits (encoded by the dnaX and dnaN genes respectively) decrease tandem repeat recombination (Saveson and Lovett, 1997). These differences might result from the fact that the mutations tested affect different activities of the proteins in E. coli and B. subtilis. Alternatively, the proteins assumed to be equivalent could play different functions in the two hosts. Thus, dnaX encodes only the τ-subunit in Gram-positive bacteria, whereas it also encodes the γ-subunit in E. coli by translational frameshifting (Bruck and O'Donnell, 2000). Mutations in the α- and ε-subunits of the E. coli DNA polymerase III, encoded by the dnaE and dnaQ genes, respectively, stimulate tandem repeat deletion independently of RecA (Bierne et al., 1997a; Saveson and Lovett, 1997). It is thought that the B. subtilis counterparts of the E. coliα- and ε-subunits are encoded by the same polypeptide, the product of the polC gene (Sanjanwala and Ganesan, 1991). We found that the polC33 mutation stimulated recombination, but could not test the RecA dependence of hyper-recombination in this mutant. The dnaE gene encodes a protein highly similar to the E. coliα-subunit, but its role in DNA replication is currently unknown (Bruck and O'Donnell, 2000). However, it should be noted that the B. subtilis dnaE mutation, in contrast to E. coli dnaE mutations, stimulated recombination in a RecA-dependent manner.
The RecA-dependent mechanisms of stimulation of recombination in the B. subtilis polymerase mutants are not known. Nevertheless, like E. coli, B. subtilis possesses several pathways of RecA-dependent recombination (for a recent review, see Fernández et al., 2000). The counterpart of the RecBCD enzyme of E. coli has been shown, in vivo and in vitro, to be encoded by the B. subtilis addAB operon (Chédin et al., 2000 and references therein), whereas the counterpart of the RecFOR system of E. coli would be encoded by the recFLOR genes (Fernández et al., 1999). Therefore, the models proposed previously to explain RecA-dependent homologous recombination between tandem repeats in E. coli (see Introduction) could apply to B. subtilis as well: mutations in the DNA polymerase subunits could possibly block replication forks, leading to breakage and AddAB-dependent double-strand break repair, or leave small gaps of unreplicated DNA that are repaired by a RecFLOR-dependent mechanism. During these processes, mispairing of the repeats by RecA leads to the deletion of one repeat.
In addition to its roles in recombination, RecA is involved in the induction of the SOS response in B. subtilis as in E. coli (for reviews, see Yasbin et al., 1993; Walker, 1996). Accumulation of single-stranded DNA as a possible consequence of some of the replication defects might induce, via RecA, the SOS response. In E. coli as in B. subtilis, some genes involved in RecA-dependent recombination, in particular RecA itself, are induced during the SOS response, and this probably contributes to stimulate the recombination processes described above. Additionally, several error-prone polymerases involved in DNA repair are activated during the SOS response in E. coli (for reviews, see Goodman, 2000; Goodman and Tippin, 2000). These polymerases could be involved in recombination through slipped mispairing of repeated sequences during DNA synthesis. If such polymerases are present in B. subtilis, they might be responsible for some recombination events. Therefore, further genetic studies are needed to elucidate the different role(s) played by RecA in the process of stimulation of recombination in replication mutants.
In contrast to polymerase mutants, the dnaG20 and dnaD23 mutations stimulated recombination in a RecA-independent manner. In this case, slipped-mispairing during replication is likely to be the appropriate model, because it need not involve RecA at any stage (see Introduction). An alternative is the single-strand annealing model, in which DNA is subject to breakage in one of the repeats. The free DNA ends could then be degraded by exonucleases and generate single-stranded regions containing the repeats. Erroneous annealing of complementary repeats in the absence of any recombination function generates the deletion of one of the repeats. Both these models were proposed previously in bacteria to account for RecA-independent recombination between short homologous sequences (for a review, see Michel, 1999).
The dnaG20 mutation lies in the polymerase domain of the primase (Price and Doi, 1985; Wang et al., 1985; Keck et al., 2000) and therefore probably affects the efficiency of primer synthesis. In an in vitro reconstituted E. coli replication system, decreasing the efficiency of primer synthesis resulted in longer Okasaki fragments, which reflect an increase in the distance between consecutive primers on the lagging strand (Wu et al., 1992; Zechner et al., 1992). If the dnaG20 mutation has the same effect in B. subtilis in vivo, it might result in large single-stranded regions on the lagging strand template, thus increasing the probability that both repeats are exposed in a single-stranded form at the same time, a prerequisite for slipped-mispairing. An alternative possibility is that these large regions of single-stranded DNA are susceptible to cleavage by cellular endonucleases, thus favouring the single-strand annealing model. In E. coli, the primase mutant dnaG3903 did not show any stimulation of tandem repeat recombination (Saveson and Lovett, 1999). However, as suggested by the authors, this could result from the fact that this mutant is not affected in primer synthesis but is rather a partition mutant, whereas the B. subtilis dnaG20 mutant tested here was isolated as a replication mutation (Karamata and Gross, 1970).
The RecA-independent stimulation of tandem repeat deletion in the dnaD23 mutant is more difficult to interpret. In B. subtilis, DnaD is involved, together with DnaB and DnaI, in the loading of the DnaC helicase onto DNA, either in a DnaA-dependent manner during initiation of replication at oriC (for a review, see Moriya et al., 1999) or in a PriA-dependent manner during reinitiation of inactivated replication forks (Bruand et al., 1995b; P. Polard and C. Bruand, unpublished). The initiation defect at oriC is unlikely to be responsible for the observed hyper-recombination phenotype. However, the priA1 mutation did not stimulate recombination, which shows that the defect in reinitiation of inactivated forks is not primarily responsible for hyper-recombination. What could be the reason for the stimulation of recombination in the dnaD23 and dnaB19 mutants, but not in the priA1 mutant? The DnaB and DnaD proteins have single-stranded DNA-binding activities (S. Marsin, S. McGovern, C. Bruand and P. Polard, unpublished) and could thus protect single-stranded DNA against nucleases or impair DNA polymerase slippage and, consequently, prevent recombination. Another possibility is that, in the absence of DnaD or DnaB, PriA binding to inactivated forks does not lead to primosome assembly. Instead, in these conditions, if the helicase activity of PriA is activated, the newly synthesized lagging strand may be unwound. Such an activity has been observed in vitro on forked DNA substrates with the E. coli PriA protein in the absence of one or more other primosomal proteins (McGlynn et al., 1997; Jones and Nakai, 1999). This unwinding would generate large regions of single-stranded DNA on the lagging-strand template, a situation similar to that described above in the dnaG20 mutant. Although the RecA dependence of recombination in the dnaB19 mutant could not be tested, we predict that hyper-recombination in this mutant would be as in dnaD23, i.e. RecA independent. Interestingly, the priA mutation in E. coli stimulated tandem repeat recombination 10-fold (Saveson and Lovett, 1997). It has been shown that the loading of the replication fork helicase in E. coli and B. subtilis occurs through different mechanisms (Bruand et al., 1995b; Sandler, 2000; C. Bruand, unpublished). This could be the source of the different recombination phenotypes of E. coli and B. subtilis priA mutants.
No stimulation of recombination was observed in the dnaC14 mutant, which encodes an elongation-deficient replication fork helicase (Gross et al., 1968; Sakamoto et al., 1995). This is rather surprising as, in E. coli, the dnaB107 mutant showed a stimulation of tandem repeat deletion. However, another elongation-deficient allele of E. coli dnaB did not (Saveson and Lovett, 1999). Such an allele specificity could apply to the dnaC mutants of B. subtilis as well, and dnaC14 would not be a hyper-recombinogenic allele.
Experimental procedures
Bacterial strains
The strains used in this work are listed in Table 5. The Kndup513 structure was transferred in the different strains by transformation with chromosomal DNA from strain CBB345 to spectinomycin resistance. Correct insertion of the structure was verified by Southern blot analysis. The recA::tet mutation was transferred by transformation to tetracycline resistance with chromosomal DNA from strain HVS567. The presence of the recA mutation in the resulting strains was verified by their UV sensitivity and the presence of a disrupted copy of recA by Southern blot analysis. During this study, we observed that the DNA fragment carrying the TetR gene inserted in recA from strain HVS567 was 3 kb long, as expected (E. Dervyn, personal communication), and not 1.7 kb long, as published by mistake (Chédin et al., 1994). All strains were grown in LBT medium or minimal medium (Spizizen's minimal salts; Bron, 1990; supplemented with 0.1% d-glucose, 0.01% l-tryptophan, 0.1% casamino acids, 18 mg l−1 ammonium-ironIII citrate (iron ≈ 17%; Merck). Media were supplemented when required with spectinomycin (Spc, 60 µg ml−1), kanamycin (Kn, 5 µg ml−1), tetracycline (Tet, 7.5 µg ml−1) or erythromycin (Em, 0.6 µg ml−1).
| Strain | Relevant genotype | Reference or source |
|---|---|---|
| B. subtilis strains | ||
| 168 | trpC2 | C. Anagnostopoulos |
| PPBJ120 | trpC2 priA1::pAPJ11 (EmR) | P. Polard |
| EDJ72 | trpC2 dnaE1 | E. Dervyn |
| SB202 | trpC2 tyrA1 aroB2 hisH2 | This laboratory |
| L1430 | ilvA metC lys21 | Mauël and Karamata (1984) |
| L1432 | metC ilvA dnaB19 | Mauël and Karamata (1984) |
| L1433 | metC lys21 dnaC14 | Mauël and Karamata (1984) |
| L1434 | metC lys21 dnaD23 | Mauël and Karamata (1984) |
| L1435 | metC ilvA dnaG20 | Mauël and Karamata (1984) |
| L1437 | metC lys21 dnaN5 | Mauël and Karamata (1984) |
| L1438 | metC ilvA dnaX51 | Mauël and Karamata (1984) |
| L1439 | metC ilvA dnaI2 | Mauël and Karamata (1984) |
| 1A217 | trpC2 thr5 polC133 | Bacillus Genetic Stock Center |
| 1A74 | hisA1 thr5 dnaX8132 | Bacillus Genetic Stock Center |
| 1A224 | trpC2 hisH2 pheA1 | Bacillus Genetic Stock Center |
| 1A226 | trpC2 hisH2 pheA1 polA5 | Bacillus Genetic Stock Center |
| HVS567 | leuA8 metB5 recA::tet | Chédin et al. (1994) |
| CBB345 | trpC2 tyrA1 aroB2 hisH2 thyA ΔilvA Kndup513 | This work |
| CBB350 | metC lys21 ΔilvA Kndup513 | This work |
| CBB353 | metC ΔilvA dnaB19 Kndup513 | This work |
| CBB356 | metC lys21 ΔilvA dnaC14 Kndup513 | This work |
| CBB360 | metC lys21 ΔilvA dnaD23 Kndup513 | This work |
| CBB362 | metC ΔilvA dnaG20 Kndup513 | This work |
| CBB368 | metC lys21 ΔilvA dnaN5 Kndup513 | This work |
| CBB371 | metC ΔilvA dnaX51 Kndup513 | This work |
| CBB374 | metC ΔilvA dnaI2 Kndup513 | This work |
| CBB377 | trpC2 hisH2 pheA1 ΔilvA Kndup513 | This work |
| CBB380 | trpC2 hisH2 pheA1 ΔilvA polA5 Kndup513 | This work |
| CBB383 | trpC2 ΔilvA Kndup513 | This work |
| CBB494 | trpC2 ΔilvA dnaE1 Kndup513 | This work |
| CBB569 | hisA1 thr5 ΔilvA dnaX8132 Kndup513 | This work |
| CBB571 | hisA1 thr5 ΔilvA Kndup513 | This work |
| CBB573 | metC ΔilvA dnaB19 recA::tet Kndup513 | This work |
| CBB575 | trpC2 thr5 ΔilvA polC133 Kndup513 | This work |
| CBB577 | trpC2 thr5 ΔilvA Kndup513 | This work |
| CBB500 | lys21 metC ΔilvA recA::tet Kndup513 | This work |
| CBB506 | trpC2 ΔilvA recA::tet Kndup513 | This work |
| CBB509 | trpC2 ΔilvA dnaE1 recA::tet Kndup513 | This work |
| CBB532 | metC ΔilvA dnaX51 recA::tet Kndup513 | This work |
| CBB536 | metC ΔilvA dnaG20 recA::tet Kndup513 | This work |
| CBB595 | metC lys21 ΔilvA dnaN5 recA::tet Kndup513 | This work |
| CBB601 | trpC2 thr5 ΔilvA polC133 recA::tet Kndup513 | This work |
| CBB607 | trpC2 ΔilvA priA1::pAPJ11 Kndup513 | This work |
| CBB612 | metC lys21 ΔilvA dnaD23 recA::tet Kndup513 | This work |
To determine the semi-permissive temperatures of the thermosensitive mutants used in recombination experiments, the different strains were grown at the permissive temperature (30°C) and plated at 30°C, 37°C, 46°C and 51°C. The highest temperature giving a plating efficiency identical to that determined at 30°C was the highest temperature used in recombination experiments. It should be noted, however, that some strains had a slow growth rate at this temperature. Thus, the OD600 doubling times were calculated from growth curves established by growing strains in microplates at the indicated temperature in either LB or minimal medium supplemented with Spc (or Em for the priA mutant) and monitoring OD600 using a Bioscreen apparatus. Doubling times were 56–72 min at 30°C, 32–49 min at 37°C, except the priA1 and polC133 mutants (164 and 141 min at 37°C respectively) and 27–39 min at 42°C, except the dnaB19, dnaD23, and dnaG20 mutants (≥ 160, 156 and 72 min at 42°C respectively). This slow growth reflected the reduced viability of the strains at these temperatures. Indeed, the plating efficiencies at 30°C of the strains grown at semi-permissive temperatures were between 1.1 and 3.7 cfu × 108/OD650, except for the dnaB19, dnaC14, dnaD23, dnaG20, dnaI2 and polC133 mutants (0.2–0.9 cfu × 108/OD650). The plating efficiency at 37°C was particularly low for the priA1 mutant (0.09 cfu × 108/OD650).
Construction of Kndup513
Strain CBB345, carrying the Kndup513 structure, was constructed as follows. First, pK1, a KnR SpcR derivative of pBR322, was constructed. For this purpose, the SpcR gene from pIC156 (Steinmetz and Richter, 1994) was first cloned as an XbaI–SspI (blunted) fragment into the BamHI (blunted) site of pBR322, giving plasmid pBRSpc. Afterwards, a DNA fragment carrying a KnR gene was amplified by polymerase chain reaction (PCR) from plasmid pUB110 using oligonucleotides (1) and (2) as primers. This fragment was then digested by BamHI and HindIII and inserted in plasmid pBRSpc cut with BamHI and HindIII, giving plasmid pBRKnSpc. Then, the KnR gene of pBRKnSpc was placed under the control of the PenP promoter. For this, a fragment carrying the PenP promoter was amplified from plasmid pDG148 (Stragier et al., 1988) using oligonucleotides (3) and (4) as primers. This PCR fragment was digested by EcoRI and HindIII and inserted into the EcoRI–HindIII-digested plasmid pBRKnSpc, giving plasmid pK1. The plasmid pM1 was then constructed, which is analogous to pK1 but contains a BamHI site at position 324 of the KnR gene (co-ordinates are according to GenBank accession pb0kan.gb_ba), which was introduced by oligomutagenesis using oligonucleotide (5). Finally, the 721 bp BamHI fragment of pM1 carrying the 3′ end of the KnR gene was cloned into pK1 cut with BglII–BamHI, giving the plasmid pK1M1, which contains Kndup513.
To insert Kndup513 into the B. subtilis chromosome, a recipient strain containing an integrated copy of pBR322 was first constructed as follows. The PstI (blunted) fragment of pHV438 (Niaudet et al., 1982), which contains two DNA fragments from the chromosomal thyB region flanking the ilvA gene, was cloned in the PvuII site of pBR322 to give the plasmid pBRthy. The CmR gene of pC194 was then inserted into the BamHI site of pBRthy giving the plasmid pBRthyCm. Finally, pBRthyCm was linearized at the BglII site present in its chromosomal moiety and used to transform the B. subtilis strain SB202 to CmR. The CmR transformants were tested for isoleucine auxotrophy to prove that the plasmid was integrated in the chromosome by double crossing over. Finally, the resulting strain was transformed by pK1M1 linearized at its PvuII site with selection for SpcR. The SpcR transformants were tested for sensitivity to chloramphenicol, which indicates that Kndup513 was integrated in the chromosome by double crossing over between pBR322 sequences. The resulting strain was designated CBB345.
Oligonucleotides: (1) 5′-CATAAGCTTCGGGCCAGTTTG-3′; (2) 5′-CGGGATCCGCGCCATGACAG-3′ (3): 5′-GGAAT TCCGGTGGAAACG-3′; (4): 5′-ATCAAAGCTTACAAATGT AGTC-3′ (5): 5′-GGGGATCCTGTTAAGGC-3′. Underlined sequences correspond to relevant restriction sites described in the text.
Deletion assay
Strains to be tested were grown on plates containing Spc (LB or minimal medium, as indicated in the text and table legends). In the case of the priA mutant, plates of minimal medium containing Em were used. Plates were then incubated for 16–72 h at the test temperature (i.e. 30°C, 37°C or 42°C, as indicated). Whole isolated colonies were inoculated in 5 ml of liquid medium (either LB or minimal medium) supplemented with Spc (Em for the priA mutant) and cultured at the same temperature up to OD650≈ 0.6 (average value). The cultures were diluted in LB or minimal medium, and appropriate dilutions were plated in duplicate on LB or minimal medium supplemented with Spc or Spc + Kn (Em or Em + Kn for the priA mutant). Plates were then incubated at 30°C for 16–72 h. The recombinant proportion is the ratio of the numbers of SpcR KnR cells over SpcR cells (EmR KnR cells over EmR cells for the priA mutant).
To ensure that we do not underestimate recombinant proportions in this test, we isolated a recombinant derivative (KnR) of each strain tested in this study and verified that these strains plated efficiently (i.e. ≈ 100%) on plates containing Spc + Kn relative to plates containing only Spc (Em + Kn relative to Em for the priA mutant).
Acknowledgements
We thank E. Dervyn for providing the dnaE1 mutant, A. Schmautz for technical help, M.-A. Petit, B. Michel, P. Polard and S. Marsin for stimulating discussions and/or critical reading of the manuscript, and P. Bellenand for help with statistical analyses of some results. This work was supported, in part, by a grant from the European Commission (BIO4-CT98-0250).




