Ecology and conservation of common bottlenose dolphins Tursiops truncatus in the Mediterranean Sea
Editor: JD
ABSTRACT
- 1
Bottlenose dolphins Tursiops truncatus are amongst the best-known cetaceans. In the Mediterranean Sea, however, modern field studies of cetaceans did not start until the late 1980s. Bottlenose dolphins have been studied only in relatively small portions of the basin, and wide areas remain largely unexplored.
- 2
This paper reviews the ecology, behaviour, interactions with fisheries and conservation status of Mediterranean bottlenose dolphins, and identifies threats likely to have affected them in historical and recent times.
- 3
Whilst intentional killing was probably the most important cause of mortality until the 1960s, important ongoing threats include incidental mortality in fishing gear and the reduced availability of key prey caused by overfishing and environmental degradation throughout the region. Additional potential or likely threats include the toxic effects of xenobiotic chemicals, epizootic outbreaks, direct disturbance from boating and shipping, noise, and the consequences of climate change.
- 4
The flexible social organization and opportunistic diet and behaviour of bottlenose dolphins may allow them to withstand at least some of the effects of overfishing and habitat degradation. However, dolphin abundance is thought to have declined considerably in the region and management measures are needed to prevent further decline.
- 5
Management strategies that could benefit bottlenose dolphins, such as sustainable fishing, curbing marine pollution and protecting biodiversity, are already embedded in legislation and treaties. Compliance with those existing commitments and obligations should be given high priority.
INTRODUCTION
The common bottlenose dolphin Tursiops truncatus (hereafter ‘bottlenose dolphin’; Fig. 1) has been studied intensively in numerous locations around the world, and today is one of the best known of the 85+ living cetacean species (Leatherwood & Reeves, 1990; Wells & Scott, 1999; Reynolds, Wells & Eide, 2000). In the Mediterranean Sea (Fig. 2), modern cetacean field studies started only in the late 1980s (Notarbartolo di Sciara & Bearzi, 2005) and relatively little is yet known about bottlenose dolphins in the region. Most of the research to date has been conducted in northern portions of the basin, confined to coastal areas.
These common bottlenose dolphins photographed in the eastern Ionian Sea show the characteristic morphology of the species: robust body, short beak, fading brown/grey coloration, white belly (occasionally pinkish), dorsal cape and flank stripes. Photos: (a) Stefano Agazzi, (b, d) Marina Costa, (c) Joan Gonzalvo/Tethys Research Institute.
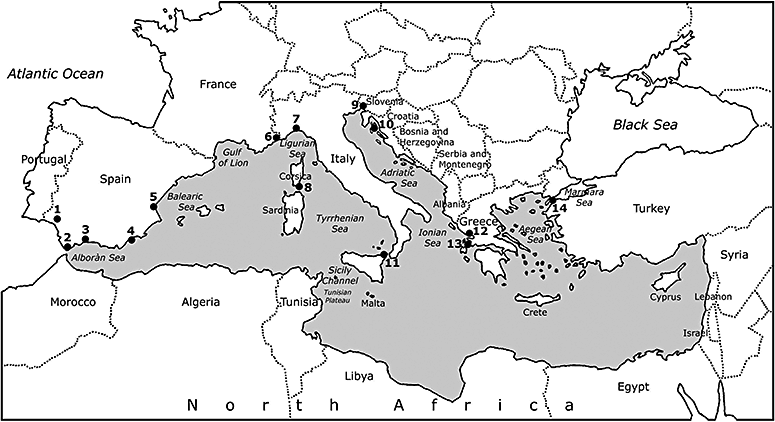
Map of the Mediterranean Sea (highlighted in grey and delimited by the Straits of Gibraltar to the west and by the Dardanelles to the east) showing some of the locations cited in the text. Numbers indicate the following localities: (1) Huelva; (2) Gibraltar; (3) Almería; (4) Gulf of Vera; (5) Valencia; (6) Principality of Monaco; (7) Genoa; (8) Bonifacio; (9) Trieste; (10) Kvarneric, islands of Losinj and Cres; (11) Messina; (12) Amvrakikos Gulf; (13) island of Kalamos; (14) Dardanelles.
A first partial attempt to assess the status of bottlenose dolphins in the Mediterranean was made in the context of a regional Red List workshop in March 2006 (Reeves & Notarbartolo di Sciara, 2006). Participants agreed that the Mediterranean ‘subpopulation’ of bottlenose dolphins qualified as ‘Vulnerable’ according to the International Union for Conservation of Nature (IUCN) Red List criteria (Bearzi & Fortuna, 2006). This proposed classification was based primarily on suspected population declines caused by (i) directed kills and extermination campaigns conducted until at least the early 1960s in portions of the basin and (ii) recent and ongoing incidental mortality in fishing gear (generally known as ‘by-catch’). The assessment also took into consideration ongoing threats from overfishing of prey populations (overlap between prey species of bottlenose dolphins and target species of commercial fisheries has been demonstrated in several cases) and from more generalized habitat degradation, understood to include environmental contamination by chemical pollutants and the disturbance caused by marine traffic (Bearzi & Fortuna, 2006). Although not easily quantified or linked to the survival or fitness of individual animals, these latter factors were suspected of contributing to an overall decline in numbers of bottlenose dolphins in the region.
The scattered and uneven character of the literature on bottlenose dolphins in the Mediterranean complicates understanding of their ecology and population trends, and further delays the already slow process of implementation and enforcement of regional conservation actions. Although some studies of bottlenose dolphins are long-running and of high scientific quality, only a small proportion of the data has been published in peer-reviewed scientific journals. Here, we summarize the published information with the intention of facilitating further studies and stimulating and helping to guide conservation efforts. The available information also includes a number of unpublished abstracts and short contributions from conference proceedings, reports and academic theses or dissertations. Such material is not formally peer-reviewed, often presents preliminary information and is of variable quality, making it difficult or impossible to evaluate its reliability. Most of the ‘grey literature’ has been excluded from this review, although in a few instances, unpublished sources of essential information have been cited.
This article (i) reviews and synthesizes information on the distribution, ecology, behaviour, interactions with fisheries and status of Mediterranean bottlenose dolphins; (ii) identifies threats likely to have affected the dolphin population in both historic and present times; (iii) calls for the enforcement of existing obligations on the part of Mediterranean States with regard to the protection of the marine environment; and (iv) describes other actions needed to protect bottlenose dolphins in the region.
DISTRIBUTION
The bottlenose dolphin is one of the most frequently observed cetaceans in the Mediterranean (Notarbartolo di Sciara & Demma, 2004; Reeves & Notarbartolo di Sciara, 2006). Bottlenose dolphins occur in most coastal waters of the basin and have been reliably reported in the waters of Albania, Algeria, Croatia, Cyprus, France, Gibraltar (United Kingdom), Greece, Israel, Italy, Montenegro, Morocco, Slovenia, Spain, Tunisia and Turkey. They occur regularly around many of the region's offshore islands and archipelagos.
Many of the Mediterranean areas inhabited by these dolphins are subject to intensive human use, e.g. the straits of Gibraltar, Bonifacio and Messina, and the gulfs of Lion, Genoa and Trieste. Within their overall range, gaps with very low densities of animals have been documented, e.g. in the north-western Ligurian Sea (France and Italy) and in the north-western Gulf of Vera (Spain). Variation in density is likely related to several factors, including (i) habitat characteristics, (ii) local availability of suitable prey and (iii) the generally gregarious nature of bottlenose dolphin communities. Moreover, the effects of past extermination campaigns (Bearzi, Holcer & Notarbartolo di Sciara, 2004) and a variety of ongoing threats (see ‘Present threats’) have probably contributed to the patchiness of the current distribution of bottlenose dolphins across the region.
POPULATION STRUCTURE
Of the two species recognized within the genus Tursiops, the common bottlenose dolphin T. truncatus and the Indo-Pacific bottlenose dolphin T. aduncus, only the former is known to occur in the Mediterranean Sea and North Atlantic Ocean (Rice, 1998).
Mediterranean bottlenose dolphins are genetically differentiated from those inhabiting the contiguous eastern North Atlantic Ocean and Scottish waters. Based on nuclear and mitochondrial DNA analyses, distinct populations have been identified across the Black Sea and the Mediterranean Sea (Natoli et al., 2005). The genetic analysis of 145 samples along a continuous distributional range from the Black Sea to the eastern North Atlantic (16 samples from the Black Sea, 74 from the Mediterranean Sea, 35 from the eastern North Atlantic and 20 from Scotland) found population structure with boundaries that coincided with the transitions between different types of habitat. The different zones can be characterized by ocean floor topography and by features such as surface salinity, productivity and temperature. Five populations were identified: Black Sea, eastern Mediterranean, western Mediterranean, eastern North Atlantic and Scottish. The Black Sea population showed the highest differentiation from other populations. Significant genetic differentiation was observed between populations from the eastern and the western Mediterranean. The boundary between the western Mediterranean and the eastern North Atlantic was the weakest observed, although the two populations still showed significant genetic differentiation. Despite no obvious physical barriers, the eastern North Atlantic and the Scottish populations also showed genetic differences. There was genetic evidence of directional emigration of females at the extreme of the range, although neither sex showed a strong bias for greater dispersal (Natoli et al., 2005).
Population structure of bottlenose dolphins around the Iberian Peninsula was investigated through isotopic signatures and organochlorine pollutant loads in tissues of stranded animals from Catalonia, Valencia and the Balearic Islands and adjacent Atlantic waters (Huelva and Portugal; Borrell et al., 2006). Significant differences in stable isotopes of carbon (13C/12C) and in PCB congener profiles indicated that dolphins from the Atlantic and the Mediterranean do not intermingle. In the Mediterranean, dolphins from Catalonia and Valencia were indistinguishable, suggesting a common distribution area. However, dolphins from the Balearic Islands differed from those of mainland Spain in their DDT/PCB ratio and from all the other sample groups in their PCB congener profiles, suggesting that the deep waters between the Balearic Islands and the Iberian Peninsula represent an effective barrier for the species (Borrell et al., 2006).
Evidence of population structure has also been found in other Mediterranean delphinids, including short-beaked common dolphins Delphinus delphis (Natoli et al., 2008), striped dolphins Stenella coeruleoalba (Fossi et al., 2004; Gaspari et al., 2007b) and Risso's dolphins Grampus griseus (Gaspari, Airoldi & Hoelzel, 2007a). These findings suggest not only that the more obvious physical boundaries such as the Strait of Gibraltar (minimum width about 45 km and sill depths less than 145 m) and the Turkish Straits system (Dardanelles minimum width about 450 m and sill depths less than 55 m) represent barriers to the movement of individuals, but also that the much wider Sicily Channel (143 km and sill depths less than 200 m), oceanic features such as the Almería-Orán front (Tintoréet al., 1988), and more generally, differences in habitat characteristics restrict the movements of bottlenose dolphins (Natoli et al., 2005). Further evidence of genetic discontinuities, especially in relation to the Almería-Orán front, comes from mtDNA studies on other highly mobile top predators such as bluefin tuna Thunnus thynnus (Carlsson et al., 2004) and Atlantic bonito Sarda sarda (Viñas, Alvarado Bremer & Pla, 2004) as well as from a variety of bottlenose dolphin prey species (Chikhi, Agnese & Bonhomme, 1997; Rolda'n et al., 1998; Naciri et al., 1999; Pérez-Losada et al., 2002; Zardoya et al., 2004; Bargelloni et al., 2005; Atarhouch et al., 2006; Magoulas et al., 2006).
NUMBERS AND POPULATION TRENDS
Little is known about the numbers of bottlenose dolphins in the Mediterranean Sea. There is no basin-wide estimate and the most reliable information comes from a few local studies (Table 1). Numbers in the published and unpublished literature are based on different methodological approaches, including estimates of absolute abundance obtained through mark-recapture methods or Generalized Additive Models (GAMs), maximum number of photo-identified individuals, and discovery curves (i.e. curves showing the cumulative number of individuals identified as a function of photo-identification effort, usually expressed as survey days with photos). Useful information on past and present occurrence also comes from stranding records from Algeria, Croatia, France, Greece, Italy, Malta, Morocco, Spain, and Tunisia (however, a stranded carcass is not necessarily indicative of the dead animal having lived nearby). Virtually nothing is known for large portions of the south-eastern part of the basin.
| Absolute measures | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Geographic area | Study area (km2) | Area type | Years | Density (animals / km2) | n | CV | 95% CI | Estimation method | Source |
| Alborán Sea (Spain) | 11 821 | In- and offshore | 2000–03 | 0.049 | 584 | 0.28 | 278–744 | Distance sampling and GAMs | Cañadas & Hammond, 2006 |
| Almería (Spain) | 4 232 | In- and offshore | 2001–03 | 0.066 | 279 | 0.28 | 146–461 | Distance sampling and GAMs | Cañadas & Hammond, 2006 |
| Spanish waters between Gulf of Valencia and Gulf of Vera | 32 270 | In- and offshore | 2001–03 | 0.041 | 1333 | 0.31 | 739–2407 | Distance sampling | Gomez de Segura et al., 2006 |
| Balearic Islands (Spain) | 16 659 | Inshore | 2002 | 0.085 | 1030 | 0.35 | 415–1849 | Distance sampling | Forcada et al., 2004 |
| Balearic Islands and Catalan Sea (Spain) | 86 000 | In- and offshore | 2002 | 0.088 | 7654 | 0.47 | 1608–15766 | Distance sampling | Forcada et al., 2004 |
| Tunisian waters | ∼750 | Inshore | 2001 & 2003 | 0.19 | – | – | – | Distance sampling (uncorrected) | Ben Naceur et al., 2004 |
| Asinara Island (Italy) | 480 | Inshore | 2001 | – | 22 | 0.26 | 22–27 | Mark-recapture (closed population) | Lauriano, Mackelworth & Fortuna, 2003 |
| Amvrakikos Gulf (Greece) | 400 | Inshore | 2005 | 0.37 | 148 | – | 132–180 | Mark-recapture (closed population) | Bearzi et al., 2008a |
| Relative measures | |||||||
|---|---|---|---|---|---|---|---|
| Geographic area | Study area (km2) | Area type | Years | Encounter rate | Max # of photo-identified | Additional information | Source |
| Strait of Gibraltar | 1670 | In- and offshore | 2001–04 | 1.3 groups/100 km | – | Frequent association with long-finned pilot whales | De Stephanis et al., 2008 |
| North-eastern Adriatic Sea (Kvarneric, Croatia) | 800 | Inshore | 1990–94 | 1.4 groups/100 km | 106; discovery curve asymptotic at about 100 marked individuals | Population size estimated at about 140 based on 30% of unmarked individuals | Bearzi et al., 1997 |
| Eastern Ionian Sea (Greece) | 480 | Inshore | 1997–2004 | 0.6 groups/100 km | 48; discovery curve not asymptotic due to transients | ∼20 resident; others transient | Bearzi et al., 2005, 2006 |
- GAMs, Generalized Additive Models.
Most studies in coastal waters are limited to relatively small areas of 400–1000 km2 and probably do not cover the entire range of the groups under study. Relatively recent, broad-scale shipboard surveys (4000–80 000 km2) showed that in some Mediterranean areas bottlenose dolphins are present both near shore and offshore, and densities can range between 4 and 20 animals per 100 km2 (Ben Naceur et al., 2004; Forcada et al., 2004; Cañadas & Hammond, 2006; Gomez de Segura et al., 2006; and see Table 1). Studies tend to focus on areas of relatively high dolphin density, although study site preferences may also depend on logistical and other considerations. In this situation, the total population size in the Mediterranean remains uncertain, but it is unlikely to exceed the low 10 000 s (Bearzi & Fortuna, 2006).
Indirect but convincing evidence of dolphin abundance in historical times can be found in early accounts describing interactions with fisheries and systematic attempts to exterminate dolphins (including bottlenose dolphins) in Mediterranean coastal waters (De Marchesetti, 1882; Gourret, 1894; Del Rosso, 1905; Cuculic, 1960; Stoka, 1962; and see ‘Past culling campaigns’ below).
The only Mediterranean area with quantitative historical information that can be used to infer population trends is the northern Adriatic Sea. There, bottlenose dolphin numbers probably declined by at least 50% in the second half of the 20th century, largely as a consequence of deliberate killing initially, followed by habitat degradation and overfishing of prey species (Bearzi et al., 2004; Bearzi & Fortuna, 2006). For other parts of the northern Mediterranean, e.g. Spain, Italy and southern France, the available information is less precise but suggests similar trends (Blanco & González, 1992; Borrell et al., 2000). The nature and extent of threats vary with region, but the overall pattern has led to the suspicion that the Mediterranean population has been reduced by at least 30% over the last 60 years (Reeves & Notarbartolo di Sciara, 2006).
ECOLOGY AND BEHAVIOUR
Bottlenose dolphins in the Mediterranean are often regarded as predominantly ‘coastal’ or ‘inshore’ animals but this designation may be misleading as they can be encountered in continental shelf and shallow plateaux waters at any distance from the coast (Notarbartolo di Sciara et al., 1993; Bearzi et al., 2004; Ben Naceur et al., 2004; Gomez de Segura, Tomas & Raga, 2004; Gannier, 2005; Gnone et al., 2006; Azzellino et al., 2008b). They inhabit a wide variety of habitats including continental shelf waters (Gomez de Segura et al., 2006; Azzellino et al., 2008b), lagoons and enclosed seas (Bearzi et al., 2008), and the waters surrounding islands and archipelagos (Bearzi, Notarbartolo di Sciara & Politi, 1997; Mussi & Miragliuolo, 2003; Forcada et al., 2004; Fortuna et al., 2007). In the Alborán and Balearic Seas they occur across the entire shelf, along the shelf edge and in deep waters of the continental slope, and in productive waters 200–500 m deep (Cañadas, Sagarminaga & García-Tiscar 2002; Forcada et al., 2004; Cañadas et al., 2005). In the Strait of Gibraltar, at or near the western limits of what is defined here as the Mediterranean region, bottlenose dolphins occur mainly in water 200–600 m deep, over steep slopes (de Stephanis et al., 2008). In Greek waters they are found in coastal areas, straits, gulfs, semi-closed eutrophic waters, and steep coasts with no continental shelf (Frantzis et al., 2003; Bearzi et al., 2005, 2008a).
Genetic or other evidence of coastal and offshore ‘ecotypes’, as found in other areas (e.g. Duffield, Ridgway & Cornell, 1983; Van Waerebeek et al., 1990; Mead & Potter, 1995), has not been reported in the Mediterranean (Natoli et al., 2005). However, there has not been an appropriate effort to investigate this possibility using a large sample of specimens, as has been attempted, e.g. in striped dolphins (Gaspari et al., 2007b). Movements into and across pelagic waters may occur, and bottlenose dolphin groups are sighted occasionally in offshore waters deeper than 2000 m. At the other extreme, bottlenose dolphins sometimes enter sea enclosures, estuaries, lagoons and rivers (Bearzi et al., 2004; Sackl, Smole & Saveljic, 2007). In the semi-closed Amvrakikos Gulf, a 400 km2 lagoon in western Greece, bottlenose dolphins show high levels of site fidelity and have adapted to murky eutrophic waters about 30 m deep (Bearzi et al., 2008a).
The size of bottlenose dolphin groups varies according to biogeographic region, prey availability, activity and other factors. Most encounters have been with groups of fewer than 10 individuals (Table 2). As the definition of ‘group’ can vary among studies and sampling methods are often undefined, group sizes in Table 2 (and those reported in a number of unpublished contributions) represent relative values that cannot be directly compared.
| Geographic area | Area | Years | n | Mean | Error | Range | Platform | Source |
|---|---|---|---|---|---|---|---|---|
| Strait of Gibraltar | In- and offshore | 2001–04 | 59 | 23.5 | S.D. = 33.3 | 1–200 | Motorboat | De Stephanis et al., 2008 |
| Spanish waters between Gulf of Valencia and Gulf of Vera | Inshore | 2000–02 | 19 | 10.8 | – | 2–40 | Airplane | Gomez de Segura et al., 2006 |
| Balearic Islands (Spain) | Offshore | 2002 | 6 | 6.9 | CV = 0.15, 95% CI = 5.11–9.37 | 2–29 | Airplane | Forcada et al., 2004 |
| Balearic Islands and Catalan Sea (Spain) | Inshore | 2002 | 19 | 6.4 | CV = 0.20, 95% CI = 4.14–9.11 | 1–15 | Airplane | Forcada et al., 2004 |
| Asinara Island (Italy) | Inshore | 2001 | 15 | 4.9 | S.D. = 3.0 | 1–10 | Motorboat | Lauriano et al., 2003 |
| North-eastern Sardinia (Italy) | Inshore | 1999–2004 | 437 | 4.95 | S.E. = 0.3 | 1–20 | Motorboat | Díaz López, 2006b |
| Villasimius, Tyrrhenian Sea (Italy) | Inshore | 1996–97 | 39 | 2.7 | S.D. = 2.0 | 1–8 | Motorboat | Arcangeli, Marini & Nannarelli, 1997 |
| Maddalena Island, Tyrrhenian Sea (Italy) | Inshore | 1995–96 | 11 | 1.7 | S.D. = 0.7 | 1–3 | Motorboat, land | Arcangeli et al., 1997 |
| Tavolara Island, Tyrrhenian Sea (Italy) | Inshore | 1995–96 | 13 | 2.3 | S.D. = 1.9 | 1–7 | Motorboat, land | Arcangeli et al., 1997 |
| Golfo Aranci, Tyrrhenian Sea (Italy) | Inshore | 1995–96 | 92 | 2.6 | S.D. = 1.9 | 1–10 | Motorboat | Arcangeli et al., 1997 |
| Aeolian Islands (Italy) | Inshore | 2002–03 | 5 | 12.0 | S.D. = 7.0 | 3–20 | Ship | Fortuna et al., 2007 |
| Delta of Bojana/Buna river (Albania and Montenegro) | Inshore | 2003–04 | 4 | – | – | 1–6 | Motorboat | Sackl et al., 2007 |
| North-eastern Adriatic Sea (Kvarneric, Croatia) | Inshore | 1990–94 | 787 | 6.8 | S.D. = 5.9, S.E. = 0.2 | 1–65 | Motorboat | Bearzi et al., 1997 |
| Italian waters | In- and offshore | 1986–89 | 67 | 6.64 | S.D. = 7.7, S.E. = 0.9 | 1–40 | Motorsailer | Notarbartolo di Sciara et al., 1993 |
| Eastern Ionian Sea (Greece) | Inshore | 1993–2003 | 364 | 6.8 | S.D. = 4.2, S.E. = 0.2 | 1–24 | Motorboat | Bearzi et al., 2005 |
Associations with other cetacean species are uncommon, although in some areas mixed aggregations with short-beaked common dolphins (Notarbartolo di Sciara et al., 1993; Bearzi & Notarbartolo di Sciara, 1995; Cañadas et al., 2002; Bearzi et al., 2003) and long-finned pilot whales Globicephala melas (Cañadas et al., 2002; de Stephanis et al., 2008) have been observed.
The only available information on the behaviour and social organization of bottlenose dolphins in the Mediterranean comes from groups living in coastal waters, and little is known about offshore groups. In two coastal studies, groups were highly fluid, and a change in group size and/or composition occurred on average every hour (Bearzi et al., 1997, 2005). Little is known about social organization. In at least one study, sexual segregation did not appear to be the rule, as groups included animals from both sexes (Bearzi et al., 1997).
Lone ‘sociable’ animals (Lockyer, 1990) that leave their affiliates and establish residency in ports or bays, where they may interact regularly with humans, have been reported fairly often in the Mediterranean. Although these occurrences often end prematurely when the animal is killed, some individuals have survived for several years under unusual circumstances (Müller et al., 1998; Müller & Bossley, 2002).
The behaviour of bottlenose dolphins can differ considerably depending on the habitat in which the animals are living (Shane, Wells & Würsig, 1986). Feeding habits, in particular, seem to shape the behaviour of these dolphins, whose diet has been described as catholic or opportunistic (e.g. Leatherwood, 1975; Barros & Odell, 1990). In Mediterranean coastal waters, bottlenose dolphins target primarily demersal prey (e.g. Miokovic, Kovacic & Pribanic, 1999; Blanco, Salomón & Raga, 2001) during feeding sessions characterized by dives lasting 3–5 min and occasionally up to 8 min, depending on water depth (Bearzi, Politi & Notarbartolo di Sciara, 1999; Bearzi et al., 2005). Reported prey items include demersal species such as European hake Merluccius merluccius, European conger Conger conger, red mullet Mullus barbatus, striped red mullet Mullus surmuletus, common cuttlefish Sepia officinalis, common octopus Octopus vulgaris and a variety of other bony fishes and molluscs. As most studies have relied on stomach contents from stranded animals, inferences may be subject to bias (Barros & Clarke, 2002). In some Mediterranean areas, bottlenose dolphins also feed on Clupeidae or other epipelagic prey (Díaz López, 2006a; Bearzi et al., 2008a). Diet and foraging behaviour appear to vary widely depending on area, season or trophic niche occupied by the local dolphins. Even geographically contiguous groups may show dramatically different foraging behaviour and prey preferences. For instance, epipelagic species including European pilchard Sardina pilchardus and round sardinella Sardinella aurita are important prey for bottlenose dolphins living in the Amvrakikos Gulf (Bearzi et al., 2008a), whilst those around the Greek island of Kalamos – less than 100 km distant – predominantly target demersal prey (Bearzi et al., 2005). In north-eastern Sardinia, Italy, bottlenose dolphins were observed feeding on wild fish attracted by a fish farm facility, including flathead mullet Mugil cephalus, salema Salpa sarpa, European pilchard and garpike Belone belone, and occasionally on farmed fish escaping from cages or being discarded at sea, including European seabass Dicentrarchus labrax, gilthead seabream Sparus aurata and brown meagre Sciaena umbra (Díaz López, 2006a).
Predation by sharks is a significant cause of mortality of bottlenose dolphins (Tursiops spp.) and other small cetaceans in several areas around the world (e.g. in Australia: Corkeron, Morris & Bryden, 1987; Mann & Barnett, 1999; Heithaus, 2001; in the south-eastern United States: Wood, Caldwell & Caldwell, 1970; Wells, Scott & Irvine, 1987). In the Mediterranean, however, predation is regarded as a minor cause of mortality. Bottlenose dolphins, as well as other small Mediterranean delphinids, are preyed upon occasionally by sharks, principally great white sharks Carcharodon carcharias but also several other species (Fergusson, 1994; Morey et al., 2003). The numbers of large sharks have declined drastically in Mediterranean waters over the past century (Ferretti et al., 2008) and this may have reduced predation pressure considerably. Other potential dolphin predators, such as the killer whale Orcinus orca and the false killer whale Pseudorca crassidens, are rare in the Mediterranean (Notarbartolo di Sciara, 1987; Reeves & Notarbartolo di Sciara, 2006).
IMPACT ON FISHERIES
In this section, we consider a range of activities by bottlenose dolphins that may result in real or perceived economic losses for Mediterranean fishermen. Targeted kills of dolphins and incidental mortality of dolphins in fishing gear, i.e. ‘interactions’ with fisheries that cause damage to dolphins, are treated separately under ‘Past culling campaigns’ and ‘Present threats’.
Gear damage and depredation
Consistent with their varied diet (Blanco et al., 2001) and adaptable behaviour (Shane et al., 1986; Leatherwood & Reeves, 1990; Reynolds et al., 2000), bottlenose dolphins in the Mediterranean have become involved in depredation of fish in various fishing gear, most notably gill and trammel nets (Lauriano et al., 2004; Lauriano & Bruno, 2007; Brotons, Grau & Rendell, 2008; Gazo, Gonzalvo & Aguilar, 2008). Reports of bottlenose dolphins either removing or damaging the catch, damaging fishing gear and disturbing fishing activities come from several Mediterranean areas, but the available information is largely unpublished and sometimes difficult to evaluate. Impacts have been reported on catch per unit effort of species such as striped red mullet, red mullet, common sole Solea solea, blotched picarel Spicara maena, European anchovy Engraulis encrasicolus, European pilchard, greater amberjack Seriola dumerili, common cuttlefish and the small octopus Eledone sp. As a note of caution, the link between depredation events (e.g. gear damage) and actual involvement of dolphins may be inferred rather than directly observed or proven. In at least some cases, damage caused by other species (such as fish, sharks or invertebrates) or even by entanglement with bottom debris or natural substrate may be wrongly ascribed to dolphins (Gazo et al., 2008). In one study in the Balearic Islands, there was a large increase of catch per unit effort of common octopus in the presence of dolphins and investigators speculated that some cephalopods may be attracted to nets by odours released from damaged fish after dolphin depredation (Bozzano & Sardà, 2002; Brotons et al., 2008).
Gear damage, reduced catch, and loss of fishing time (Reeves, Read & Notarbartolo di Sciara, 2001) seem primarily to affect seasonal fisheries (Lauriano et al., 2004; Gazo et al., 2008), but year-round fisheries are also affected (Brotons et al., 2008). The combined cost of catch loss and net damage caused by bottlenose dolphins to small-scale fisheries in the Balearic Islands in 2001–03 was estimated as 6.5% of the total catch value (95% CI: 1.6–12.3%) and the annual loss as 3.4% of the total catch by weight (95% CI: 0.1–6.5%; Brotons et al., 2008). Another study conducted in the Balearic Islands in September–October 2001 (coincident with the red mullet fishing season) reported economic damage caused by dolphins of 1094 Euro per trammel boat (Gazo et al., 2008). In a study carried out in north-eastern Sardinia, the catch loss in 1999–2001 was estimated to be about 1170 Euro per boat per fishing season based on direct observations (Lauriano et al., 2004). Unpublished studies conducted in Morocco reported annual economic losses to bottlenose dolphins in the purse seine fishery as high as 36%, with annual losses per ship owner varying between 9 and 19% (Najih, 2003; Zahri et al., 2004). Significant economic losses also have been reported in unpublished studies in Italy and Greece.
The most informative studies are those that provide relative rather than absolute estimates of economic loss because they give a basis for evaluating significance with regard to profitability. Therefore, studies focusing on the damage caused by dolphins to fisheries should provide information that helps frame the reported losses in the appropriate socio-economic context (as done, e.g. by Brotons et al., 2008). Interviews with fishermen are only the first (and often biased) step in studies aiming to evaluate the actual nature and extent of damage (Smith, 1995; Reeves et al., 2001). Estimates of damage in local areas are highly variable (depending, e.g. on method, duration or timing of the study) and cannot necessarily be extrapolated to other portions of the basin, or to other seasons or years. Moreover, it is important to consider that studies tend to focus on areas of relatively severe conflict, and that situations where interactions are infrequent or non-existent may not be studied (and even when they are, the results may not reach print).
Impact on trawlers
In many areas around the world, bottlenose dolphins have learned to follow bottom trawlers to take advantage of fish caught, stirred up and attracted by the net, or discarded at sea after hauling (Leatherwood, 1975; Fertl & Leatherwood, 1997). This kind of behaviour has also been observed in several Mediterranean areas, including the Alborán Sea, Balearic Islands, Sardinia, Corsica, Tyrrhenian Sea, Lampedusa Island, the northern Adriatic Sea, and Israel. In the northern Adriatic Sea, bottlenose dolphins have been estimated to spend around 5% of their time following trawlers (Bearzi et al., 1999) and in some areas, they have been observed feeding on discarded fish. It is sometimes claimed that bottlenose dolphins cause reduced catches when they feed behind trawlers. However, few Mediterranean studies have investigated differences in trawl catch with or without dolphins present, although one unpublished investigation in the northern Adriatic indicated a significant reduction in catch per unit effort of Eledone sp. (Casale & Giovanardi, 2001).
Impact on aquaculture facilities
Bottlenose dolphin visits to aquaculture facilities in the Mediterranean appear to be occurring with increasing frequency, probably owing to the rapid expansion of fish farming in coastal waters (EAA/UNEP, 2000; UNEP/MAP/MEDPOL, 2004; Karakassis, Pitta & Krom, 2005) and to the opportunistic behaviour of the dolphins. Increased nutrient levels, complex substrate and provision of fish-feed in the proximity of the cages trigger trophic enrichment and can attract potential bottlenose dolphin prey (Karakassis et al., 2000; Würsig & Gailey, 2002; Kemper et al., 2003; Dempster et al., 2004). Bottlenose dolphins have been observed visiting fish farm cages in search of prey occasionally or regularly in several coastal areas in the northern Mediterranean (e.g. Díaz López & Bernal Shirai, 2007). Whether reported or not, visits of this kind are likely to occur in most Mediterranean areas where coastal fish farming overlaps with bottlenose dolphin distribution. Observations made in a single study have included dolphins catching farmed fish that had escaped from the cages, targeting fish that had escaped from nets during transfer operations from one cage to another, and even consuming dead, discarded fish (Díaz López, 2006a).
There is little information on whether bottlenose dolphins cause damage to fish farm infrastructure in Mediterranean areas. In most cases where dolphins have been seen feeding near fish farms, no damage has been reported. A single study consisting of about 5 hours of observations around a fish farm in north-western Sardinia reported three cases of dolphins biting and making small holes in the nets (Díaz López, 2006a). There, fish farmers also claimed that bottlenose dolphins caused stress to farmed fish but there was no evidence to validate this claim (Díaz López, 2006a). Antipredator nets have been deployed at coastal fish farms in north-eastern Sardinia to keep bottlenose dolphins away (Díaz López & Bernal Shirai, 2007), resulting in some dolphin mortality (see ‘Fishery related mortality’).
No information seems to be available regarding the occurrence and behaviour of bottlenose dolphins around other kinds of aquaculture facilities commonly used in the Mediterranean, e.g. shell farming (reported to result in habitat loss for small cetaceans elsewhere; Würsig & Gailey, 2002; Kemper et al., 2003; Watson-Capps & Mann, 2005), tuna farming and fattening in offshore areas.
Mitigation of dolphin depredation
A widely used method to reduce depredation and net damage by marine mammals is the deployment of acoustic devices: acoustic harassment devices (AHDs), acoustic deterrent devices (ADDs, including ‘pingers’) or acoustic mechanical systems (e.g. the ‘dolphin tube’; Zahri et al., 2004). Pingers, in particular, have become increasingly popular, and have been used in Mediterranean coastal areas with varying and sometimes contrasting outcomes in relation to different brands, types and duration of deployment. A number of European Commission-funded projects have focused on, or included, studies of pinger use as a way of reducing depredation by dolphins. One recent study found that nets equipped with functional pingers received less damage (87% fewer holes) than nets with non-functional devices or without pingers (Gazo et al., 2008).
Concern has been expressed that the widespread use of acoustic deterrent devices could result in habitat loss for cetacean populations (International Whaling Commission, 2000, p. 204; Carlström et al., 2002). Indeed, some evidence suggests that pingers (low power ADDs, as opposed to AHDs) have exclusion effects on harbour porpoises Phocoena phocoena (Culik et al., 2001). However, no clear effects have been reported so far for bottlenose dolphins. There is also potential for a ‘dinner bell’ effect resulting in increased interactions after habituation (as reported for pinnipeds; Richardson et al., 1995), given the highly opportunistic character of these dolphins (Reeves et al., 2001).
Food-web effects on fishery yields
There has been much debate about whether dolphins ‘compete’ with fisheries and affect fishery landings to a significant extent. Views vary depending on individual perspectives, perceptions and agendas. The belief that cetaceans reduce fishery yields is often based on ‘common sense’ scenarios that are overly simplistic and do not consider the enormous complexity of marine food-webs (Lavigne, 2003). Although there is undisputed evidence of widespread ecosystem damage caused by overfishing (see ‘Prey depletion’), there is no evidence that dolphins negatively affect fishery yields through ‘food-web competiton’ (Trites, Christensen & Pauly, 1997). In addition, it has never been demonstrated that the culling of cetaceans or other high-order predators improves fishery yields (Plaganyi & Butterworth, 2002). A great deal of uncertainty surrounds the subject of ecological interactions in marine systems. Such systems consist of numerous trophic levels with species that interact in multiple ways, and the influence of a particular predator on the production of a commercially desirable prey is hard to determine. Healthy and diverse ecosystems typically include thriving stocks of both predators and prey, and the assumption that natural predators may be removed and/or replaced for the benefit of fisheries is hard to support on scientific (let alone ethical) grounds.
In some Mediterranean areas, ecosystem damage resulting from overfishing and habitat degradation (Briand, 2000) has probably exacerbated the perception that dolphins reduce fishery yields (Northridge, 1991; Reeves et al., 2001). Despite suggestions by some scientists and claims by some fishermen, no robust scientific investigations exist to confirm that present-day bottlenose dolphin populations, in the Mediterranean and elsewhere, reduce fishery catches by removing fish that would otherwise be available to fishermen. One recent analysis of ecosystem structure and fishing impacts in the Adriatic Sea suggested that the role of dolphins is minor and the greatest pressure comes from fisheries (Coll et al., 2007). In another study in the eastern Ionian Sea, the total estimated biomass removed by both bottlenose dolphins and short-beaked common dolphins (the only two cetacean species regularly present in the area) amounted to about 3% of that removed by local fisheries (Bearzi et al., 2008b; Tethys Research Institute, unpublished data). (Correction added after online publication 22 Dec 2008: 0.03% changed to 3%.)
PAST CULLING CAMPAIGNS
Numerous early reports and artefacts from the Mediterranean refer to dolphins interacting with fishermen and people. What has been portrayed as a rather idyllic relationship appears to have changed dramatically as fisheries developed in the region. In 1587, a Papal Decree was issued ‘anathematizing the vermin’ in response to concerns in France about the effects of dolphins on fisheries. Starting in the late 18th century, dolphins came to be regarded as competitors and a major concern of fishery managers. Hence, new means were developed and deployed to kill the largest possible number of dolphins (Smith, 1995). Nineteenth century reports describe attempts by fishermen to keep dolphins away from their nets using, e.g. loud noises, weapons, modifications of fishing techniques and schedules, and large-mesh nets surrounding the fishing nets to protect them from dolphin incursions (Bearzi, 2002). Direct killing (culls) and bounties were supported by several governments for at least a century (Smith, 1995). Conflicts with fisheries reportedly were acute in several areas off Spain (A. Aguilar, personal communication), France (Gourret, 1894; Duguy et al., 1983; Bompar, 2000), Italy (Barone, 1895; Del Rosso, 1905; Bearzi et al., 2004), and particularly the former Yugoslavia (today's Slovenia, Croatia, Serbia and Montenegro) where thousands of bottlenose dolphins and short-beaked common dolphins were killed and landed for state bounties (Bearzi et al., 2004).
For most areas, the historical record is far from complete. The northern Adriatic Sea is an exception (Bearzi et al., 2004). Dolphins there were long viewed as pests deserving systematic extermination. Although most of the records fail to specify the species involved, in a few cases there is clear reference to both short-beaked common dolphins and bottlenose dolphins (Faber, 1883; Brusina, 1889; Ninni, 1901; Bearzi et al., 2004). The first record of a monetary reward offered for a killed dolphin dates back to 1872 (Crnkovic, 1958). No information is available to indicate the scale and duration of the first culling campaigns, but at least 335 dolphins were killed between 1933–35 (Crnkovic, 1958). The main campaign was launched in 1949, with the intent of eradicating dolphins from the region. Reported landings include 788 dolphins killed between 1955–60. Of these, 153 were killed in the district of Rijeka (at that time a relatively small city) between 1956–57 (Crnkovic, 1958; Marelic, 1961). There is no record of bounties after 1960. However, even after that time, fishermen in the eastern Adriatic often carried guns on board and shot dolphins frequently (Bearzi et al., 2004). At least until the early 1960s the animosity towards dolphins was tremendous, and every opportunity was taken to harm them (Peksider-Srica, 1931; Cuculic, 1960; Marelic, 1961). Although it is true that the perception of dolphins as mere competitors and game trophies progressively changed in subsequent years, deliberate killing remained legal until 1995 when all marine mammals became protected under Croatian law. Bounty hunting also occurred in Italy from the early 1930s (Brunelli, 1932). In the late 1950s and early 1960s, the reward for killing a dolphin was said to be equivalent to an entire week's pay for an Italian fisherman (Stanzani & Piermarocchi, 1992). An unknown number of dolphins were caught in the northern Adriatic between 1964–78 for live display in captive facilities (Greenwood & Taylor, 1978; Duguy et al., 1983). Also, killing dolphins for food or sport was a relatively common practice in Italy until a few decades ago, and it was only in 1979 that the government prohibited unauthorized killing (Bearzi et al., 2004).
Although the available numbers refer only to a few years in a series of culling campaigns that encompassed almost a century, there is no doubt that a great number of dolphins were killed along the eastern Adriatic coast alone between 1930–60, especially when wounded animals that sank or escaped and died later are taken into account (Mitchell, 1975; Bearzi et al., 2004). In the 1950s, dolphins (including bottlenose dolphins and short-beaked common dolphins) in the eastern portion of the Adriatic were said to number in the thousands (Crnkovic, 1958; Marelic, 1961). Although such claims have no scientific basis, they are nevertheless consistent with the high numbers reportedly killed and they support the general perception of substantial abundance in the recent past. Today, the available evidence suggests that the number of bottlenose dolphins in the entire Adriatic is unlikely to exceed a few hundred.
PRESENT THREATS
Owing to their occurrence in coastal waters, bottlenose dolphins in the Mediterranean are exposed to a wide variety of human activities. Whilst intentional killing was likely the most important cause of mortality until the 1960s (see previous section), important ongoing threats include incidental mortality in fishing gear and the reduced availability of key prey caused by region-wide overfishing and environmental degradation. Additional potential or likely threats include the toxic effects of xenobiotic chemicals, epizootic outbreaks, direct disturbance from boating and shipping, noise, and the consequences of climate change. It is worth noting that this same array of known and potential threats applies to riverine, estuarine and coastal cetaceans in many other parts of the world as well (e.g. Reeves et al., 2003).
Fishery related mortality
Due to their opportunistic behaviour and predominantly coastal occurrence, bottlenose dolphins in the Mediterranean are at risk of entanglement in many types of fishing gear. In addition to incidental mortality, depredation and damage caused by dolphins to fishing gear may result in animals being shot or harassed in retaliation (Di Natale & Notarbartolo di Sciara, 1994). However, depredation and damage to gear does not always lead to open hostility. Attitudes towards dolphins along the Mediterranean coasts vary widely according to cultural, religious and other factors. Most fishermen from Libya, for instance, respect dolphins for traditional reasons, reportedly ‘accept’ gear damage and depredation, and claim that they would never kill a dolphin (Bearzi, 2006). Even when not part of the local cultural heritage, some degree of tolerance may develop depending on education, awareness and attitude towards nature (Lavigne, Scheffer & Kellert, 1999).
Incidental mortality of bottlenose dolphins has been reported from Algeria, Croatia, France, Greece, Israel, Italy, Malta, Morocco, Spain, Tunisia and Turkey (Di Natale & Notarbartolo di Sciara, 1994; Roditi-Elasar et al., 2003; Tudela et al., 2005; Díaz López, 2006b; Van Canneyt & Peltier, 2006; Brotons et al., 2008). By-catch in set nets reportedly is frequent in coastal waters throughout the basin (Di Natale & Notarbartolo di Sciara, 1994; Díaz López, 2006b; Brotons et al., 2008). Significant mortality also was reported in pelagic driftnets off Morocco, Spain, Malta, Italy and Turkey (Di Natale & Notarbartolo di Sciara, 1994; Di Natale, 1995).
Few attempts have been made to assess the impact of fishery-related mortality on local populations of bottlenose dolphins (Díaz López, 2006b; Díaz López & Bernal Shirai, 2007; Brotons et al., 2008); the actual magnitude of by-catch and retaliation events is unknown in most cases. Even when they are available, by-catch estimates are partial in terms of geographic and gear coverage. However, the available studies and circumstantial evidence for local populations raise serious concern, suggesting that annual fishery-induced mortality is locally unsustainable in at least some cases (e.g. Brotons et al., 2008). Rigorous studies of by-catch rates using reliable methods, which must normally include on-board observers and a statistically robust sampling design, are needed to obtain credible estimates of mortality. It is then required that ‘sustainability’ be determined by reference to the population size, and taking into account other existing threats.
Data from strandings can be informative with regard to the occurrence and relative scale of by-catch. Along the Italian coasts in the years 1986–2005, bottlenose dolphins were the second most numerous species classified as bycaught after the striped dolphin (71 and 301 records, respectively; Podestà, 2007). Of 694 bottlenose dolphins stranded in Italy during the same period, 71 (11%) showed signs of by-catch (Podestà, 2007). Of a total of 21 bottlenose dolphins stranded in France in 2003, eight reportedly had been bycaught (Dhermain, 2003). Signs included specimens gutted, missing peduncles or fins, obvious net scars, and/or ropes tied to the tails. These percentages are probably underestimates, considering that the majority of the remaining carcasses were in an advanced state of decomposition and therefore signs, if present, would not necessarily have been observable.
By-catch in trawl nets appears to be infrequent in most Mediterranean areas, but may be locally significant. For instance, of 67 bottlenose dolphins found dead, stranded or adrift along the coasts of Israel between 1993 and 2004, 26 (39%) were judged to have been taken in trawl nets (Feingold et al., 2005).
Bottlenose dolphins have not been reported entangled in fish farm gear in the Mediterranean, except when antipredator nets (mesh size 15 cm) are deployed (Díaz López & Bernal Shirai, 2007).
The overall frequency of intentional killing (see ‘Past culling campaigns’) has drastically declined over the years, due in part to protective legislation in most Mediterranean countries. However, targeted kills still occur in certain areas (e.g. Tudela, 2004; Gazo et al., 2008). In addition to killing in retaliation to damage to fisheries, killing with harpoons or guns for local consumption of meat was reported as recently as the early 1990s in the Ligurian and Tyrrhenian seas, notwithstanding legal protection (Di Natale, 1991; Di Natale & Notarbartolo di Sciara, 1994). Such occurrences seem to have become rare in more recent times.
The illegal use of dynamite for fishing in several Mediterranean areas (e.g. Reynolds et al., 1994; Tudela, 2004) represents another fishery-related threat to bottlenose dolphins. Though impact at the basin level is probably low, it may be significant locally and a few bottlenose dolphin deaths suspected to have been caused by explosives have been reported.
Prey depletion
Overlap between dolphin prey species and fishery target species does not imply direct competition (Briand, 2004). However, it is reasonable to infer competitive effects when key prey becomes scarce and remain subject to heavy fishing pressure (Trites et al., 1997). In this regard, we note that about 95% of marine fish catches globally come from continental shelf regions (Roberts & Hawkins, 1999) where bottlenose dolphins occur, and that the total biomass removed by fisheries in such regions may exceed that taken by dolphins by orders of magnitude (see ‘Food-web effects on fishery yields’).
Excessive fishing pressure and the resulting worldwide decline in fish stocks and loss of marine biodiversity is a growing concern worldwide (Pauly et al., 1998, 2002; Jackson et al., 2001; Pitcher, 2001; Csirke, 2005; Worm et al., 2006). Recent reports by the European Environment Agency concede that more fishing has been allowed than is recommended by scientific advice, and that this is due to the lobbying influence of the fishing industry (EEA, 2003, 2004). Overfishing is having profound direct and indirect impacts on Mediterranean ecosystems (Sala, 2004). In the Mediterranean, there is an acute lack of historical data and fishery statistics are generally incomplete and unreliable, data on fishing effort being almost absent (Briand, 2000, 2003; Lleonart, 2005). Nonetheless, it is generally acknowledged that unsustainable fishing has contributed significantly to dramatic ecological changes and caused the decline of many fish stocks (Caddy & Griffiths, 1990; De Walle, Nikolopoulou-Tamvakli & Heinen, 1993; Stanners & Bourdeau, 1995). According to the United Nations Food and Agriculture Organization, approximately 35% of the Mediterranean stocks are exploited beyond maximum sustainable yields (MSY), and 43% at MSY (Garcia, de Leiva Moreno & Grainger, 2005). Some of the Mediterranean fish stocks that have been either ‘overexploited’ or ‘fully exploited’ include important bottlenose dolphin prey such as European hake, striped red mullet, European pilchard, common pandora Pagellus erythrinus, annular seabream Diplodus annularis, and Atlantic horse mackerel Trachurus trachurus (Lleonart, 2005). While declines in prey species caused by fisheries may be expected to affect predators negatively, the great complexity of marine food-webs gives reason to be cautious about simplistic interpretations.
A resilient, opportunistic species such as the bottlenose dolphin may be able to survive in areas with strong anthropogenic pressure as long as prey is abundant (e.g. Bearzi et al., 2008a). Reduced carrying capacity (i.e. fewer prey available) due to overfishing was proposed as one explanation for the low densities of bottlenose dolphins in the Adriatic and Ionian Seas (Bearzi et al., 1999, 2005). Conversely, dolphin densities tend to be high in areas where prey is still abundant. For instance, bottlenose dolphin density in the prey-rich Amvrakikos Gulf, Greece – where effective fishery management measures including the prohibition of purse seining and trawling are in place – is an order of magnitude higher than dolphin density in the overfished waters of the nearby island of Kalamos (Bearzi et al., 2006, 2008a).
Contamination by xenobiotics and epizootic outbreaks
Toxic contamination can affect reproduction and health (Gauthier et al., 1999; O'Shea, Reeves & Long, 1999; Fossi & Marsili, 2003; Newman & Smith, 2006). Contaminant levels, particularly of organochlorine compounds, in Mediterranean bottlenose dolphins are very high compared to the levels reported for bottlenose dolphins in some other areas (Corsolini et al., 1995; Marsili & Focardi, 1997; Aguilar, Borrell & Reijnders, 2002; Fossi & Marsili, 2003; Wafo et al., 2005; Borrell et al., 2006; Borrell & Aguilar, 2007; Storelli et al., 2007). At concentrations similar to or lower than those documented for Mediterranean bottlenose dolphins, compounds such as PCBs or PAHs have been associated with reproductive disorders, immune-system suppression and neoplasia (Lahvis et al., 1995; Reddy et al., 2001; Schwacke et al., 2002; Jaber et al., 2005; Hall et al., 2006). Although organochlorine contamination is decreasing in some areas, levels in Mediterranean bottlenose dolphins remain high (Tolosa et al., 1997; Aguilar & Borrell, 2004; Borrell & Aguilar, 2007; Storelli et al., 2007). Monitoring of toxic chemicals, risk assessment and intervention protocols therefore represent important precautionary measures (Schwacke et al., 2002; Fossi & Marsili, 2003; Jaber et al., 2005; Porte et al., 2006).
Various, and sometimes high, levels of heavy metals have been found in stranded bottlenose dolphins from the Mediterranean (e.g. Leonzio, Focardi & Fossi, 1992; Frodello, Viale & Marchand, 2002; Roditi-Elasar et al., 2003; Lahaye et al., 2006). The effects of these metals at the population level are unknown.
Epizootic outbreaks appear to have affected bottlenose dolphins to a lesser extent than other Mediterranean delphinids, such as the striped dolphin (Aguilar & Raga, 1993; Van Bressem et al., 1993). Morbillivirus infections have been reported in a bottlenose dolphin stranded on the Mediterranean coast of Israel in 1994 (Tsur et al., 1997) and another stranded in Mauritania (Atlantic coast of West Africa) in 1988 (Van de Bildt et al., 2001). Bottlenose dolphins elsewhere have experienced mass mortality from such outbreaks, e.g. in Black Sea waters (Birkun et al., 1998; Birkun, 2006) and on the Atlantic coast of the United States, where more than half of one local population may have died (Lipscomb et al., 1994; Duignan et al., 1996; Schulman et al., 1997). As epizootic phenomena may be related to some compromise of the immune-system induced by exposure to xenobiotics and/or by stress from poor nutrition (Aguilar & Borrell, 1994; Calzada et al., 1996; O'Shea & Aguilar, 2001), the risk of disease outbreaks in bottlenose dolphins in the Mediterranean may be considerable.
Boat traffic and acoustic disturbance
There is growing evidence that prolonged direct (or physical) disturbance and noise caused by boat traffic can affect the behaviour and habitat use of cetaceans including bottlenose dolphins (Bejder & Samuels, 2003; Nowacek et al., 2007). Various alterations in behaviour have been shown to be related to boat disturbance in groups of both common and Indo-Pacific bottlenose dolphins (Janik & Thompson, 1996; Nowacek, Wells & Salow, 2001; Hastie et al., 2003; Lusseau, 2003, 2006; Constantine, Brunton & Dennis, 2004; Bejder et al., 2006a; Lemon et al., 2006). Disturbance by boats also has been correlated with changes in vocal behaviour (Scarpaci et al., 2000; Buckstaff, 2004; Morisaka et al., 2005), but the nature and extent of such changes remain largely undefined.
There has been a great expansion of recreational boat traffic and shipping in the Mediterranean in recent decades (Dobler, 2002) but the potential for resultant behavioural disruption and habitat loss has been investigated only to a limited extent. Permanent or temporary avoidance of one Mediterranean area by bottlenose dolphins as a consequence of a large seasonal increase in boat traffic was reported in coastal waters of Croatia (International Whaling Commission, 2007). Similar negative effects have been reported from other places around the world (Allen & Read, 2000; Lusseau, 2004, 2005; Bejder et al., 2006b). The noise from various human activities in addition to boating/shipping – e.g. seismic surveys, dredging, drilling, underwater explosions, and the use of military or other sonars – is also a cause for concern (Richardson et al., 1995; Nowacek et al., 2007).
Climate change
Some of the effects of global warming have become dramatically apparent in recent years (Pollack, Huang & Shen, 1998; Barnett, Pierce & Schnur, 2001; IPCC, 2007). Climate change has the potential to affect a range of biological processes and cause significant shifts in marine and other biota (e.g. Peñuelas, Filella & Comas, 2002; Parmesan & Yohe, 2003; Díaz-Almela, Marba & Duarte, 2007). Increased seawater temperature has been observed in Mediterranean deep (Bethoux et al., 1990) and surface waters (Metaxas, Bartzokas & Vitsas, 1991; Bethoux & Gentili, 1996), and there is increasing evidence of biological responses to such warming (e.g. Francour et al., 1994; Díaz-Almela et al., 2007).
Effects of climate change on cetaceans, often mediated via changes in prey abundance and distribution, have become apparent in several non-Mediterranean areas (Learmonth et al., 2006; Simmonds & Isaac, 2007) and similar effects may be occurring in Mediterranean waters (Azzellino et al., 2008a; Cañadas & Hammond, 2008). At present, however, it is impossible to predict the net effect of climate change on bottlenose dolphins – either in the Mediterranean Sea or elsewhere.
Live capture
The removal of live cetaceans from the wild – whether for captive display, for ‘dolphin-assisted therapy’ (Marino & Lilienfeld, 2007) or for research purposes – is equivalent to incidental or deliberate killing, as the animals brought into captivity or killed during capture operations are no longer available to help maintain their wild populations (Reeves et al., 2003). ‘Takes’ of bottlenose dolphins are prohibited in most Mediterranean riverine States by national legislation or international agreements (Table 3). However, live captures (and any mortality and social disruption associated with capture operations) still occur occasionally. For instance, bottlenose dolphins were removed from Turkish Mediterranean waters following issue of a permit in early 2006 by the Turkish Ministry of Agriculture and Rural Affairs to capture a total of 30 dolphins ‘for the rehabilitation of physically and mentally handicapped and for show purposes’. Though such live captures do not represent a threat at the basin scale, they can have a significant impact on local populations, both directly and indirectly (e.g. female bottlenose dolphins are more successful at rearing calves in large, stable groups; Wells, 2003).
| Wildlife protection treaties and international treaties relevant to the conservation of Mediterranean bottlenose dolphins (BD) and/or their habitat | |
| CMS or Bonn Convention: 1979a, 1983f | Mediterranean BD population listed in Appendix II; ACCOBAMS (see below) is one of the CMS agreements. Supports multilateral agreements for the conservation, management and cooperation in research on migratory species in Appendix II (including all dolphin species inhabiting the Mediterranean). ‘Taking’ as defined in the CMS can be interpreted to include by-catch and disturbance. |
| ACCOBAMS: 1996a, 2001f | BD fully protected. Aims to reduce threats to cetaceans in agreement area, protect them and establish a network of protected areas important for feeding, breeding and migration. Contracting parties are urged to develop research and monitoring as well as public awareness and capacity building programmes. |
| Convention on the Conservation of European Wildlife and Natural Habitats (Bern Convention): 1979a, 1982f | BD listed in Appendix II (Strictly Protected Fauna Species). Based on its listing (and as a migratory species), BD should benefit from (i) duty to establish areas that protect its habitat and (ii) prohibitions intended to ensure ‘special protection’. 1989 recommendation (#18) to parties to advance designation of Areas of Special Interest for Conservation, particularly where these were important for the survival of threatened or endemic species or those included in Appendices I and II. |
| Agreement for the creation of a Sanctuary for marine mammals in the Mediterranean Sea (Pelagos Sanctuary): 1999 | Establishes protection regime for marine mammals, encompasses 88 000 km2 of north-western Mediterranean Sea, extending between south-eastern France, Monaco, north-western Italy and northern Sardinia, and surrounding Corsica and the Tuscan Archipelago. |
| CITES: 1973 | Controls international trade in fauna and flora. Since 1979 BD in Appendix II, which includes all species of cetaceans not in Appendix I and requires trade documentation and monitoring. |
| Barcelona Convention: (i) Protocol for the Protection of the Mediterranean Sea against Pollution Resulting from Exploration and Exploitation of the Continental Shelf and the Seabed and its Subsoil (Offshore Protocol): 1994a; (ii) Protocol for the Protection of the Mediterranean Sea against Pollution from Land-Based Sources and Activities (LBS Protocol): 1980a, 1983f | Provides a framework under which specific Protocols, addressing issues of direct relevance to BD, may be implemented. Definition of ‘pollution’ refers to both substances and energy. As such, it arguably includes noise. |
| Barcelona Convention: (iii) Protocol for SPA and Biological Diversity in the Mediterranean (SPA and Biodiversity Protocol): 1982a, 1999f | Includes regimes for the protection of both area and species. Duty on parties to establish ‘specially protected areas’ in their waters; further duties flow once a SPA has been established. Obligation on parties to draw up a list of ‘specially protected areas of Mediterranean importance’; such sites can extend to the high seas.General duty on parties to ‘manage species of flora and fauna with the aim of maintaining them in a favourable state of conservation’. Furthermore, ‘protected status’ must be accorded to endangered or protected species, and specific protection duties apply to species listed in Annex II (endangered or threatened, which includes BD). |
| UN Convention on the Law of the Sea: 1982a, 1994f | Provides for States in their EEZs and on the high seas to cooperate with a view to conserving marine mammals and in the case of cetaceans work through appropriate international organizations for their conservation, management and study. Duties regarding pollution sources including noise are also relevant as is protection of habitats of ‘depleted, threatened or endangered species’. |
| UN Food and Agriculture Organisation Code of Conduct for Responsible Fisheries: 1995 | Particularly relevant to BD conservation due to provisions on conservation and management of living aquatic resources and specific focus on measures taking the wider environment into account. Deals with use of selective, environmentally safe fishing gear and practices; minimisation of catch of non-target species (including cetaceans); recognizing and seeking to resolve uncertainty (precautionary approach). |
| Agreement to Promote Compliance with International Conservation and Management Measures by Fishing Vessels on the High Seas: 1993a, 2003f | Aims at improving compliance by fishing vessels with international conservation and management measures on high seas. Particularly relevant to Mediterranean because of high proportion of high seas and competence of two regional fisheries bodies (i.e. ICCAT and GFCM). Depends for success on acceptance by large number of flag States but few significant distant water flag States have accepted Agreement so far. |
| Stockholm Convention on POPs: 2001a, 2004f | Focus on production, use and release of POPs, with the purpose of protecting human health and environment. Few Mediterranean countries have ratified. Will benefit BD to extent that POP exposure is a threat to them. |
| Protocol for the Protection of the Mediterranean Sea against Pollution Resulting from Exploration and Exploitation of the Continental Shelf and the Seabed and its Subsoil (Offshore Protocol): 1994a | Envisages a regime for control of harmful or noxious substances, materials and wastes. Would relate to exploration and/or exploitation of resources irrespective of whether their impact is likely through pollution or other environmental effects. |
| Protocol for the Protection of the Mediterranean Sea against Pollution from Land-Based Sources and Activities (LBS Protocol): 1980a, 1983f | Includes undertaking by Parties to phase out inputs of Annex I toxic, persistent and bioaccumulating substances, through regional plans and programmes. Provides for authorisation or regulation regime for point source discharges and releases into water or air. Relevant to BD to extent that these substances constitute threats to them. |
| UN Environment Programme Mediterranean Environmental Action Plan – Action Plan for the Conservation of the Cetaceans in the Mediterranean Sea: 1991 | Two broad objectives: (i) protection of cetaceans and conservation of their habitats; and (ii) protection, conservation and regeneration of the cetacean populations. Urges parties to develop co-ordinated research programmes to determine conservation status and distribution of cetaceans. |
| Agreement for the Establishment of the GFCM: 1949a, 1952f | GFCM has the power to adopt measures ‘for the conservation and rational management of living marine resources’. Measures are binding, subject to power of Commission members to object. Working groups are involved in the assessment of problems including dolphin by-catch and prey depletion. |
| A few European Community instruments that are binding for EU Member States are worth mentioning here among the many existing, due to their relevance to bottlenose dolphin conservation: | |
| Council Directive No 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora (Habitats Directive) | Includes regimes for protection of both habitats and species. BD may benefit from special areas of conservation established for Annex I habitats, or habitats of Annex II species (including BD). For species listed in Annex II (including BD), it is required to create SAC. Under Article 1(k), a site of Community importance is defined as one that ‘contributes significantly to the maintenance or restoration at a favourable conservation status of a natural habitat type in Annex I or of a species in Annex II’. In Article 1(l) a SAC is defined as ‘a site of Community importance where necessary measures are applied to maintain, or restore, to favourable conservation status, the habitats or populations of the species for which the site is designated’. To be accepted as part of the European Natura 2000 Network of protected areas, a proposed SAC should be of particular importance for conservation of species in Annex I or II. According to Article 12(4), ‘Member States shall establish a system to monitor the incidental capture and killing of the animal species listed in Annex IV (a). In the light of the information gathered, Member States shall take further research or conservation measures as required to ensure that incidental capture and killing does not have a significant negative impact on the species concerned.’ BD are listed in Annex IV (a). |
| Council Regulation (EC) No 1626/94 of 27 June 1994 laying down certain technical measures for the conservation of fishery resources in the Mediterranean | According to Article 1.2 Member States while managing fisheries in the Mediterranean ‘shall pay attention to the conservation of fragile or endangered species or environments, in particular those listed in Annex I’ (which refers to marine mammals including cetaceans). |
| Council Regulation (EC) No 338/97 of 9 December 1996 | This Regulation, referring to CITES, establishes rules for the protection of species of wild fauna and flora by regulating trade therein. Annex A of the Regulation includes all species listed in Appendix I of the Convention, including BD. |
| Council Regulation (EC) No 2371/2002 of 20 December 2002 on the conservation and sustainable exploitation of fisheries resources under the Common Fisheries Policy | The purpose of this Regulation is to ‘ensure the long-term viability of the fisheries sector by conserving, managing and exploiting fishery resources, limiting the impact of fisheries on the environment and adjusting fishing capacity’. This involves ‘progressively implementing an ecosystem-based approach to fisheries management and contributing to efficient fishing activities within an economically viable and competitive industry’. Confers powers and duties on EC institutions (e.g. regarding fisheries conservation coupled with environmental protection requirements) and Member States (including access restriction in the case of waters within 12 nautical miles of coastal Member States' baselines). |
| Council Regulation (EC) No 812/2004 of 26 April 2004 | This Regulation lays down measures concerning incidental catches of cetaceans in Mediterranean pair trawlers. Member States are obliged to assess by-catch rates and set up a monitoring system through independent observers on boats with an overall length of 15+ m. |
| Council Regulation (EC) No 1967/2006 of 21 December 2006 and its Corrigendum (Official Journal of the European Union L 409 of 30 December 2006) | This Regulation includes management measures for the sustainable exploitation of fishery resources in the Mediterranean, and calls for the creation of Fishing Protected Areas relevant for the conservation of BD. |
- BD, bottlenose dolphins; ACCOBAMS, Agreement on the Conservation of Cetaceans in the Black Sea, Mediterranean Sea and contiguous Atlantic area; CMS, Convention on the Conservation of Migratory Species of Wild Animals; CITES, Convention on International Trade in Endangered Species of Wild Flora and Fauna; EC, European Commission; EEZ, Exclusive Economic Zone; LBS, Land-Based Sources; SPA, Specially Protected Areas; UN, United Nations; GFCM, General Fisheries Commission for the Mediterranean; POPs, Persistent Organic Pollutants; SAC ‘Special Areas of Conservation’.
CONSERVATION FRAMEWORK
Large-scale campaigns to cull dolphins have stopped, cetaceans are protected by law in most Mediterranean countries and deliberate killing has become far less frequent than in the past. Several attempts have been made to ban some fishing gears or methods, such as driftnets, that cause high dolphin mortality. In addition, the existing laws and treaties in force today provide a potentially solid framework for the conservation of bottlenose dolphins and other cetaceans, and for the protection of their habitat in the Mediterranean region. Unfortunately, implementation and enforcement of those instruments have not been consistently effective. Actions taken to date have not been adequate to maintain a ‘favourable’ status of bottlenose dolphin populations (as advocated by the EU Habitats Directive) or to prevent further population decline. Tangible actions to protect bottlenose dolphins and other Mediterranean cetaceans have been surprisingly few, especially if one considers the large number of existing laws, regulations and agreements that have been in place for years and even decades (Table 3).
About a hundred national Marine Protected Areas (MPAs) of different types, sizes and purposes have been established in Algeria, Croatia, Cyprus, France, Greece, Israel, Italy, Lebanon, Malta, Monaco, Morocco, Slovenia, Spain, Syria, Tunisia and Turkey, but specific measures for cetacean conservation are rarely included in their management plans. A notable exception is the 87 000 km2 cetacean sanctuary created in 1999 by France, Italy, and the Monaco Principality in the Corso-Ligurian-Provençal Basin (the ‘Pelagos Sanctuary’; Notarbartolo di Sciara et al., 2008). In addition, 3 years of provisional protection (from July 26th, 2006) were granted to the waters east of the islands of Losinj and Cres (Croatia) following a proposal for the creation of a special zoological reserve for dolphins. After that period, the protection is expected to become permanent. If appropriately managed, MPAs could contribute to bottlenose dolphin conservation by preserving their prey and habitat, reducing the risks of mortality in fishing gear, providing refuge from noise and other types of disturbance, raising awareness, stimulating research and facilitating exchange of information (Hoyt, 2005). However, levels of enforcement are often low and many of the existing Mediterranean MPAs merely represent ‘paper parks’ (Bearzi, 2007; Guidetti et al., 2008). Other types of action that can provide direct or indirect benefits to bottlenose dolphins include area-, season-, or fishery-specific reductions in fishing effort, changes to fishing gear or fishing practices to reduce incidental mortality, curtailment of inputs of toxic pollutants, and boating regulations.
CONCLUSIONS
Bottlenose dolphins have apparently been better able than short-beaked common dolphins (the other predominantly coastal cetaceans in the Mediterranean) to persist in spite of attempts at eradication and the habitat degradation caused by constantly increasing coastal development (Airoldi & Beck, 2007) and use of destructive fishing practices. The flexible social organization and opportunistic diet and behaviour of bottlenose dolphins probably make them relatively resilient and able to adapt to changing environmental conditions. Still, bottlenose dolphins were probably much more abundant in the Mediterranean in historic times (see ‘Past culling campaigns’) and our present perception of the species' conservation status in the region may be influenced by the ‘shifting baselines syndrome’ (Pauly, 1995; Sáenz-Arroyo et al., 2005).
Bottlenose dolphins have been studied only in relatively small portions of the Mediterranean basin; wide areas remain largely unexplored. Research must play a major role in filling the gaps in knowledge. Insufficient knowledge can delay the adoption of meaningful conservation measures and limit the efficacy of those in place. However, the risks of simply perpetuating calls for more research must also be considered. Inaction is too often justified by the inadequacy of information. Waiting for more and better data can delay the management process indefinitely, and unwillingness to act based on what is known can allow the status of cetacean populations to deteriorate further.
The fate of Mediterranean bottlenose (and other) dolphins depends on range States having the political will to take action to mitigate known anthropogenic threats. Management measures that could benefit bottlenose dolphins are already embedded in a large number of existing laws and treaties. If all such measures were to be fully implemented and enforced, and the range States were doing everything to which they were committed based on multiple obligations under agreements that are already in force with regard to fishing, pollution and other forms of habitat degradation, many of the problems would be addressed and the status of dolphin populations would likely improve. Compliance with existing obligations such as those listed in Table 3 therefore represents the highest management priority. National monitoring of population status and anthropogenic threats is also needed, and should result in conservation plans followed quickly by concrete action.
Mediterranean biodiversity is undergoing rapid alteration due to human activities (Bianchi & Morri, 2000) and attempts to slow this alarming, widespread trend are encountering substantial resistance. At least in part, this is because progress in conservation requires actions with socio-economic consequences. The existing legal commitments of countries in the region might go a long way towards mitigating or even solving some of the problems facing Mediterranean bottlenose dolphins. However, provisions that run counter to what is perceived as being in the self-interest of States, institutions, companies and individuals will continue to be difficult to implement and enforce. No less than a fundamental shift in public values may be required. In this context, efforts by committed scientists and individuals are extremely important, particularly when unambiguous science-based conservation messages are conveyed directly to managers and the general public.
ACKNOWLEDGEMENTS
Frank Dhermain, Bruno Díaz López, Nicolas Entrup, Ian Fergusson, Drasko Holcer, Ada Natoli and Olivier Van Canneyt contributed useful information. Early drafts benefited from comments and suggestions by Randall S. Wells, Giuseppe Notarbartolo di Sciara, Simon Ingram, and an anonymous reviewer. Silvia Bonizzoni, Mauro Bastianini and Nino Pierantonio helped with the literature.




