Inferring population history from fine-scale spatial genetic analysis in Oryza rufipogon (Poaceae)
Abstract
Determining the genetic structure of an in situ conserved population can provide insight into the dynamics of population genetic processes associated with successful plant conservation. We used 21 microsatellite loci to analyse the genetic relationships among individuals (n= 813) collected from a small Oryza rufipogon population conserved since 1993 in Hunan Province of China. The analysis revealed four distinct genetic subpopulations (FST= 0.145) without geographic isolation. One subpopulation was composed of possible introgressed individuals, two subpopulations were composed of seed recruits and their descendants, and the fourth subpopulation consisted of reintroduced individuals, seed recruits and their descendants. Positive spatial genetic structures were detected by spatial autocorrelation statistics at the population (c. 63 m) and subpopulation levels (11–30 m), but the degree of autocorrelation was stronger at the population level. These results showed that prejudging the cryptic structure is important before autocorrelation analysis for the entire population. Our study suggests that population history can be a significant determinant on population structure for plant restoration projects.
Introduction
It is commonly thought that successful restoration of plant populations requires considerable initial genetic variability (Khurana & Singh 2001; Procaccini & Piazzi 2001; Proffitt et al. 2003). However, there is little data on the subsequent demographic and micro-evolutionary events that function to maintain biodiversity. Determining the population genetic structure of a successfully reintroduced plant can provide insight into the dynamics of population genetic processes associated with successful plant restoration (Escudero et al. 2003). Due to the availability of highly informative genetic markers combined with new statistical procedures, current gene flow and historical events, such as seed recruitment, rapid population expansion, and plant introgression, can be distinguished by analysing patterns of genetic diversity in a population (e.g. Kalisz et al. 2001; Dutech et al. 2002). Likewise, through analysis of spatial population genetic structure, the effects of dynamic natural processes can be separated from that of recent human-induced ecosystem modifications (Kalisz et al. 2001; Dutech et al. 2002). Despite their importance, little is known about the role of historical events in forming patterns of genetic diversity of a population (Knowles et al. 1992; Kalisz et al. 2001).
In situ conservation has been implemented for agriculturally important but endangered plant species (e.g. wild rice in China, see review by Song et al. 2005). Conserved wild relatives experience a wide range of conditions that may impact population genetic diversity and structure over time such as: fluctuations in plant population abundance, bottlenecks associated with the reintroduction, introgression of related plants, and rapid population expansion. In situ plant populations are also often carefully monitored, providing an ideal opportunity to infer the influence of population history on genetic structure. In order to determine population genetic structure and determine the history of events, a comprehensive sample collected at a really fine scale should be analysed by using a relatively large set of markers.
Oryza rufipogon Griff. (Poaceae) is a perennial wild rice widely distributed in the tropics and subtropics of monsoon Asia (Oka 1988). China is the northernmost extent of O. rufipogon, and within China, wild rice is found in its eight southern provinces and autonomous regions (Song et al. 2005). This species is thought to be the progenitor of the cultivated rice species, Oryza sativa (Oka 1988). However, in the past few decades, it has become threatened in China by changes in farming systems, urbanization, and other human disturbances (Gao 2004). Conservation measures for this species have been initiated in China since the 1990s. For instance, one northernmost population in Chaling county of Hunan Province was restored in 1993 and protected by in situ conservation, after the standing population temporarily disappeared (Zhou 1995). This population has rapidly expanded since then, and has become a successful model for the conservation of this species (Liu et al. 2004; Song et al. 2005). Additionally, natural gene introgression from cultivated O. sativa to this O. rufipogon population was assumed, due to their high compatibility and sympatric growing (Oka 1988; Song et al. 2002). This process has been found to influence population genetic variability and differentiation of O. rufipogon (Song et al. 2003; Gao 2004). Although practices for managing in situ and ex situ conservations of wild rice populations have been refined in the recent years and a great deal of information on genetic variation is known (Gao et al. 2000; Song et al. 2003, 2005; Zhou et al. 2003; Gao 2004), little is known about fine-scale spatial population genetic structure for this species, and the genetics associated with a successful restoration of a plant population.
Here, we study the fine-scale genetic structure of an in situ conserved common wild rice, O. rufipogon population using 21 microsatellite loci to determine the fine-scale population genetic structure of O. rufipogon in Chaling, Hunan Province, China. This study was conducted to answer the following questions: (i) Does population history lead to population differentiation? (ii) Does this population show spatial genetic structure? and (iii) If there is spatial genetic structure, what factors appear to contribute to this? Such information may provide a more solid basis for understanding population dynamics and for appropriate conservation measures for this species.
Materials and methods
Study site
Huli Marsh is a 33.4-ha shallow basin surrounded by low hills about 20–70 m high, in Chaling county in Hunan Province of China (26°51′26″N, 113°41′45″E). The marsh is 153 m above sea level (a.s.l.). The water in the marsh comes from surface runoff from the surrounding hills (Liu et al. 2004). The water depth varies between 0.1 and 0.6 m throughout the marsh. A channel, 5 m wide and 1.5 m deep, flows from east to west through the marsh (Fig. 1a). The water in north of the channel is slightly deeper than the south side (c. 0.3 m level difference). The marsh is one of the northernmost populations of Oryza rufipogon under in situ conservation. A fence was built in 2003 around the marsh to reduce anthropogenic disturbances.
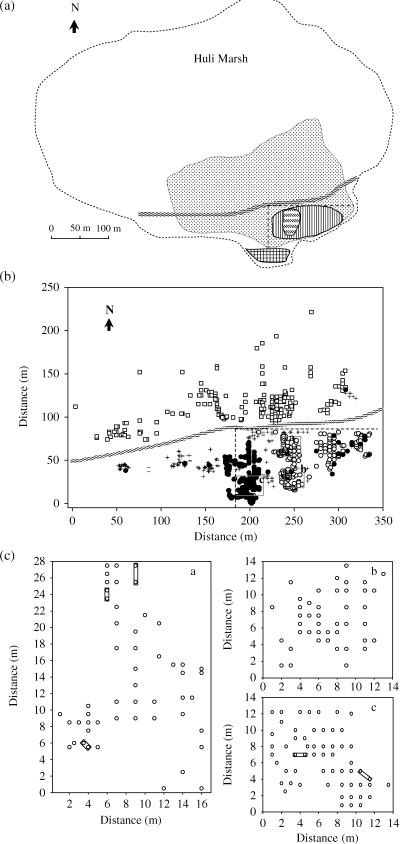
(a) A map indicating historical events and the physical distribution of Oryza rufipogon in Huli Marsh. 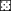 represents Oryza rufipogon distribution in 2003,
represents Oryza rufipogon distribution in 2003, 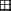 represents cultivated rice fields in 1994–1998,
represents cultivated rice fields in 1994–1998,  represents the channel,
represents the channel,  represents the original area where ex situ conserved clones were sampled in 1980s,
represents the original area where ex situ conserved clones were sampled in 1980s,  represents the reintroduction area, and the southeastern area surrounded with broken line is restoration area established in 1993.
(b) Spatial distribution of the four model-based subpopulations inferred by the program structure. Individuals assigned to different subpopulation were indicated by different icons, black circles (•) represent subpopulation A, empty circles (○) represent subpopulation B, empty panes (□) represent subpopulation C, and black crosses (+) represent subpopulation D; the southeastern area with broken line delineates the restoration area; letters a, b, and c in panes show the sample plots for clonal diversity analysis;
represents the reintroduction area, and the southeastern area surrounded with broken line is restoration area established in 1993.
(b) Spatial distribution of the four model-based subpopulations inferred by the program structure. Individuals assigned to different subpopulation were indicated by different icons, black circles (•) represent subpopulation A, empty circles (○) represent subpopulation B, empty panes (□) represent subpopulation C, and black crosses (+) represent subpopulation D; the southeastern area with broken line delineates the restoration area; letters a, b, and c in panes show the sample plots for clonal diversity analysis;  represents the channel.
(c) Maps of O. rufipogon clonal structure at the sampling plots. Each circle represents a sampled individual, and the circles included in a pane indicate putative clones. Labelled letter of plots correspond to their sampled localities.
represents the channel.
(c) Maps of O. rufipogon clonal structure at the sampling plots. Each circle represents a sampled individual, and the circles included in a pane indicate putative clones. Labelled letter of plots correspond to their sampled localities.
Population history
Oryza rufipogon in Huli Marsh was first discovered by the Hunan Academy of Agricultural Science of China (HAAS, Changsha City) during a nationwide project to collect wild Oryza species during 1978–1982. In this survey, the O. rufipogon population was found to cover a 3.3-ha area in the southern and eastern part of Huli Marsh, and 152 individuals were randomly collected and ex situ conserved as clonal strains in Changsha City by HAAS. During 1988–1990, the marsh was dammed for aquaculture, which raised the water level to 1.5–1.8 m, resulting in significant vegetation changes and O. rufipogon disappearance. In 1993, 54 O. rufipogon clones were reintroduced into a 50-m2 area in the southern part of the marsh from the Changsha ex situ population, and the water level was reduced to 0.2–0.7 m deep (Zhou 1995) (Fig. 1a). In the autumn of the same year, 10 seed recruits were found outside the reintroduction area. And, in the next autumn 84 seed recruits were observed inside and 79 seed recruits were found outside the reintroduction area in the southern part of the channel. In the following years, more O. rufipogon plants were found in other parts of the marsh where O. rufipogon had not been reintroduced; these plants were thought to have been recruited from the seed bank (Zhou 1995). By 1998, the water level was completely restored to the level before wild rice disappearance in the marsh, and many seed recruits were found in the northern area of channel (Z. P. Song, personal observation) (Fig. 1a).
Cultivated rice was grown as close as 10 m to the reintroduction area from 1994 to 1998 (Fig. 1a). Since then, some O. rufipogon individuals in the southeastern corner of the marsh, near the cultivated rice fields, showed morphological traits typically only found in cultivated rice, which was thought to be due to introgression from cultivated rice (Song et al. 2003). Recently, we used a wide collection of rice varieties (139) as control, to study the possible introgression of O. rufipogon populations (including the Huli population) from cultivated rice in China. This study applied an admixture model of the structure program to analyse consanguinity between the wild and cultivated rice samples through shared SSR (single sequence repeat) allelic frequencies. The results demonstrated that samples from the Huli population near rice fields (Fig. 1a) contained a few rare alleles that were commonly present in the rice varieties, confirming the introgression of the Huli O. rufipogon population from cultivated rice (Song et al., unpublished data). The O. rufipogon population in Huli Marsh has increased significantly since restoration activities began. In 2003, the size of the O. rufipogon stand was about 7.6 ha and nearly occupied a quarter of the marsh. Thus, analysing genetic diversity of the current O. rufipogon population in Huli Marsh may reveal the genetic history of a successful plant restoration.
Sampling
The entire stand of 813 O. rufipogon individuals in the marsh was carefully mapped within a 320 × 240 m area during September 2003 (Fig. 1b). In the restoration area, where there was a dense stand, individuals were sampled by 1 × 1 m lattices. In the areas with patchy regions, all individuals were sampled. Samples consisted of 1 g of young leaves from each individual. Samples were dried by placing them in a plastic bag containing silica gel, and stored at −80 °C until DNA extraction.
SSR genotyping
A total of 21 SSR primer pairs were selected to assay genetic variation in O. rufipogon. The SSR markers were chosen throughout the rice genome from about 200 candidates to detect cultivar-specific alleles in wild rice populations. During this screening, the SSR markers with null alleles in wild rice were excluded. About two of these SSR markers located in short or long arms of each of the 12 chromosomes, respectively (http://www.gramene.org). SSR amplification and genotyping were conducted in the same manner as described by Song et al. (2003).
Clonal diversity analysis
We selected three plots with high density of O. rufipogon samples to analyse clonal diversity in this population (Fig. 1b). The mean clone size (N/G) was calculated for each population by dividing the sample size (N) by the number of genotypes (G) detected (McClintock & Waterway 1993).
Individual assignment test
The program structure version 2.0 was used to assign the sampled individuals to their corresponding subpopulations (Pritchard et al. 2000). The admixture model was adopted, because it is the most appropriate model to use with introgressed populations, and allowed for analysing admixture and correlated allele frequencies (Pritchard et al. 2000). In this model, the number of populations, K, was cautiously chosen, because it could be easily overestimated even with slight departures from the model (Pritchard et al. 2000). To choose an appropriate K value for data analysis, we ran a series of independent analyses at a range of K values (1–5) for five independent runs. In each run, a burn-in of 10 000 and a 100 000 run length were performed. The analysis consistently showed that K = 4. Individuals were assigned to their corresponding subpopulations and subsequent analysis used the four model-based subpopulations.
Genetic diversity estimation
Genetic diversity parameters at the population and subpopulation level were estimated according to Nei (1987) for each polymorphic locus. This included allelic frequencies, observed heterozygosity (HO), expected heterozygosity (HE), and effective number of alleles [AE = 1/(1 –HE)]. We tested for single-locus differences in allele frequencies by applying G-tests (at the 5% level), which tested for differences in the distributions of allele frequencies between the model-based subpopulations.
Spatial genetic structure
We used F-statistics (FST) to estimate the degree of genetic differentiation within population (i.e. between the model-based subpopulations) using the methods of Wier & Cockerham (1984), and a upgma tree for the four subpopulations were established based on Nei's genetic similarity. These analyses were performed using the program popgene version 1.31 (Yeh et al. 1999).
For spatial genetic structure analysis, distance class intervals between individuals in the population were determined by testing to get at least 30 pairs of data in each distance class (Degen et al. 2001). In this case, 3 m was the average distance between neighbouring individuals in the four inferred subpopulations and the whole population. The autocorrelation coefficient r (ranging from −1 to 1) was calculated based on pairwise geographic and genetic distances using genalex 5.1 software (Peakall & Smouse 2001), which is available at http://www.anu.edu.au/BoZo/GenAlEx Tests for statistical significance were performed by random permutation with a Monte Carlo simulation. This is equivalent to shuffling the individual genotypes among the geographic locations and recomputing r. This generates an estimate of r about the null hypothesis of no spatial genetic structure (rp). In this case the individual rp values were compared with the observed r value to estimate the probability of achieving a value greater than or equal to the observed r. After 1000 permutations, the rp values were sorted and the 25th and 975th rp values were taken to define the upper and lower bounds of the 95% confidence interval as described by Peakall et al. (2003).
Results
Clonal diversity and the extent of clonality
The sample sizes for the three plots were 51, 43, and 57, respectively. Their clone sizes (N/G) were 1.063, 1.0, and 1.036, respectively. The largest clone consisted of two samples and spread at maximum 3 m long. Only five samples among the 151 genets contained a duplicate clone (Fig. 1c), indicating that most of the samples were distinct genotypes.
Individual assignment tests
The assignment tests showed that the population could be subdivided into four subpopulations and all individuals were assigned to a corresponding subpopulation (Fig. 1b). The numbers of individuals assigned to the corresponding subpopulations were listed in Table 1. Figure 1b showed that most individuals located in the restoration area were assigned to two subpopulations (A and B), while the remaining individuals outside the restoration area belonged to either subpopulation C or subpopulation D. It is important to point out that the model-based subpopulation A included the putative introgressed population referred to as CL-Pi in the previous study by Song et al. (2003), and subpopulation B included the reintroduced (CL-Pr) and original populations (CL-Po) in Song et al. (2003), indicating CL-Pr and CL-Po had similar genetic ancestry.
| Sample size | A E | H O | H E | F ST | |
|---|---|---|---|---|---|
| Subpopulation A | 241 | 2.785 | 0.331 | 0.581 | |
| ± 1.053 | ± 0.155 | ± 0.188 | |||
| Subpopulation B | 214 | 2.313 | 0.177 | 0.485 | |
| ± 0.952 | ± 0.142 | ± 0.229 | |||
| Subpopulation C | 177 | 2.322 | 0.139 | 0.485 | |
| ± 0.937 | ± 0.106 | ± 0.235 | |||
| Subpopulation D | 181 | 2.392 | 0.260 | 0.535 | |
| ± 0.745 | ± 0.123 | ± 0.170 | |||
| Entire population | 813 | 2.956 | 0.232 | 0.609 | 0.145 |
| ± 1.010 | ± 0.120 | ± 0.175 |
Genetic variation
All 21 SSR loci were polymorphic and generated 134 alleles in the Oryza rufipogon population. The numbers of scored alleles in the model-based subpopulations were 120 for subpopulation A, 109 for subpopulation B, 100 for subpopulation C, and 101 for subpopulation D, respectively. Unique rare alleles (frequency < 0.05) were found in subpopulation A (11), C (three), and D (one), respectively. In addition, there were five rare alleles shared by subpopulation A and B, and six shared by subpopulation C and D. The frequencies of rare allele for these polymorphic loci are listed in Table 2.
| Allele | Subpopulation | Entire population | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| RM11-E | 0.004 | 0.005 | 0.011 | 0 | 0.005 |
| -F | 0.012 | 0.009 | 0 | 0 | 0.006 |
| -H | 0 | 0 | 0.016 | 0 | 0.004 |
| -I | 0 | 0 | 0.085 | 0.116 | 0.045 |
| RM14-B | 0.120 | 0.009 | 0.022 | 0 | 0.043 |
| -G | 0.008 | 0.051 | 0 | 0 | 0.016 |
| RM17-C | 0.019 | 0 | 0 | 0 | 0.006 |
| -F | 0.010 | 0 | 0 | 0 | 0.003 |
| RM19-C | 0.004 | 0 | 0 | 0 | 0.001 |
| -D | 0.006 | 0 | 0 | 0 | 0.002 |
| -A | 0.006 | 0.030 | 0 | 0 | 0.010 |
| RM21-F | 0.006 | 0 | 0 | 0 | 0.002 |
| RM44-E | 0.056 | 0.014 | 0 | 0.017 | 0.024 |
| -F | 0 | 0 | 0.006 | 0.014 | 0.004 |
| -G | 0 | 0 | 0.006 | 0 | 0.001 |
| RM55-B | 0.177 | 0 | 0 | 0 | 0.052 |
| RM84-C | 0.008 | 0.012 | 0.006 | 0.028 | 0.013 |
| RM167-A | 0.008 | 0.002 | 0 | 0 | 0.003 |
| -E | 0.141 | 0.005 | 0.030 | 0 | 0.050 |
| -F | 0.023 | 0.005 | 0 | 0.053 | 0.020 |
| RM180-E | 0.004 | 0.056 | 0 | 0 | 0.016 |
| -F | 0 | 0.030 | 0.019 | 0.028 | 0.018 |
| -G | 0 | 0 | 0 | 0.058 | 0.013 |
| RM205-A | 0.012 | 0 | 0 | 0 | 0.004 |
| -G | 0 | 0 | 0.011 | 0.022 | 0.007 |
| RM211-B | 0.098 | 0.016 | 0 | 0.066 | 0.048 |
| -C | 0.004 | 0 | 0 | 0 | 0.001 |
| RM212-A | 0.008 | 0 | 0 | 0.006 | 0.004 |
| -E | 0.023 | 0.014 | 0.068 | 0.072 | 0.042 |
| RM219-A | 0.004 | 0.002 | 0.011 | 0.011 | 0.007 |
| -D | 0.054 | 0.047 | 0.011 | 0.006 | 0.032 |
| -F | 0.052 | 0.079 | 0.006 | 0.006 | 0.039 |
| -G | 0.021 | 0.005 | 0 | 0.017 | 0.011 |
| -H | 0.002 | 0.016 | 0 | 0 | 0.005 |
| -I | 0.025 | 0 | 0.019 | 0.077 | 0.029 |
| RM228-A | 0.004 | 0 | 0.006 | 0 | 0.002 |
| RM230-D | 0.002 | 0 | 0 | 0 | 0.001 |
| RM253-B | 0.002 | 0.051 | 0.118 | 0 | 0.040 |
| -F | 0 | 0.002 | 0 | 0.069 | 0.016 |
| RM276-E | 0.025 | 0.026 | 0.085 | 0 | 0.033 |
| -F | 0 | 0.007 | 0.011 | 0.014 | 0.007 |
| -G | 0.050 | 0.040 | 0 | 0 | 0.025 |
| -H | 0.108 | 0.056 | 0 | 0 | 0.046 |
| -I | 0 | 0.005 | 0.006 | 0 | 0.002 |
| -M | 0 | 0 | 0.006 | 0.011 | 0.004 |
| RM280-A | 0.035 | 0 | 0 | 0 | 0.010 |
| -B | 0.017 | 0 | 0 | 0 | 0.005 |
| -J | 0 | 0 | 0.019 | 0 | 0.004 |
| RM289-E | 0.002 | 0.005 | 0.003 | 0.061 | 0.016 |
| -F | 0 | 0.051 | 0.025 | 0 | 0.019 |
| -G | 0.004 | 0.005 | 0 | 0 | 0.002 |
The G-tests indicated that allele frequencies of almost all loci significantly differed between the four model-based subpopulations. The exceptions were: RM19 did not differ between subpopulation A and D (G-test, P = 0.131) and between subpopulation C and D (P = 0.117); RM84 did not differ between subpopulation A and B (P = 0.256); and RM280 did not differ between subpopulation B and D (P = 0.179). The entire population showed a high level of genetic diversity (AE = 2.956, HE = 0.609). Subpopulation A had the highest level of effective alleles (AE) and the expected heterozygosity (HE), whereas subpopulation B exhibited the lowest level of AE and HE (Table 1). A marked heterozygosity deficiency was found both at subpopulation and population levels (HO < HE), suggesting high level of selfing.
Spatial genetic structure
The F-statistics (FST = 0.145) and upgma tree (Fig. 2) also suggested considerable population subdivision in Huli Marsh. Figure 3 shows the results of the spatial autocorrelation analysis, using the distance size class of 3 m, across the entire population and subpopulations. A correlation between genetic and spatial distances among individuals was found. At the subpopulation level, the correlation was positive and significant from 11 to 30 m with an x-intercept of 11.8–50.7 m (Fig. 3a–d). For the entire population, the correlation was positive and significant up to 63 m with an intercept of 67 m (Fig. 3e).
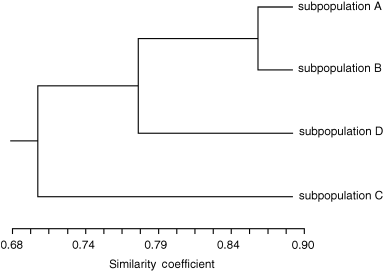
A upgma tree for the four model-based subpopulations of Oryza rufipogon in Huli Marsh.
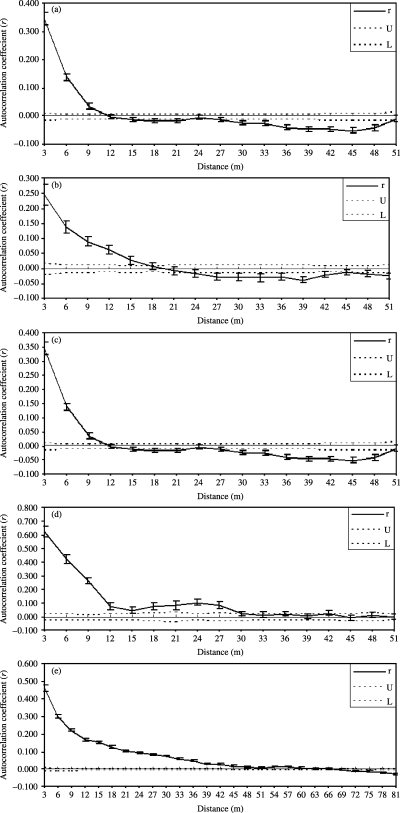
Correlograms showing the genetic correlation across the model-based subdivisions as a function of distance, 95% CI about the null hypothesis of random distribution of genotypes and individuals, and 95% confidence error bars about r were determined by bootstrapping. Fig. 3a, b, c, d, e, genetic correlation for the subpopulation A, B, C, D, and for the whole population, respectively.
Discussion
Genetic differentiation and population history
The population genetic data indicate cryptic population structure in the Huli Marsh Oryza rufipogon. All sampled individuals were assigned to their corresponding model-based subpopulations and the proportion of individuals assigned to each subpopulation was significantly asymmetric, which demonstrates real genetic structure within the O. rufipogon population (Pritchard et al. 2000). F-statistics and G-tests for allelic frequencies also strongly support this conclusion, i.e. significant genetic differentiation has occurred and such differences have not yet been eliminated by gene flow due to the short time span. In addition, individuals assigned to subpopulation D were scattered across half of the sample area and most of them mixed with the other three subpopulations (Fig. 1b). Such a cryptic genetic distribution could not be identified without the individual assignment tests.
There are several possible factors influencing the genetic differentiation pattern in this population. First, the putative introgressed individuals likely received genes from cultivated rice, resulting in genetic divergence between introgressed and pure O. rufipogon subpopulations (Song et al. 2003; Gao 2004). The finding of more relatively unique rare alleles in subpopulation A also supports this viewpoint. It is notable that the individuals classified into the introgressed subpopulation A were scattered in the areas of subpopulation B and D, but only one individual (one gene flow event) was found in the subpopulation C (see Fig. 1b). We expected that such pattern was mainly shaped by the topography and short population history, because the gene flow was about 150 m and restricted to the west–east direction. Figure 1a indicates a channel in Huli Marsh that divided the distribution of O. rufipogon into two areas. Water in the northern area of the channel is deeper than in the south and water depth is an important factor to regulate seed recruitment of the wild rice. The extensive formation of subpopulation C occurred after water was reduced to a suitable level for wild rice since 1998, and samples used in this study were collected in 2003. Due to the short time span, there should be few introgressed plants in the subpopulation C. Besides the short population history, relatively strong selfing in this wild population, indicated by the significant heterozygosity deficiency (Table 1, HO < HE), also contributed considerably to the restricted gene flow.
Second, seed recruitment may also have contributed to genetic differentiation. At another in situ conservation site in Jiangxi Province of China, significant genetic differentiation was found between the surface and seed populations, as well as among soil strata (FST = 0.154 and 0.057, respectively, Liu et al., unpublished data). Because seed recruitment occurred outside the restoration area in Huli Marsh (Zhou 1995; Song et al. 2003; Liu et al. 2004), the current expanded area likely consists of recruits from the seed bank. Therefore, our data indicate that subpopulation B consists of reintroduced individuals, seed recruits, and their descendants, whereas subpopulation C and D is composed of seed recruits and their descendants. This conclusion is supported by a study of the seed bank in Huli Marsh by Liu et al. (2005).
Although subpopulations B to D were recruited from the same seed bank, they showed significantly different allele frequencies, a high level of genetic differentiation, and alleles unique to each population. This suggests that: (i) significant population structure was historically found at Huli Marsh and this structure was retained in the seed bank; (ii) seed recruitment may vary among genotypes or subpopulations due to nonrandom germination patterns; or (iii) seed recruits may vary in their fitness. In addition, human disturbances, such as aquaculture, water control, and grazing might also influence this divergence. Therefore, factors that contribute to population differentiation should be further investigated. Nevertheless, the finding that the subpopulations retained rich genetic variation (HE = 0.485–0.535, Table 1) indicates that the seed bank acts as a genetic reservoir in retaining population genetic diversity of plant species (Levin 1990; Kalisz & McPeek 1992).
At Huli Marsh, we predict that population expansion would be achieved by the residual seed bank, rather than by extensive colonization from the reintroduced subpopulations. These predictions could have important implications for population persistence and distribution patterns in fragmented landscapes where populations may suffer local extirpation. In addition, both introgression and seed recruitment strongly affected population genetic structure and diversity, indicating that historical events can leave a strong signature on contemporary populations. Thus, the present study shows that population histories can shape present-day population structure.
Spatial pattern of genetic structure in the O. rufipogon population
We detected a significant pattern of fine-scale genetic structure within the subpopulations and population of O. rufipogon in Huli Marsh with positive autocorrelation, although the genetic clustering differed between the two levels of analysis (c. 30 m vs. 60 m, see Fig. 3). Therefore, we reject the null hypothesis that genotypes within O. rufipogon populations are randomly distributed at the scale studied. The spatial patterns of genetic variability in O. rufipogon populations are generally consistent with the prediction that plant populations often have a local structure over a short distance (see review by Ennos 2001).
For plant populations, positive spatial genetic structure can be caused by reproductive features such as inbreeding and clonal spread, restricted gene flow, population history, and other processes such as heterogeneity of habitats (Sokal et al. 1989; Perry & Knowles 1991; Reusch et al. 1999; Jin et al. 2003). For the Huli Marsh O. rufipogon population, several processes are likely responsible for the spatial genetic structure. First, the mating system is important for determining genetic variability. O. rufipogon is wind-pollinated and self-compatible with mixed mating systems (Oka & Morishima 1967; Morishima & Barbier 1990; Gao et al. 2000; Song et al. 2003). However, the outcrossing rate of the population was estimated at 0.236 (Z. P. Song, unpublished data). This indicates a nonrandom mating system of O. rufipogon and a high level of selfing in this population, which therefore resulted in significant heterozygosity deficiency in this population. Although O. rufipogon reproduces clonally (Xie et al. 2001), we found high clonal diversity in this population (Fig. 1c), indicating that inbreeding strongly contributes to genetic structuring in the O. rufipogon population.
Second, simulation and field studies confirm genetic structure within plant populations can arise from limited pollen and seed dispersal at the local scale (model of isolation by distance) (e.g. Wright 1946; Levin & Kerster 1974; Hardy & Vekemans 1999; Jin et al. 2003). In this study, pollen probably had less influence on genetic structure. As mentioned by Kalisz et al. (2001), fine-scale spatial genetic structure can develop as a result of differential effects of pollen and seed dispersal on allelic correlations among individuals within a population. O. rufipogon was found to disperse its seeds mainly by gravity with a distance about 3 m per year (Zhou 1995; Xiao & Ying 1996; Qian 2003). Wind-mediated pollen flow occurs randomly and over greater distances (c. 110 m, Song et al. 2004) than the extent of positive genetic structure of O. rufipogon detected in this study. This indirectly suggests a high selfing rate in wild rice. Hence, seed dispersal combined with a high ratio of selfing may be highly localized comparing to pollen dispersal and helps shape positive spatial genetic structure of O. rufipogon population.
Spatial genetic structure at population and subpopulation levels
Spatial genetic structure was less detectable at the subpopulation level than at the population level. One explanation for this difference might be the effect of plant density on fine-scale structure (Hamrick et al. 1993; Degen et al. 2001). As shown in Fig. 1b, all of subpopulations except subpopulation D had a high plant density. Consequently, the subpopulation D had the largest extent of spatial genetic structure than the others. A second explanation derives from the difference in between the two scales on spatial genetic clustering. Using the one-tailed probability test as Peakall et al. (2003), we found that spatial genetic structure at the population level exceeded 80 m (data not shown). This supports the viewpoint that the sampling unit should be wider than the maximum extent of detectable positive autocorrelation in order to obtain the true extent of spatial genetic structure (Peakall et al. 2003), and suggests the existence of scale effects in fine-scale structure studies. Third, the history at Huli Marsh population has resulted in pronounced population subdivision. Although the population has been established for 10 years and appears to be expanding, gene flow is too low to counteract the genetic divergence between the subpopulations due to short time span. Given this point, cryptic structure should be identified before spatial autocorrelation analysis for populations that have experienced extreme population fluctuations. Lastly, introgression may influence spatial genetic structure within O. rufipogon population. Alien gene flow will increase the departure of gene frequencies from population equilibrium, leading to significant genotypic disequilibria and aggregation (Boileau et al. 1992). Some agricultural traits, such as compact panicles, may further restrict seed dispersal leading to positive spatial genetic structure.
Conservation implications
Genetic diversity is thought to be important for plant restoration (Procaccini & Piazzi 2001; Proffitt et al. 2003). This study shows how genetic diversity can be maintained or even increased due to natural plant recruitment and mating processes. Seed banks can buffer populations from the short-term loss of genetic diversity. A large number of O. rufipogon individuals in this study were recruited from the seed bank in Huli Marsh after water levels were managed. This strongly suggests that appropriate habitat management can facilitate in situ conservation for endangered plants. In addition, by using a large set of hypervariable SSR markers and exhaustively sampling all individuals within the studied area, our study will provide a useful reference to measure the levels of genetic diversity in other O. rufipogon populations.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (grant no. 30300019, 30125029), Science and Technology Commission of Shanghai (grant no. 03DJ14014, 03DZ19309) and Ministry of Science and Technology (Grant no. 2006CBI00205).
References
Zhiping Song is Associate Professor at Fudan University with research interests in molecular ecology and conservation genetics. Professor Bao-Rong Lu focuses on plant resource conservation, molecular evolution, and biosafety assessment of transgenic crops. Xian Xu is a Postgraduate student at Fudan University studying wild rice genetics and structure.




