When log-dwellers meet loggers: impacts of forest fragmentation on two endemic log-dwelling beetles in southeastern Australia
Abstract
Anthropogenic activities continue to cause massive fragmentation and reduction of forest area worldwide. With fragmentation and reduction of habitat recognized as the greatest threats to biodiversity, the implementation of improved, informed and conservation-based forestry practices is essential, and requires a greater understanding of the responses of different organisms to forest fragmentation. While genetic techniques can add invaluable insights to fragmentation studies they have rarely been employed, particularly for multiple species. In the present study, we combined genetic information, obtained from allozyme loci and anonymous single copy nuclear DNA markers, with ecological data to investigate the impacts of forest fragmentation on two log-dwelling beetles with different life histories, in an ‘islands of bush in a sea of pine’ model, at Tumut in New South Wales, Australia. Both the relatively mobile (i.e. has high dispersal ability and/or broad habitat range) Adelium calosomoides and the less mobile Apasis puncticeps showed reduced mobility and gene flow in fragmented compared to continuous forest: there was significantly greater isolation by distance and stronger local structure revealed by spatial autocorrelation in fragmented forest. Analysis of patch and species characteristics revealed that genetic and demographic structure may be influenced by log degradation class for both species, and number of potential dispersal barriers, distance from continuous forest and desiccation intolerance/moisture preference for Ap. puncticeps. Thus the pine plantation matrix poses a barrier or filter for gene flow and mobility in both beetle species.
Introduction
Impacts on biodiversity through loss and fragmentation of forest
Fragmentation and reduction of habitat are recognized as the greatest threats to biodiversity (WCMC 1992), yet anthropogenic practices continue to cause massive fragmentation and reduction of forest area worldwide. At the same time, there is rapidly accumulating evidence that attendant negative impacts undermine the productivity and sustainability of forestry (Norton 1996; Grove 2002a, b). Thus, the implementation of informed, conservation-based forestry practices has the potential to improve both the long-term viability of forestry and retention of biodiversity.
Forest fragmentation reduces once-continuous areas of forest into smaller, less-connected and isolated remnants, usually receiving fewer migrants and less gene flow. Impeded movement of individuals and their genes have a range of demographic and genetic impacts (Frankham et al. 2002), including increased genetic differentiation between populations, reduced genetic variation within populations, and altered within-fragment population structures such as the mating system (Frankham et al. 2002; Stow & Sunnucks 2004a, b). Reduced migration can impede recolonization of patches operating in a metapopulation structure, and ultimately lead to local or broader extinction (Bradshaw & Marquet 2003).
How fragmentation of forest impacts individual organisms is dependent on the life history attributes of those organisms (Didham et al. 1998; Lindenmayer et al. 1999; Henle et al. 2004; Wiegand et al. 2005) and their interaction with a multitude of fragmentation effects, so that only those species that find the new matrix sufficiently hostile may experience impeded migration and gene flow (Berggren et al. 2001; Mabry & Barrett 2002). While there have been some extensive programmes on forest vertebrates (e.g. Lindenmayer citations herein; Gibbons et al. 2002; Ball et al. 2003; Sumner et al. 2004), direct information can be difficult to obtain for invertebrates, not least because they are less amenable to capture–mark–recapture. Rapid recent advances in molecular population biology have improved this situation by facilitating highly resolving genetic estimators of mobility and gene flow (Sunnucks 2000; Brouat et al. 2003; Keller & Largiadèr 2003). Studies that can estimate migration and gene flow can aid in understanding the complexities of impacts of fragmentation by supplying the missing information about mobility of individuals.
The importance of invertebrates and the saproxylic community
Invertebrates make key contributions to all levels of biodiversity (Erwin 1982; WCMC 1992). Key forest invertebrates include ‘saproxylic’ organisms, which inhabit decaying wood — the largest pieces of which have been referred to as coarse woody debris (CWD) and diminish through a number of forest management practices (Siitonen 2001; Sippola et al. 2001). This resource and its community are now recognized as fundamental to forest function through critical processes such as nutrient cycling (Wallace 1953; Yee et al. 2001; Grove et al. 2002). We hereafter refer to CWD as fallen timber owing to negative connotations of both ‘coarse’ and ‘debris’ used to describe this ecologically critical resource.
Understanding the impacts of habitat alteration on invertebrates that depend on rotting wood and leaf litter is critical. As well as being ecologically (and as a consequence, commercially) important, the saproxylic community contains a disproportionate fraction of species of conservation concern (Yen et al. 1990; Grove 2002a). The organisms also have the practical advantages (as models/indicators) of being relatively aseasonal, and distributed in a convenient hierarchy of countable logs in measurable patches within regions.
Experimental approaches to fragmentation: manipulations and pseudo-experiments
Ideally, fragmentation studies should be carried out in manipulation experiments, but for logistic reasons they are rare (Margules 1992; Holt & Debinski 2003). Instead, studies of habitat fragmentation have been largely based on pseudo-experiments, utilizing fragments created through the exploitation of resources (e.g. Niemeläet al. 1993; Abensperg-Traun et al. 1996; Sumner et al. 2004). An important consideration in such studies is the quality and quantity of prefragmentation data. The absence of such data may be compensated for where forest remnants adjoin unfragmented forest representing prefragmented habitat. Buccleuch State Forest (BSF) near Tumut in New South Wales, Australia, meets these requirements, having a large number of eucalypt forest patches in a Pinus radiata plantation matrix adjoining large areas of continuous native forest (Lindenmayer 2000).
Studies of the impacts of forest fragmentation have been carried out for a diversity of plant and vertebrate species at BSF (e.g. Lindenmayer 2000 and references within; Lindenmayer & Peakall 2000; Lindenmayer et al. 2001a, b; Fischer & Lindenmayer 2002a, b; Gibbons et al. 2002; Lindenmayer et al. 2002; Ball et al. 2003; Banks et al. 2005), but there have been no published studies on the effects of habitat fragmentation on invertebrates. Moreover, studies on how fragmentation affects the mobility and gene flow of terrestrial invertebrates are generally lacking globally. A few recent studies have demonstrated significantly altered genetic structure and reduced persistence of populations in fragmented habitats for a range of generalist and specialist as well as high and low gene flow species in recently and historically fragmented systems (Knutsen et al. 2000; Brouat et al. 2003; Keller & Largiadèr 2003; Williams et al. 2003). Together they have shown that genetic responses to fragmentation are species-specific and can be complex, just like the demographic effects with which they are associated (Davies & Margules 1998; Didham et al. 1998; Henle et al. 2004). Thus, comparative studies and syntheses are needed to formulate useful generalizations (Henle et al. 2004).
While genetic techniques can add complementary and novel insights in fragmentation studies (e.g. Stow & Sunnucks 2004a, b and references within), these approaches have rarely been applied in the present context, particularly for multiple species (Brouat et al. 2003). In the present study, a combination of genetic and ecological techniques were utilized to examine the effects of forest fragmentation at BSF on the mobility and gene flow of two species of flightless tenebrionid beetles differing in life histories: Adelium calosomoides Kirby (1818) and Apasis puncticeps Lea (1896). If pine plantation in Australia is hostile to them, Ap. puncticeps and Ad. calosomoides should display reduced mobility and gene flow among the native forest patches embedded in pine.
Materials and methods
Study site and organisms
Buccleuch State forest (BSF) contains 192 eucalypt forest patches of different size, shape, spatial configuration and fragmentation history, embedded in a 50 000+ ha pine plantation matrix (Lindenmayer 2000). Two tenebrionid beetle species of the subfamily Lagriinae, tribe Adeliini, were collected in a southern section of BSF from patches ranging from 21 to 36 years since their creation, and from adjacent continuous native forest (Fig. 1). Adelium calosomoides is the smaller species and occurs largely in dry microhabitats, while we find Apasis puncticeps more in moist microhabitats. The different habitat use is consistent with the natural histories of the two genera, from which it might also be inferred that Adelium are the more generalist and mobile (Matthews 1998). These beetles are dependent on fallen timber and, being among the major invertebrate consumers of fallen timber at BSF, have the ability to cause physical changes in their environment and influence the availability of resources to other species.
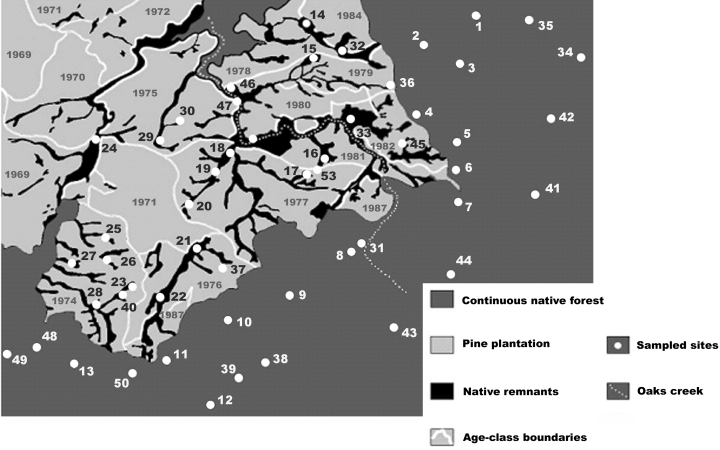
Location of sampled sites.
We obtained 2323 adult beetles — 707 Ap. puncticeps and 1616 Ad. calosomoides— from 142 rotting logs in 52 sites. Logs were dismantled by hand in March 2001 (sites 25–30, 32–33, 53; Fig. 1), in March 2003 (sites 1–23) and in April–June 2004 (sites 24, 31, 34–51). For each log, GPS coordinates, dimensions, percentage sampled and degradation class (Barclay et al. 2000) were recorded. Live specimens were transported to the laboratory and stored at 6 °C, where they were frozen at −80 °C or retained alive for a physiological experiment (below).
Desiccation/heat stress experiment
Beetles were housed for approximately 2 weeks in ventilated containers (r = 7.25 cm, L = 21 cm) containing rotting log material and sliced carrot, then at room temperature (22 °C ± 0.5 °C) for 2 h prior to the experiment. In all, 132 Ad. calosomoides and 43 Ap. puncticeps were tested for desiccation/heat stress response. Individuals were weighed (accurate to 0.1 mg) and then placed in open containers (r = 2 cm, H = 5.5 cm), which sat in tubs (L = 26 cm, W = 19 cm, H = 10 cm) containing 200 g silica gel desiccant. The tubs were sealed and placed in a 40 °C oven for 2.5 h. Beetles were subsequently weighed, recorded as alive or dead and stored at −80 °C. The experiment was designed following a pilot study (data not shown) that identified the narrow range of conditions causing >0% and <100% mortality in both species.
Allozyme electrophoresis and anonymous single-copy nuclear DNA markers
The 23 enzyme systems tested (available upon request) yielded five presumptive polymorphic loci of satisfactory resolution for subsequent population screening: glucose phosphate isomerase (GPI; E.C. 5.3.1.9), phosphoglucomutase (PGM; E.C. 2.7.5.1), malate dehydrogenase (MDH; E.C. 1.1.1.37), malic enzyme (ME; E.C. 1.1.1.40) and adenylate kinase (AK; E.C. 2.7.4.3) (abbreviations follow Harris & Hopkinson 1976).
Genotypes for each beetle at each locus were determined using cellulose acetate electrophoresis (Titan III system, Helena Laboratories). GPI, PGM, MDH and ME were all resolved using a Tris Glycine buffer of pH 8.0 (Richardson et al. 1986) diluted 1:4 with distilled water. GPI and MDH gels were run at 4 °C for 1 h at 200 V, and PGM and ME at room temperature for 35 min at 200 V. AK was resolved using the same buffer diluted 1:2 for 30 min at 4 °C and 200 V. Stain was applied in an agar overlay, and recipes followed Richardson et al. (1986) with minor modifications. In all, 1641 beetles (up to 20 per log: 678 Ap. puncticeps and 963 Ad. calosomoides) were genotyped at the five loci. All those Ap. puncticeps were genotyped at single-copy nuclear (scn) DNA loci AP38, A1Mp20U2, S2Gm49 and all Ad. calosomoides at AD 19 and AD 32 as per Schmuki et al. (in press).
Analysis of genetic data
Whether genetic structure followed a genetic isolation-by- (geographic) distance (IBD) pattern was estimated using Mantel tests (10 000 permutations) in genepop (version 3.4; updated version cf. Raymond & Rousset 1995), based on logarithm of geographic distances and FST/(1 −FST) (Rousset 1997). Tests for different IBD slopes in continuous and fragmented forest were conducted in poptools (version 2.5.9; Hood 2003): regression and resample functions were used to generate real and randomized slopes, respectively, and the Monte Carlo function calculated 1000 randomized slope-differences and the number of randomized slopes > the real slope. IBD analyses for sex differences were conducted in the same way.
Spatial autocorrelation analyses were implemented in genalex (Peakall & Smouse 2001; version 6.0, Peakall et al. 2003) and performed with 9999 permutations for tests of significance, and 999 bootstraps for estimating 95% confidence intervals. Differences in continuous vs. fragmented and male vs. female autocorrelation coefficients (r) within distance classes (1000–10 000 m) were assessed relative to 10 000 permuted r values; sorted permuted r values from genalex were shuffled using the Resample function in poptools.
Tests for sex-biased dispersal were performed using fstat (version 2.9.3.2; Goudet 1995). Allelic richness (using rarefaction to overcome problems associated with uneven sample sizes) and FIS were calculated in fstat and observed and expected heterozygosities calculated in popgene (version 1.31; Yeh et al. 1995).
Uncovering factors contributing to differential dispersal abilities
Two-way analyses of variance (anova) (using the GLM command in spss 11.5, SPSS Inc.) were used to test for effects of habitat types (continuous vs. fragmented) on three response variables: allelic richness, FIS and density (all animals sampled per m3 wood at each site; required a third-root transformation to meet the assumptions of anova).
For the fragmented sites, we investigated the effects of three variables (distance of fragmented sites from continuous habitat, number of potential dispersal barriers between fragmented sites and continuous habitat and the area of eucalypt (m2) within a 500-m radius of sampled sites) on densities, allelic richness and FIS of the two beetles using multiple regression. Altitude and fragment age varied little among fragments and were thus excluded from the analysis. Distances from continuous forest were taken as shortest air-line distances. Barriers comprised roads and creeks and were counted if they intersected shortest air-lines between continuous forest and fragmented sites; most were given a value of one barrier, but major gravel roads that were wider and used with high frequency by logging trucks were given a value of two. To avoid patterns of FIS for Ad. calosomoides being driven by homozygous excess at AD 19 and AD 32 (Schmuki et al. in press), we excluded these loci from this species’ data set when examining possible impacts of the three predictor variables (above) on FIS, and for species comparisons of FIS. As explained in Schmuki et al. (in press), continuous and fragmented forest sites were equally deficient in heterozygotes at AD 19 and AD 32; we thus found it legitimate to include these loci when comparing the two in other analyses.
To avoid the pitfalls of automated procedures for variable selection (Quinn & Keough 2002), we used model selection based on Akaike's information criterion (AIC, Burnham & Anderson 1998) to evaluate which combination of predictor variables best represented our data. All eight possible additive combinations of predictors (including a ‘null’ model only containing the intercept) were compared. Each model was fitted with maximum-likelihood estimation of parameters in proc genmod of sas (SAS Institute 1996) using the identity link and normal errors. From the log-likelihood of each model, AICc (AIC adjusted for small sample size) was calculated to identify the most parsimonious model (lowest AICc), i.e. the one best representing the data using the fewest parameters, properly balancing the errors of over- and underfitting (Burnham & Anderson 1998). Akaike weights were calculated to assess the relative support for each model (Burnham & Anderson 1998). Where model selection provided evidence for a particular predictor to be important, its parameter estimate was obtained by ‘model averaging’ (Burnham & Anderson 2001), using the estimator of Buckland et al. (1997). This method accounts for model selection uncertainty and provides an average across all candidate models containing the predictor, weighted by the relative evidence supporting each model (Burnham & Anderson 2001).
The impact of log degradation class on beetle density was assessed for the combined data set of logs from fragmented and continuous habitat using analysis of covariance (ancova), accounting for the effects of habitat type and site identity.
Results
Number of alleles ranged from two to three, and two to five for Apasis puncticeps allozymes and nuclear DNA markers, respectively, and two to six, and eight to ten for Adelium calosomoides. Neither species exhibited significant differences in allelic richness or FIS in continuous vs. fragmented forest (two-way anova, F2,83 = 0.12, P = 0.89 and F2,83 = 1.84, P = 0.17, respectively). Ad. calosomoides was sampled at significantly (F1,88 = 11.7, P < 0.001) greater densities than Ap. puncticeps in both continuous (mean = 2.2 ± 0.21 beetles/m3 wood searched, cf. 1.85 ± 0.21) and fragmented (3.07 ± 0.21 cf. 2.01 ± 0.21) forest, although there is apparently such seasonal variation in the relative abundance of the two species that in some sampling periods many more Ap. puncticeps are sampled than Ad. calosomoides. Ap. puncticeps showed significantly (P < 0.0001) stronger patterns of IBD in both forest contexts than did Ad. calosomoides (continuous: b = 0.017 vs. b = −0.00067, P = 0.014; fragmented: b = 0.038 vs. b = 0.012, P < 0.0001) indicating significantly poorer dispersal ability than Ad. calosomoides.
Analyses of spatial genetic structure indicated male-biased dispersal for Ad. calosomoides: females displayed significant genetic IBD (b = 0.005, P = 0.040), while males did not (b = −0.005, P = 0.72). This sex difference was not formally significant (P = 0.083) perhaps in part because genetic–geographic relationships in the sexes have different shapes: autocorrelation coefficients (r) were significantly (P < 0.004) higher for male Ad. calosomoides over the 1000–2000 m and 8000–10 000 m distance classes (Table 1). There were no significant (P > 0.40) sex differences in mean assignment index (AIC), variance in AIC, FST, relatedness, observed heterozygosity (HO) and gene diversity (HS).
| Distance class (m) | Apasis puncticeps | Adelium calosomoides | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | F | P | Female | Male | P | C | F | P | Female | Male | P | |
| 1000 | 0.0489 | 0.0398 | 0.003 | 0.0499 | 0.0447 | 0.046 | 0.0477 | 0.0176 | < 0.001 | 0.0190 | 0.0285 | 0.002 |
| 2000 | 0.0196 | 0.0275 | 0.015 | 0.0080 | 0.0245 | < 0.0001 | −0.0036 | −0.0033 | 0.661 | −0.0004 | 0.0086 | 0.001 |
| 3000 | −0.0074 | 0.0132 | < 0.0001 | 0.0075 | 0.0124 | 0.058 | −0.0158 | –0.0081 | < 0.001 | −0.0035 | −0.0052 | 0.513 |
| 4000 | 0.0117 | 0.0008 | < 0.001 | 0.0097 | 0.0097 | 0.643 | 0.0153 | 0.0051 | < 0.001 | 0.0017 | 0.0010 | 0.745 |
| 5000 | –0.0019 | −0.0159 | < 0.0001 | −0.0079 | 0.0033 | < 0.0001 | −0.0018 | −0.0007 | 0.418 | 0.0027 | 0.0004 | 0.311 |
| 6000 | −0.0178 | −0.0175 | 0.660 | 0.0003 | –0.0033 | 0.022 | −0.0092 | –0.0053 | 0.015 | 0.0019 | –0.0027 | 0.037 |
| 7000 | 0.0105 | –0.0155 | < 0.0001 | −0.0064 | –0.0002 | 0.029 | −0.0087 | 0.0052 | < 0.0001 | –0.0010 | –0.0065 | 0.023 |
| 8000 | –0.0012 | –0.0344 | < 0.001 | −0.0118 | –0.0056 | 0.020 | 0.0047 | –0.0080 | < 0.0001 | −0.0054 | –0.0005 | 0.002 |
| 9000 | –0.0003 | –0.0345 | < 0.0001 | −0.0085 | –0.0161 | 0.004 | −0.0005 | −0.0029 | 0.093 | −0.0032 | 0.0022 | 0.003 |
| 10 000 | –0.0123 | –0.0434 | < 0.001 | −0.0188 | −0.0189 | 0.387 | N/A | N/A | N/A | −0.0082 | –0.0005 | 0.002 |
The same measures revealed no evidence of sex-biased dispersal for Ap. puncticeps (P > 0.20 for mean AIC, variance AIC, FST, relatedness, HO and HS). The sexes displayed similar, significant IBD (M: b = 0.035, P < 0.0001; F: b = 0.032, P = 0.003; P = 0.43 for the difference) and spatial autocorrelation revealed no consistent sex differences in genotypic structure (Table 1). Since sex differences are slight or absent in both species, data will not be analysed separately by sex below.
Comparison of genotypic structure in continuous and fragmented forest
Adelium calosomoides. A strong significant pattern of genetic IBD was found for this species in fragmented forest (b = 0.012, P = 0.0007), but there was no sign of genetic differentiation with distance in the continuous forest (b = -0.00067, P = 0.46). Regression slopes for the two forest contexts differed significantly (P = 0.002, permuted differences in slopes). Spatial autocorrelograms showed similar genotypic structure in both forest contexts (Fig. 2), with oscillation of positive and negative autocorrelation across both continuous and fragmented forest, but genotypes were significantly more similar over shorter distances in the continuous forest than in the fragmented one (continuous: r = 0.048; fragmented: r = 0.016; P = 0.0001). The difference in genetic structure in the two forest types can be seen more easily by examination of the outcomes of the Peakall et al. technique (2003) of iteratively increasing distance bins and plotting r only for the first bin in each case — this approach revealed positive structure up to 9.0 km in continuous forest compared to only 2.0 km in fragmented forest (Fig. 3).
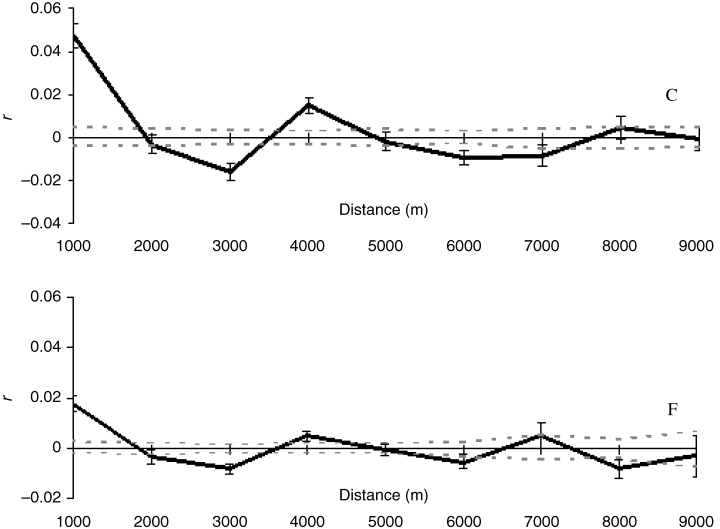
Spatial autocorrelograms for Adelium calosomoides in continuous (C) and fragmented (F) forest: black solid lines, autocorrelation coefficient (r); grey broken lines, upper and lower confidence.
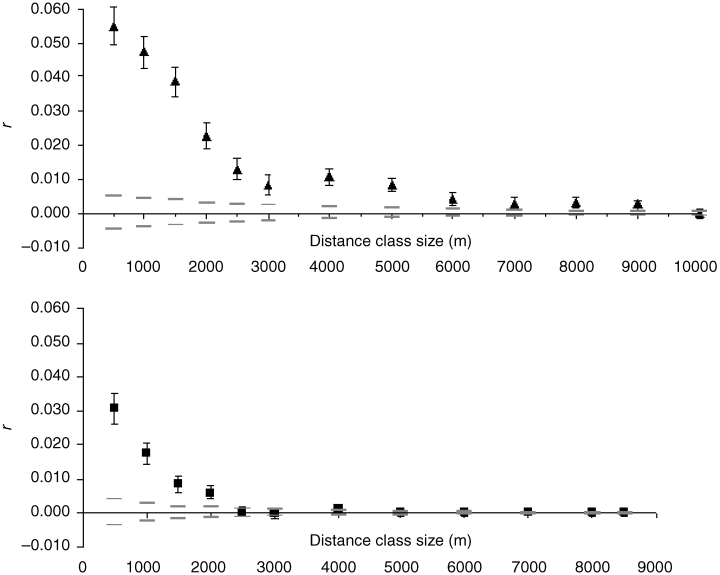
Autocorrelation for increasing distance-class sizes in continuous (▴) compared to fragmented (▪) forest for Ad. calosomoides: black shapes, autocorrelation coefficient (obtained from 1st distance bins only); grey lines, upper and lower confidence.
Apasis puncticeps. A pattern of genetic IBD was revealed for Ap. puncticeps in both continuous and fragmented forest (P = 0.003 and P < 0.0001, respectively), but the IBD regression slope for fragmented forest was significantly steeper than that for continuous forest (b = 0.038 cf. 0.017; P = 0.004 by permuting differences in slopes). Consistent with this, autocorrelograms for Ap. puncticeps showed positively autocorrelated genotypes over short geographic distance classes in both forest contexts, but there was a strong pattern of genotypic dissimilarity with distance in the fragmented forest compared to the continuous forest, which showed oscillation of positive and negative autocorrelations (Fig. 4). Tests for differences in continuous- and fragmented-forest autocorrelation coefficients (r) for distance classes 1000–9000 m were performed by permutation. In fragmented forest, positive structure was significantly lower over the smallest (up to 1000 m) and largest distances (4000–5000 m, 7000–10 000 m) and significantly higher over intermediate ones (2000–3000 m) (Table 1). This nonlinear pattern could be generated by dispersers necessarily undergoing atypically long dispersal to the next patch, with increased habitat ‘viscosity’ over long distances.
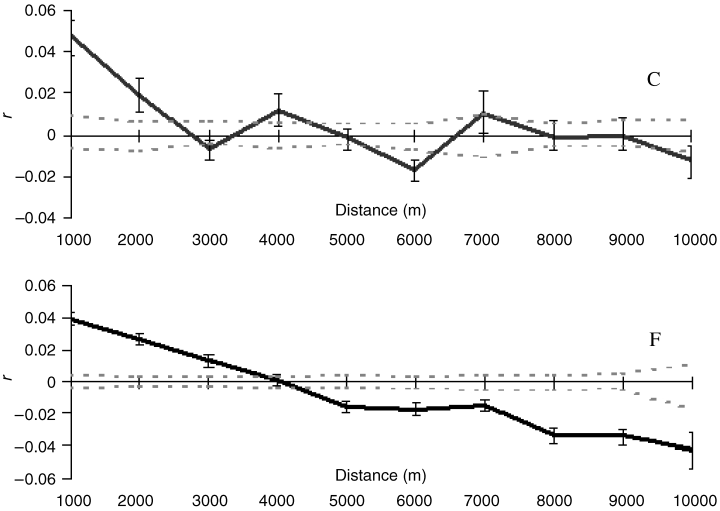
Spatial autocorrelograms for Apasis puncticeps in continuous (C) and fragmented (F) forest: black solid lines, autocorrelation coefficient (r); grey broken lines, upper and lower confidence.
Contributions to reduced mobility and gene flow in fragmented forest
Patch and habitat characteristics. Species-specific patterns were seen for variation in each of allelic richness, FIS and beetle density. Model selection showed that allelic richness of Ap. puncticeps was mainly affected by distance from continuous forest, since the regression model best supported by the data was one containing the intercept and distance from continuous forest as the only explanatory variable. This conclusion is further supported by the fact that the combined Akaike weights of all models containing this variable amount to 0.966, leaving very little support for any model without that variable. Model averaging estimated an increase in allelic richness of 0.113 ± 0.035 for every kilometre from continuous forest (Table 2). In Ad. calosomoides, none of the environmental variables had much effect on allelic richness. The best regression model contained the intercept and area of eucalypt, but was only marginally better supported than the ‘null model’ containing the intercept alone (Table 2).
| Model | Apasis puncticeps | Adelium calosomoides | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density | Allelic richness | F IS | Density | Allelic richness | F IS | |||||||
| Delta AICc | Akaike weight | Delta AICc | Akaike weight | Delta AICc | Akaike weight | Delta AICc | Akaike weight | Delta AICc | Akaike weight | Delta AICc | Akaike weight | |
| c | 9.070 | 0.006 | 7.235 | 0.012 | 2.174 | 0.102 | 0.000 | 0.452 | 0.361 | 0.226 | 0.000 | 0.321 |
| c + dist | 8.169 | 0.009 | 0.000 | 0.455 | 4.686 | 0.029 | 2.407 | 0.136 | 2.283 | 0.086 | 0.215 | 0.289 |
| c + barr | 0.000 | 0.551 | 9.065 | 0.005 | 0.000 | 0.304 | 2.158 | 0.154 | 0.888 | 0.174 | 2.483 | 0.093 |
| c + euc05 | 8.334 | 0.009 | 7.264 | 0.012 | 1.841 | 0.121 | 2.425 | 0.134 | 0.000 | 0.271 | 2.460 | 0.094 |
| c + dist + barr | 2.252 | 0.179 | 2.152 | 0.155 | 2.842 | 0.073 | 4.854 | 0.040 | 3.616 | 0.044 | 2.644 | 0.086 |
| c + dist + euc05 | 7.565 | 0.013 | 1.088 | 0.264 | 4.520 | 0.032 | 5.104 | 0.035 | 2.460 | 0.079 | 2.873 | 0.076 |
| c + barr + euc05 | 2.245 | 0.179 | 8.936 | 0.005 | 0.148 | 0.282 | 4.837 | 0.040 | 2.030 | 0.098 | 5.245 | 0.023 |
| c + dist + barr + euc05 | 4.635 | 0.054 | 3.203 | 0.092 | 3.337 | 0.057 | 7.847 | 0.009 | 5.114 | 0.021 | 5.784 | 0.018 |
In Ap. puncticeps, FIS for a patch increased with the number of potential dispersal barriers between a fragment and the continuous forest. The model containing the number of barriers as the only predictor was best supported and the combined Akaike weight of all models containing this predictor was 0.716. Model averaging estimated an increase in FIS of 0.025 ± 0.013 for each additional barrier. In Ad. calosomoides, none of the patch characteristics appeared to affect FIS (Table 2).
Density of Ap. puncticeps in fragments was also impacted by the number of potential dispersal barriers from continuous forest, declining by 0.225 ± 0.092 with each additional barrier (model-averaged estimate). The regression model only containing this predictor was by far the most parsimonious and the combined Akaike weights of models containing barriers amounted to 0.963 (Table 2). Density of Ad. calosomoides was not strongly influenced by any of the three predictor variables (Table 2).
Analysis of log degradation class showed that beetles of both species occurred at higher densities in less decomposed logs (Ap. puncticeps: P < 0.001; Ad. calosomoides: P = 0.011, ancova, accounting for the effects of habitat type and site). Highest densities occurred in logs of degradation class 2.
Species desiccation/heat shock tolerance. Approximately equal proportions of body weight were lost by each species during the dry heat treatment (Fig. 4), but the effects were profoundly different. Of 134 Ad. calosomoides desiccated, 125 (93.3%) remained alive throughout the experiment, compared with only 9 of 41 (22%) Ap. puncticeps (χ2 = 82.6, d.f. = 1, P < 0.0001). Thus the prediction that Ap. puncticeps should be less tolerant than Ad. calosomoides of hot/dry conditions was validated.
For a summary of the main characteristics of and findings for each organism, see Table 3.
| Apasis puncticeps | Adelium calosomoides | ||||
|---|---|---|---|---|---|
| Habitat preference | Moist | Dry | |||
| Dispersal ability | Lower | Higher | |||
| Densities | Lower | Higher | |||
| Isolation by distance (IBD)? | C | F | C | F | |
| 3 | 3 | ✗ | ✓ | ||
| IBD greater in F? | ✓P = 0.004 | ✓P = 0.002 | |||
| Spatial autocorrelation | • Strong pattern of genotypic dissimilarity with distance in F but not C | Positive structure up to 9 km in C vs. 2 km in F | |||
| • Autocorrelation (r) in F significantly lower than in C over smallest (up to 1000 m) and largest (7000–10 000 m) distance classes | |||||
| Patch factors that explain patterning of variables (positive/negative effect) | F IS | Number of barriers (+) | None | ||
| Allelic richness | Distance from C (+) | Area (m2) of surrounding eucalypt within 0.5 km (+) | |||
| Density | Number of barriers (–) | None | |||
Discussion
There is a growing catalogue of measurable impacts of forest fragmentation on organisms, based on demographic and ecological information (e.g. Margules et al. 1994; Davies et al. 2000; Grove 2002a). Increasingly the same can be said for genetic data (e.g. Knutsen et al. 2000; Brouat et al. 2003; Keller & Largiadèr 2003). While our focus is on saproxylic (dead-wood dependent) invertebrates, larger and potentially more mobile species can be impacted by habitat disruption. For example, in as few as 10 generations, prickly forest skinks (Gnypetoscincus queenslandiae) displayed disruption of gene flow in fragmented forest (Sumner et al. 2004). Even organisms that occupy naturally patchy habitat may rapidly show measurable genetic signals of reduced dispersal and gene flow, in the face of removal of natural vegetation (e.g. Cunningham's skink Egernia cunninghami, Stow et al. 2001; Stow & Sunnucks 2004a, b).
To the list of species that show impacts of forest fragmentation, we can now add two wood-boring, flightless tenebrionid beetles in Australian eucalypt forest, fragmented in the previous 21–36 years. The species differences reported here reiterate that the impacts of fragmentation interact with the ecology and biology of the species (Schiegg 2000a, b; Kattan & Murcia 2003). Notwithstanding idiosyncratic responses, Apasis puncticeps and Adelium calosomoides both showed evidence of decreased mobility in the face of replacement of natural forest with plantations of pine. This potentially negative impact is consistent with the results of the majority of ecological studies on saproxylic beetles and other forest organisms (references in the Introduction; Cunningham & Moritz 1998; Zanette et al. 2000).
Population genetic structure in continuous native forest
Adelium calosomoides showed very little genetic structuring in continuous forest over the spatial scale of ∼10 km represented in the samples: little to no genetic differentiation with distance and positive structure up to 9 km as shown by spatial autocorrelation both agreed. As predicted from natural history, Ap. puncticeps showed a more genetically localized structure, which may be due to the apparent preference for or limitation to moister microhabitat. Such microhabitats are relatively rare through a large proportion of BSF. The inferred differences in localization of population structure of the present two target species is consistent with the findings for a large number of organisms, including forest-dwelling carabid beetles, in which habitat specialists (for whom habitat is naturally relatively fragmented) tend to be more strongly structured than generalists (Brouat et al. 2003).
Reduced mobility and gene flow through the pine matrix
Both Ap. puncticeps and Ad. calosomoides showed evidence of reduced mobility and gene flow in fragmented compared to continuous forest, including significantly greater IBD, significantly stronger local structure in spatial autocorrelation, and significant patterning of FIS, allelic richness and density (see below). It is perhaps surprising that common and robust insects could be measurably impacted after 21–36 years of habitat modification. This is likely to represent only in the order of 10 or 15 generations — although their life cycle is very poorly known (E. Matthews, personal communication), many saproxylic beetles have extended life cycles, e.g. the fungivorous beetle Bolitophagus reticulatus is inferred to have a 2-year development cycle (Knutsen et al. 2000). Such a life cycle is likely to apply in the present cases: the species are large and live at generally low temperatures, feeding on decaying wood.
The observed spatial genetic patterns imply that the pine plantation matrix for both beetle species poses a barrier or filter to gene flow and mobility. A major reason that pine plantation is likely to impede mobility is its low levels of leaf litter and mature fallen timber. These materials are relatively rare even in mature pine stands, but essentially absent for some years after initial clearance of native forest and burning of residue, or after harvesting of pine and planting of subsequent rotations. Other factors may also contribute to the pine matrix presenting a barrier: tree genus, diameter of fallen timber and temporal continuity of fallen timber have all shown to be key factors in saproxylic beetle richness and community composition (Grove et al. 2002).
We predicted fragmentation of forest to have a considerably greater impact on the less-mobile Ap. puncticeps than the more desiccation-tolerant Ad. calosomoides (that difference being validated here for the first time, albeit by a fairly crude desiccation/heat tolerance experiment), and this was indeed the case. While Ad. calosomoides showed consistently higher autocorrelation of genotypes in continuous compared to fragmented forest over most distance classes, it was Ap. puncticeps that showed consistent significant negative spatial autocorrelation over the intermediate and larger distances in fragmented but not continuous forest. The FIS, AR and density analyses are relevant here, but are discussed below since they involve impacts of habitat factors.
Predictors of genetic diversity and population density
In the current data set, we detected statistically significant effects of distance from continuous forest and number of barriers (creeks and roads) on Ap. puncticeps allelic richness, density and FIS. Related studies have shown creeks, streams and roads to be strong barriers to gene flow (e.g. in carabid beetles) and reduced genetic variation for a range of different taxa in fragments compared to continuous areas have been reported (e.g. Kane et al. 1992; Keller & Largiadèr 2003; Williams et al. 2003; Wilson & Provan 2003). Similarly, creeks and roads featured in well-supported multiple regression models that explain patterns of FIS and density in Ap. puncticeps.
Increased number of potential barriers was accompanied by reduced density and increased FIS within sites for Ap. puncticeps. This pattern of FIS might be explained if more-isolated fragments receive fewer migrants, favouring stochastic deviations from panmixia by initial founder effect and preservation of transient family structure. The FIS pattern would also be promoted if Ap. puncticeps naturally disperse for distances greater than roads and/or creeks, acting as barriers, allow (this would maintain the stochastic impact and would slow panmixia) or alternatively if inbreeding avoidance is naturally driven by dispersal (rather than by, e.g. kin recognition), and inbreeding occurs as a consequence of impeded dispersal (Stow & Sunnucks 2004b and references). While the finding of increased allelic richness further from continuous forest is potentially surprising at first, it is consistent with the pattern in FIS if it reflects local genetic drift wherein more isolated units, farthest from continuous habitat, retain different alleles by chance and less isolated units, closer to continuous habitat, are less amenable to genetic drift (analogous with the higher retention of allelic diversity in ‘several small’ captive breeding designs than ‘single large’ ones; Frankham et al. 2002). Reduced density of Ap. puncticeps with increased number of barriers integrates well with a range of related studies demonstrating declines in species richness and relative abundance accompanying habitat modification and isolation, particularly for species of low mobility and/or high habitat specificity (see, e.g. Davies et al. 2000; Thomas 2000; Rainio & Niemelä 2003). In a specific example, the density of roads significantly reduced the occupation probability of ponds by the moor frog Rana arvalis (Vos & Chardon 1998).
Adelium calosomoides showed significantly elevated densities in fragmented forest, but not with number of barriers, distance from continuous forest or area of surrounding eucalypt within 500 m. Increased population density after fragmentation are not unusual for forest beetles, but may be artefactual and transitory, for example given the decay of habitat logs that may not be replaced in altered forests (Grove 2002b). Indeed for both species, densities were highest in logs of decay class 2, which reflects field observations that larvae and adults of both species consume hard wood and adults prefer to shelter in structurally strong galleries in logs with existing but fractured outer layers.
The associations detected between number of barriers and Ap. punticeps density and heterozygosity indicate that populations of this species may be further fragmented by increased number of (access) roads usually associated with plantation forests. That density and patterns of FIS and/or allelic richness for Ad. calosomoides were apparently not impacted by roads and/or creeks is likely due to this species being the better disperser and therefore less impacted by fragmentation of forest. Beetles may be forced to travel greater distances in fragmented forest for suitable logs or habitat patches, which would further differentiate impacts on efficient vs. limited dispersers. The slight male-biased dispersal detected for Ad. calosmoides may also increase dispersal of this species relative to Ap. puncticeps if the process is not disrupted by fragmentation and Ad. calosomoides males have to travel further for suitable mates. Despite its dispersal ability, Ad. calosomoides nonetheless showed detectably impeded mobility through patch-and-pine matrix relative to adjacent continuous forest.
Concluding remarks
Spatial analysis of genetic data was able to detect significant impacts on mobility and gene flow in each species even with a relatively weak genetic assay. We take this as an indication of the value of molecular population genetic approaches in detecting impacts of human activities on natural population structures, perhaps in advance of serious ecological and evolutionary adverse effects (see also Stow & Sunnucks 2004a, b). Spatial and genetic data together were able to detect evidence of modest sex-biased dispersal, where genetic data alone did not, highlighting the value of analysing spatial information in population genetic patterning.
In the current analyses, Ad. calosomoides was found to be a much better disperser than was Ap. puncticeps. Both showed significant signals of inhibition of mobility and gene flow in only 21–36 years. While natural habitat removal at BSF has been extensive, there are still substantial networks of riparian vegetation, and a generally high level of connectivity of remnant vegetation (Fig. 1). Thus, the impacts we have detected do not bode well for invertebrates of equal or lesser mobility than the present species in equally or more disturbed environments. As well as the potential for changing genetic structures and altering micro-evolutionary processes, reduced dispersal is likely to decrease the probability of and/or increase the time to recolonization of patches that have suffered stochastic extinction. It should not be assumed that such impacts will not affect species of appreciable mobility such as those studied here: life histories of mobile animals may be geared to require high levels of gene flow to maintain variation and adaptive and evolutionary potential (Waples 1998).
Acknowledgements
This work was done as part of the Honours and PhD research of C.S., and was funded by an Australian Research Council grant (#DP0343560) awarded to P.S. We would like to thank Dr Eric Matthews for the identification of Adelium calosomoides and Apasis puncticeps. We are also grateful to Ryan Garrick, Chester Sands and Jody Taylor for time and effort contributed to the project, and to Andrea Taylor, Sam Banks, Graham Wallis and two anonymous reviewers for comments that greatly improved the ms. Beetles were collected under permit from NSW State Forests whom we thank for logistical support.
References
Christina Schmuki is a PhD student working on fragmentation of log-dwelling invertebrates. Dave Runciman is a research associate with a broad interest in the molecular ecology of Australian animals. Sean MacEachern conducted Honours on the invertebrates at Tumut, and is now working on a PhD in cattle genomics. Christoph Vorburger is a senior research associate at the University of Zürich, Switzerland. He studies evolutionary ecology and genetics, preferentially in organisms with weird reproductive modes. Paul Sunnucks is Senior Lecturer in Zoology whose molecular population biology research programs encompass saproxylic invertebrates.




