Pharmacokinetics of azithromycin in lactating dairy cows with subclinical mastitis caused by Staphylococcus aureus
Abstract
Lucas, M. F., Errecalde, J. O., Mestorino, N. Pharmacokinetics of azithromycin in lactating dairy cows with subclinical mastitis caused by Staphylococcus aureus. J. vet. Pharmacol. Therap.33, 132–140.
Azithromycin is a time-dependent antimicrobial with long persistence. The main characteristics of azithromycin suggest that it could be useful for treating bovine mastitis caused by Staphylococcus aureus. To investigate this possibility, its pharmacokinetic (PK) behavior was studied. Six Holstein lactating cows with subclinical mastitis were administered two 10 mg/kg intramuscular (i.m.) doses of azithromycin, with a 48-h interval. Milk and plasma concentrations were measured by microbiological assay. The MIC90 was determined in 51 S. aureus isolations to calculate pharmacokinetic/pharmacodynamic (PK/PD) parameters. Milk maximal concentration (Cmax) was 7.76 ± 1.76 μg/mL (16.67 h post-first administration) and 7.82 ± 2.18 μg/mL (14 h post-2nd administration). In plasma Cmax was 0.18 ± 0.03 μg/mL (2 h post-1rst administration) and 0.11 ± 0.03 μg/mL (14 h post-2nd administration). Azithromycin was eliminated from the milk with a half-life (T½λ) of 158.26 ± 137.7 h after 2nd administration, meanwhile plasma T½λ resulted shorter(13.97 ± 11.1 h). The mean area under the concentration vs. time curve from 0 to 24 h (AUC0-24h) was 153.82 ± 34.66 μg·h/mL in milk secretion and 2.61 ± 0.59 μg·h/mL in plasma. Infection presence in the quarters had a significant effect (P < 0.05) on the area under the concentration vs. time curve from 0 to infinity (AUC0-∞) and clearance from the mammary gland (Clmam/F). Moreover, it had influence on milk bioavailability (Fmilk), T½λ, AUC0-∞ and mean residence time (MRT) in milk, which values resulted increased in mastitic quarters. In this study, it was determined that the production level and the mammary health status have an influence on PK parameters of azithromycin treatments in bovine mastitis.
Introduction
Azithromycin is a macrolide belonging to a group of semi-synthetic antimicrobial agents called azalides which were derived from erythromycin by adding an endocyclic nitrogen atom in position 9 (Mulazimoglu et al., 2005). Azithromycin formulation is not available for use in production animals. It has a particular pharmacokinetic profile (Biskri & Mazel, 2003) and it has a clear in vivo postantibiotic effect (PAE) (Van Bambeke & Tulkens, 2001). According to the pattern of antimicrobial activity, azithromycin is a time-dependant antimicrobial agent with prolonged persistent effect. The PK/PD measure that best correlates with azithromycin′s clinical efficacy is the area under the curve/minimum inhibitory concentration (AUC0-24/MIC) (Ambrose et al., 2004).
Azithromycin offers a number of advantages that differentiate it from conventional macrolides. As a consequence of the structural changes above mentioned, this drug is more potent against gram-negative bacteria than other conventional macrolides such as erythromycin, while its action remains the same against Gram-positive microorganisms (Retsema et al., 1987). In addition, it has higher resistance against acid degradation in comparison with erythromycin (Fiese & Steffen, 1990); it has wider distribution and a longer elimination half-life.
Like traditional macrolides, azithromycin is capable of concentrating in phagocytic cells (McConnell, 1999) where it penetrates by nonionic diffusion (Mulazimoglu et al., 2005). In cells, it is found in lysosomes, where it concentrates by ion trapping (Gómez-Lus et al., 2005). It was postulated that macrolide accumulation inside leukocytes may increase the activity of cell immunity-related mechanisms. Leukocytes, mainly neutrophils, macrophages and monocytes, accumulate antibiotic molecules in their lysosomes and transport them by chemotactic response to the infection site (Labro, 1998; Shryock et al., 1998).
The above mentioned properties suggest that azithromycin may be effective for treating infections caused by Gram-positive aerobic microorganisms which survive inside lysosomes, as the case of S. aureus bovine mastitis.
The objective of this study was to investigate the azithromycin pharmacokinetic profile in lactating dairy cows with subclinical mastitis caused by S. aureus, and calculate the corresponding PK/PD parameters.
Materials and methods
Isolation of Staphylococcus aureus
Approximately 300 lactating Holstein cows were sampled and cultured for S. aureus to obtain subclinical mastitis-causing isolations. The cows were part of herds of commercial dairy farms from the areas of Tandil and San Vicente, Buenos Aires Province, Argentina. Milk samples were aseptically collected according to the National Mastitis Council (NMC) procedures (NMC, 1981). Composite premilking samples were collected for somatic cells count (SCC), performed with a Fossomatic 5000 electronic cell counter (Foss Electronic, Hillered, Denmark). Postmilking samples were collected from each mammary quarter of all lactating cows. The teat ends were disinfected with 70% alcohol swabs. The first few streams were discarded and then 2–4 mL of milk was collected in sterile tubes. Quarter milk samples (10 μL) were streaked on quarter plates of 5% bovine blood agar, and plates were then incubated at 37 °C for 48 h. Identification of S. aureus was performed using colony morphology, catalase test and tube coagulase test (NMC, 1981) being the control strain for the coagulase test S. aureus ATCC 25923. Cases exhibiting presence of five or more S. aureus colonies per mammary quarter (NMC, 1981), absence of clinical signs of mastitis and SCC equal or higher than 200 000 were considered as subclinical mastitis positive. Fifty-one S. aureus isolations were used to perform the antimicrobial susceptibility tests.
Animals
The selection of the animals to be treated with azithromycin was based on the S. aureus isolations susceptibility. The 51 S. aureus isolations were evaluated by antibiogram using the Kirby–Bauer disk diffusion method and 15 μg azithromycin disks (Laboratorios Britania, Buenos Aires, Argentina). The disk-agar diffusion method was performed according to the Clinical and Laboratory Standards Institute (CLSI, 2008), formerly the National Committee for clinical laboratory Standards (NCCLS). The control strain for the disk-diffusion method was S. aureus ATCC 25923. This strain allows controlling method accuracy, and the inhibition zone around the 15 μg azithromycin disk should be 21–26 mm (NCCLS, 2000).
Six Holstein lactating cows with subclinical mastitis in at least one mammary quarter were selected. These animals were neither part of any other parallel experiment nor had received any medication for the previous 30 days. The animals mean body weight was 471.67 ± 18.77 kg. The SCC in all cows was higher than 200 000, with a minimum value of 222 000 and a maximum value of 2 203 000. The milk production was 17.07 ± 2.42 L/day and the days after calving were 204.50 ± 92.83.
During the experiment the animals were subject to their normal milking routine (twice a day), and minimizing sampling-related stress factors was intended.
Determination of the minimum inhibitory concentration
The MIC is defined as the lowest antimicrobial concentration that is capable of preventing the assessed microorganism from growing (García Rodríguez et al., 2000). The MIC50 is the minimum inhibitory concentration capable of inhibiting 50% of the assessed isolations, and the MIC90 is the one which allows inhibiting 90% of them. Determination of azithromycin MIC against the 51 S. aureus isolations was performed by means of broth macrodilution method (NCCLS, 2002). The range of concentrations of azithromycin used was 0.0625–8 μg/mL. The S. aureus ATCC 29213 strain was used as a control and the MIC acceptable value was 0.5 μg/mL (NCCLS, 2000).
Antibacterial agent administration and sample collection
All animals were weighted after the morning milking and before the administration of the antimicrobial agent. Azithromycin hydrogecitrate (75.2–82% of the anhydrous substance, as anhydrous azithromycin) was formulated as a 10% experimental solution in aqueous phase (30% ethanol in propylenglycol). This solution was transparent and its stability and potency were assessed by microbiological methods. The solution was administered intramuscularly in the gluteal area in two doses of 10 mg/kg body weight with a 48 h interval. Blood samples were collected from the coccygeal vein using heparinized sterile syringes at 0, 0.5, 1, 2, 4, 8, 12, 24, 36, 48, 60, 72, 84 and 96 h after drug administration. At the same times, quarter milk samples were aseptically collected into sterile tubes. The blood samples were centrifuged at 1500 g for 15 min and the plasma was transferred to sterile glass tubes. All samples were stored at −20 °C until assayed.
The protocol was following the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (Federation of Animal Science Societies – FASS).
Analytical method
Milk and plasma azithromycin concentrations were measured by microbiological assay (Grove & Randall, 1955). The test microorganism was Micrococcus luteus ATCC 9341. The M. luteus inoculum was 1 mL of a dilution with 75% transmittance at 670 nm (2–2.6 × 107 cfu/mL) per 120 mL of Antibiotic Assay Medium N° 1 (Laboratorios Britania, Buenos Aires, Argentina). Incubation was at 35 °C for 18 h.
The reference standard of azithromycin was dissolved in methanol to a concentration of 1000 μg/mL. This solution was stable for at least 6 months at −20 °C. The reference standard was further diluted in phosphate buffer pH 8 and then separate standard curves for azithromycin were prepared in antimicrobial free plasma and milk (working standard solutions). Standard curves were made using azithromycin concentrations ranging from 0.125 to 2 μg/mL of milk and 0.0625 to 1 μg/mL of plasma. Standards were tested in three different occasions and for quadruplicate. Calibration graphs were constructed by plotting the mean diameter of the inhibition zones against the azithromycin concentrations. The intra-assay variation must be lower than 15%. All standard curves were linear from 0.125 to 2 μg/mL in milk and from 0.0625 to 1 μg/mL in plasma. The milk standard curves had correlation coefficients of 0.9757 ± 0.0128 and the intra-assay coefficient of variation was 3.87%. The plasma standard curves had correlation coefficients of 0.9354 ± 0.0152 and the intra-assay coefficient of variation was 12%. The limit of quantification (LOQ) in milk was 0.125 μg/mL and in plasma it was 0.0625 μg/mL.
Animal samples were tested in duplicate and the mean zone diameter was used to calculate drug concentrations (μg/mL). Concentrations were calculated from zone diameters using a fresh standard curve, which was run on the same day of the analysis.
Pharmacokinetic analysis
Pharmacokinetic parameters were determined by noncompartmental analysis using WinNonlin Professional 5.2 (Pharsight Corporation, Mountain View, CA, USA) software. Each mammary quarter was considered independently (n = 24) (Mestorino, 1993; Berry et al., 2002; Rivas et al., 2006), as our purpose was to compare the findings in mastitic quarters (n = 8) against healthy ones (n = 16) and in quarters of high-producing cows (n = 12) against low-producing cows (n = 12). Simultaneously, the azithromycin PK profile in plasma was determined. PK parameters calculated from milk and plasma concentrations were: maximum concentration reached post-1rst administration (Cmax1), time at which Cmax1 is reached (Tmax1) and elimination half-life post-1rst administration (T½λ1); Cmax, Tmax and T½λ post-2nd administration (Cmax2, Tmax2 and T½λ2), area under the concentration vs. time curve from zero to 24 h (AUC0-24h), area under the concentration vs. time curve from zero to infinity (AUC0-∞), mean residence time (MRT), body clearance (ClB/F), mammary clearance (Clmam/F), and milk bioavailability (Fmilk) as the relationship AUC0-∞ in milk/AUC0-∞ in plasma. The PK/PD parameters calculated were AUC0-24h/MIC and T > MIC.
Statistical analysis
The experimental design was a completely randomized design with a factorial 2 × 2 arrangement. Mammary quarters were grouped according to two factors: level of milk production (as applicable to a high or low-producing animal) and health status (mastitic quarter or healthy quarter). To evaluate the effect of each factor, an anova based on the ranked data was performed.
Bacteriological cure control
Milk samples were aseptically collected according to the NMC procedures (NMC, 1981). Composite premilking samples were collected for SCC, performed with a Fossomatic 5000 electronic cell counter (Foss Electronic, Hillered, Denmark). Postmilking samples were collected from each mammary quarter to perform bacteriological cultures. Identification of S. aureus was performed according to the NMC (NMC, 1981). Bacteriological cure was confirmed when an infected mammary quarter, of any of the treated cows, was bacteriologically negative for S. aureus at 20 and 30 days post-treatment. Additionally, SCC was evaluated in composite samples at 20 days post-treatment. Previous infected quarters that exhibited presence of one or more S. aureus colonies in at least one of the two samples collected (20 and 30 days post-treatment) were considered as positives.
Results
According to the interpretative criteria set by CLSI (2008), the 83.6% of the S. aureus evaluated would be classified as sensitive, 9.1% showed intermedia sensitivity and 7.3% would be classified as resistant. Out of a total of 51 isolations assessed, an MIC90 of 1 μg/mL and an MIC50 of 0.5 μg/mL were determined. The MIC in the S. aureus ATCC 29213 strain was 0.5 μg/mL.
The mean concentrations of azithromycin reached in quarter milk (n = 24) and plasma(n = 6) of the six experimental cows treated are shown in Fig. 1. Pharmacokinetic parameters determined by means of a noncompartmental analysis of milk and plasma concentrations are shown in Table 1. A high Cmax and a large AUC demonstrate major azithromycin penetration into the mammary gland.
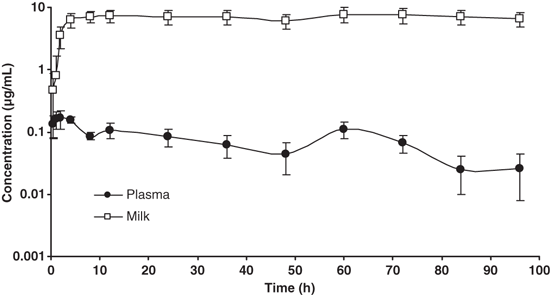
Experimental concentrations of azithromycin in quarter milk (n = 24) and plasma(n = 6) after two 10 mg/kg i.m. doses with a 48-h interval.
| Parameters | X milk ± SD | X plasma ± SD |
|---|---|---|
| C max1 (μg/mL) | 7.76 ± 1.76 | 0.18 ± 0.03 |
| T max1 (h) | 15.33 ± 10.33 | 2 ± 1.56 |
| C max 2 (μg/mL) | 7.82 ± 2.18 | 0.11 ± 0.03 |
| T max2 (h) | 64.5 ± 6.91 | 62 ± 4.90 |
| T½λ1 (h) | 166.07 ± 58.30 | 32.66 ± 21.63 |
| T½λ2 (h) | 158.26 ± 137.70 | 13.97 ± 11.10 |
| AUC 0-24h (μg·h/mL) | 153.82 ± 34.66 | 2.61 ± 0.59 |
| AUC 0-∞ (μg·h/mL) | 1931.22 ± 1194.08 | 3.39 ± 1.23 |
| MRT (h) | 242.52 ± 144.94 | 26.74 ± 16 |
| Cl B/F (mL/h/kg) | – | 3295.23 ± 1255.42 |
| Cl mam/F (mL/h/kg) | 6.88 ± 3.85 | – |
| F milk | 685.13 ± 673.16 | – |
| RCmax1 | 43.75 ± 12.28 | |
| RCmax2 | 71.65 ± 20.21 |
- C max1, maximum concentration following i.m. first administration; Tmax1, time to reach maximum concentration following i.m. first administration; T½λ1, elimination half-life following i.m. first administration; Cmax2, maximum concentration following i.m. second administration; Tmax2, time to reach maximum concentration following i.m. second administration; T½λ2, elimination half-life following i.m. second administration; AUC0-24h, area under the concentration time curve from zero to 24 h; AUC0-∞, area under the concentration time curve from zero to infinity; MRT, mean residence time; ClB/F, body clearance; Clmam/F, mammary clearance; Fmilk, bioavailability of azithromycin in milk (AUC0-∞ in milk/AUC0-∞ in plasma); RCmax1, ratio of azithromycin Cmax1(Cmax1 in milk/Cmax1 in plasma); RCmax2, ratio of azithromycin Cmax2 (Cmax2 in milk/Cmax2 in plasma).
The mean concentrations of azithromycin determined in milk of mastitic quarters (n = 8) and in milk of healthy quarters (n = 16) are shown in Fig. 2. It can be observed that the concentrations in mastitic quarters are higher than that in healthy ones. PK parameters calculated in mastitic quarters and healthy quarters are shown in Table 2.
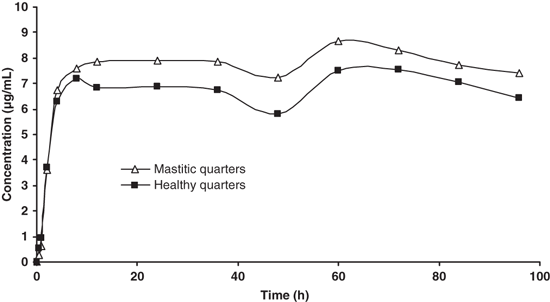
Experimental concentrations of azithromycin in mastitic quarters (n = 8) and healthy quarters (n = 16) after two 10 mg/kg i.m. doses with a 48-h interval.
| Parameters | X mastitic ± SD | X healthy ± SD |
|---|---|---|
| C max1 (μg/mL) | 7.8 ± 1.86 | 7.74 ± 1.76 |
| T max1 (h) | 16.5 ± 8.40 | 14.75 ± 11.38 |
| C max2 (μg/mL) | 8.09 ± 2.47 | 7.68 ± 2.10 |
| T max2 (h) | 60.00 ± 0.00 | 66.75 ± 7.55 |
| T½λ1 (h) | 229.00 ± 211.74 | 122.89 ± 64.41 |
| T½λ2 (h) | 210.03 ± 122.63 | 144.83 ± 84.64 |
| AUC 0-24h (μg·h/mL) | 156.53 ± 39.68 | 151.66 ± 32.30 |
| AUC 0-∞ (μg·h/mL) | 2520.79 ± 1672.31 | 1636.44 ± 776.61 |
| MRT (h) | 304.65 ± 176.31 | 211.45 ± 120.89 |
| Cl mam/F (mL/h/kg) | 5.71 ± 4.24 | 7.46 ± 3.62 |
| F milk | 997.09 ± 1065.89 | 529.15 ± 294.57 |
| RCmax1 | 45.49 ± 12.89 | |
| RCmax2 | 74.11 ± 21.99 |
- C max1, maximum concentration following i.m. first administration; Tmax1, time to reach maximum concentration following i.m. first administration; T½λ1, elimination half-life following i.m. first administration; Cmax2, maximum concentration following i.m. second administration; Tmax2, time to reach maximum concentration following i.m. second administration; T½λ2, elimination half-life following i.m. second administration; AUC0-24h, area under the concentration time curve from zero to 24 h; AUC0-∞, area under the concentration time curve from zero to infinity; MRT, mean residence time; Clmam/F, mammary clearance; Fmilk, bioavailability of azithromycin in milk (AUC0-∞ in milk/AUC0-∞ in plasma); RCmax1, ratio of azithromycin Cmax1 (Cmax1 in milk/Cmax1 in plasma); RCmax2, ratio of azithromycin Cmax2 (Cmax2 in milk/Cmax2 in plasma).
The mean concentrations of azithromycin determined in plasma of high-producing (n = 3) and low-producing cows (n = 3) and in quarters of high-producing cows (n = 12) and low-producing cows (n = 12) are shown in Fig. 3. The pharmacokinetic parameters of azithromycin in quarter milk of high-producing cows, in quarter milk of low-producing cows, in plasma of high-producing cows and in plasma of low-producing are shown in Table 3.
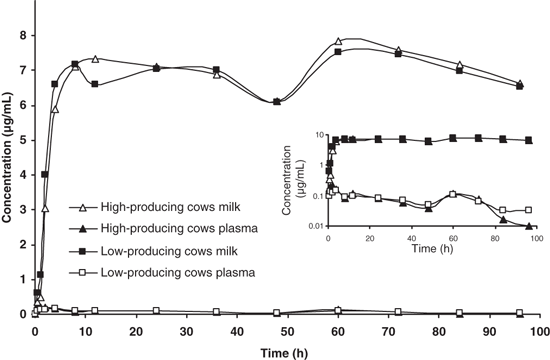
Experimental concentrations of azithromycin in plasma in high-producing (n = 3) and low-producing cows (n = 3); and quarters of high-producing cows (n = 12) and low-producing cows (n = 12) after two 10 mg/kg i.m. doses with a 48-h interval.
| Parameters | X milk high-prod ± SD | X milk low-prod ± SD | X plasma high-prod ± SD | X plasma low-prod ± SD |
|---|---|---|---|---|
| C max1 (μg/mL) | 7.87 ± 2.07 | 7.65 ± 1.46 | 0.21 ± 0.02 | 0.16 ± 0.03 |
| T max1 (h) | 16.33 ± 10.98 | 14.33 ± 10.01 | 1.17 ± 0.76 | 3.00 ± 1.73 |
| C max2 (μg/mL) | 7.93 ± 2.53 | 7.11 ± 1.88 | 0.12 ± 0.04 | 0.11 ± 0.03 |
| T max2 (h) | 64.00 ± 5.91 | 60.00 ± 8.02 | 60.00 ± 0.00 | 64.00 ± 6.93 |
| T½λ1 (h) | 166.86 ± 171.07 | 149.66 ± 101.10 | 7.19 ± 1.05 | 20.76 ± 13.0 |
| T½λ2 (h) | 155.54 ± 104.47 | 163.93 ± 101.09 | 22.02 ± 8.68 | 43.30 ± 27.47 |
| AUC 0-24h (μg·h/mL) | 152.53 ± 38.93 | 155.11 ± 31.51 | 2.87 ± 0.78 | 2.35 ± 0.27 |
| AUC 0-∞ (μg·h/mL) | 1890.04 ± 1471.84 | 1972.40 ± 900.72 | 2.80 ± 0.93 | 3.98 ± 1.37 |
| MRT (h) | 226.55 ± 149.99 | 258.49 ± 144.46 | 17.93 ± 2.07 | 35.54 ± 20.08 |
| Cl B/F (mL/h/kg) | – | – | 3894.96 ± 1495.57 | 2695.51 ± 790.24 |
| Cl mam/F (mL/h/kg) | 7.30 ± 3.97 | 6.45 ± 3.82 | – | – |
| F milk | 823.70 ± 907. 31 | 546.56 ± 287.02 | – | – |
| RCmax1 | 38.89 ± 12.23 | 48.61 ± 10.69 | ||
| RCmax2 | 69.95 ± 23.84 | 73.35 ± 16.72 |
- C max1, maximum concentration following i.m. first administration; Tmax1, time to reach maximum concentration following i.m. first administration; T½λ1, elimination half-life following i.m. first administration; Cmax2, maximum concentration following i.m. second administration; Tmax2, time to reach maximum concentration following i.m. second administration; T½λ2, elimination half-life following i.m. second administration; AUC0-24h, area under the concentration time curve from zero to 24 h; AUC0-∞, area under the concentration time curve from zero to infinity; MRT, mean residence time; ClB/F, body clearance; Clmam/F, mammary clearance; Fmilk, bioavailability of azithromycin in milk (AUC0-∞ in milk/AUC0-∞ in plasma); RCmax1, ratio of azithromycin Cmax1 (Cmax1 in milk/Cmax1 in plasma); RCmax2, ratio of azithromycin Cmax2 (Cmax2 in milk/Cmax2 in plasma).
PK/PD parameters such as AUC0-24h/MIC90 and T > MIC90 are shown in Table 4.
| Parameters | X mastitic ± SD | X healthy ± SD | X high-prod ± SD | X low-prod ± SD |
|---|---|---|---|---|
| AUC 0-24h/MIC90 | 156.53 ± 39.68 | 152.46 ± 33.19 | 152.53 ± 38.93 | 155.11 ± 31.51 |
| T > MIC90 | 96.09 ± 0.74 | 96.48 ± 1.20 | 95.83 ± 0.00 | 96.88 ± 1.80 |
- *MIC90 of azithromycin against the 51 S. aureus isolated.
The statistical results are shown in Table 5. There was a significant difference (P = 0.043) between mastitic quarters and healthy quarters regarding Tmax post-2nd administration. The health status factor also had a significant effect on the AUC0-∞ (P = 0.0331) and Clmam (P = 0.0331). The productive level factor appeared to have some influence on the T > MIC90 as well as the health status over the Fmilk. However, in these cases no statistically significant differences were observed.
| Parameters | Health status | Level of production |
|---|---|---|
| C max1 (μg/mL) | 0.9432 | 0.5702 |
| T max1 (h) | 0.3505 | 0.4056 |
| C max 2 (μg/mL) | 0.7766 | 0.8009 |
| T max2 (h) | 0.0043* | 0.8605 |
| T½λ1 (h) | 0.1331 | 0.8960 |
| T½λ2 (h) | 0.1506 | 0.5571 |
| AUC 0-24h (μg·h/mL) | 0.7229 | 0.8007 |
| AUC 0-∞ (μg·h/mL) | 0.0331* | 0.3063 |
| MRT (h) | 0.1506 | 0.5571 |
| Cl body/F (mL/h/kg) | 0.0331* | 0.3063 |
| F milk | 0.0740 | 0.5269 |
| RCmax1 | 0.4308 | 0.0178* |
| RCmax2 | 0.5641 | 0.3398 |
| AUC 0-24h/MIC90 | 0.7228 | 0.8007 |
| T > MIC90 | 0.3404 | 0.0814 |
- *α = 0.05.
Bacteriological cure control showed that seven of the eight infected quarters were negatives. One mammary quarter of one cow was positive at 20 and 30 days post-treatment. At 20 days post-treatment, the SCC in all cows ranged between 58 000 and 223 000, which represented a statistically significant diminution tested by a nonparametric test (Mann–Whitney).
Discussion
There are no azithromycin susceptibility studies on S. aureus isolated from bovine mastitis cases. However, there are data for other macrolides. Usually when running antibiograms, an erythromycin disk is used to evaluate susceptibility to the macrolide family. Variable percentages of macrolide resistant S. aureus ranging from 1.9% to 26.3% have been reported in several studies (Ziv., 1980; Owens et al., 1997; Teagle & David, 1999; Andrade et al., 2000). At present there are highly specific methods for susceptibility testing of veterinary pathogens (CLSI, 2008). The methods and interpretive criteria used to evaluate the antimicrobial susceptibility are determinant factors and might have strong influence on the results. However, the same techniques are not always used and the same study protocols are not strictly followed. This is a common finding in the literature, so, the variability factors related to the used method should be considered.
The MIC50 calculated for the 51 S. aureus isolations was 0.5 μg/mL and the MIC90 was 1 μg/mL. Although it is not advisable to compare MICs of different antimicrobial agents, it is worth stating that erythromycin MIC90 for bovine isolated S. aureus was reported as 0.5 μg/mL in several publications (Watts et al., 1995; Watts & Salmon, 1997; Salmon et al., 1998; Ruiz et al., 2001; Pitkäläet al., 2004). A study made in Argentina reported an erythromycin MIC90 of 0.75 μg/mL (Gentilini et al., 2000).
Comparison between bioassay and HPLC methods of analysis of azithromycin have also been reported by Riedel et al. (1992), showing no antimicrobial activity from azithromycin metabolites. In human, up to 10 metabolites of azithromycin have been identified and all were microbiologically inactive (Ballow & Amsden, 1992; Lalak & Morris, 1993).
The most prominent pharmacokinetic characteristic of azithromycin is the presence of high tissue concentrations which are maintained a long time after serum concentrations decline to very low levels. This characteristic was demonstrated by the same authors in lactating dairy cows (Turic et al., 2003a), in goats (Cárceles et al., 2005) and humans (Foulds et al., 1990). Azithromycin T½λ was long, an expected finding, according to the characteristics of this antimicrobial.
Milk azithromycin levels resulted much higher than those found in plasma (between 40 and 70 times in milk that in plasma concentrations), which is a logical finding according to the lipophilicity and wide distribution of the drug. Previous research made by our group showed that after one i.m. dose of azithromycin at 10 mg/kg to lactating Holstein cows, the Cmax in milk were 4.72 μg/mL in healthy cows and 3.51 μg/mL in mastitic cows and the Cmax in plasma were 0.35 μg/mL and 0.44 μg/mL in healthy and mastitic cows, respectively (Turic et al., 2003a).
A study carried out in goats (Cárceles et al., 2005) reported that, after i.m. dose of 20 mg/kg azithromycin, the Cmax in plasma was 0.65 ± 0.09 μg/mL which was higher than the one observed in the present work 0.18 ± 0.16 μg/mL. Furthermore the different species the higher dose could contribute to the explanation of this difference. The Tmax in goats was 1.25 ± 0.25 h, whereas in this study it was 2 ± 1.56 h.
Another study performed in foals (Davis et al., 2002) suggests that azithromycin may be an alternative to erythromycin for treatment of Rhodococcus equi pneumonia. R. equi is a Gram-positive aerobe that behaves as a facultative intracellular microorganism, resembling S. aureus. The PK profile of this antibiotic in foals was determined in plasma, polymorphonuclear leukocytes (PMN), bronchoalveolar lavage (BAL) fluid, and alveolar cells after oral dose of 10 mg/kg. After 4 and 12 h oral administration, azithromycin concentrations in PMN were 89 and 202 times the corresponding plasma concentrations, respectively. In addition, azithromycin concentrations in PMN persisted for 120 h after oral administration, with a half-life of more than 49 h, although the drug was detectable in plasma for only 12–24 h (the plasma half-life was 16 ± 4.54 h). Azithromycin concentrations in PMN and alveolar cells at 120 h after oral administration were four times above the MIC reported for human isolated R. equi, ≤1 μg/mL (Mascellino et al., 1994).
In our study, milk azithromycin concentrations were more than six times the S. aureus MIC at 48 h after 2nd i.m. administration. The PK/PD surrogate markers should be used with caution in the case of macrolides for which milk and tissue pharmacokinetics have an important role to predict clinical efficacy. Therefore, further studies are necessary for a better integration of PK and PD parameters to predict clinical efficacy for macrolides, especially for azithromycin (Benchaoui et al., 2004).
Azithromycin exhibited major penetration into milk and it was cleared rather slowly. Pharmacokinetic parameters indicated a high retention of the drug in peripheral compartments. The T½λ in milk after first administration was at least four times longer than that in plasma. Azithromycin T½λ suggested that milk concentrations exhibited a tendency to decrease more slowly than plasma ones. The same pattern was observed after a single 10 mg/kg i.m. dose of azithromycin to lactating Holstein cows (Turic et al., 2003a).
When comparing PK parameters by grouping quarters according to health status, it was observed that azithromycin was eliminated more slowly from mastitic quarters. Although this was an unexpected finding (the pKa partition hypothesis suggests the opposite), it is coincident with previously reported data (Turic et al., 2003a). Milk pH in the experimental animals ranged between 6.5 and 7.5 with the majority of values around 7.0. Average pH from all mastitic quarters was 7.13 ± 0.23 and from healthy quarters was 6.90 ± 0.21 (see Table 6). This is a normal finding for animals carrying subclinical mastitis. Azithromycin is a weak base with a pKa value of 8.74, as a consequence, by application of the Henderson–Hasselbach equation, there would be approximately double azithromycin molecule dissociation in mastitic milk and more than three times in milk of healthy animals in comparison with plasma (see Table 6). Alkaline drugs (like azithromycin) are trapped in acidic compartments. This theoretical considerations could not, however, be confirmed by the experimental findings reported here. Azithromycin (i.m.) gave rise to very low plasma AUCs, which could be explained by its very high liposolubility and penetration into tissues. Although higher AUC was expected in milk of healthy animals (more acidic), which is a common finding with the classic macrolide antibacterials, we found exactly the opposite end. In our experiment, the highest concentrations were determined in the milk of mastitic animals, with an AUCmilk/AUCplasma ratio of 743.60 (see Table 6). Our explanation for this unexpected finding is the amount of somatic cells (SCC) present in mastitic milk in comparison with the normal milk. In the former case, the number of SCC was several times above those in normal milk. Mastitic milk normally exhibits very high cell counts as consequence of the inflammatory reaction. As it is known, azithromycin is able to reach high concentrations at infected sites, as a result of increased delivery from phagocytes (Wildfeuer et al., 1994, 1996; Labro, 1998; Shryock et al., 1998; Davis et al., 2002). On this basis, we consider that the inflammatory reaction (and the high amount of cells) in the infected quarters is the main reason for the differences found between mastitic and healthy quarters. A significant azithromycin fraction could be trapped in the milk-cell compartment without participating of the plasma:milk equilibrium, largely dependent on the pKa– pH relationship. The AUC0-∞ (P ≤ 0.05) and the MRT were higher in whole milk from mastitic quarters, which may indicate that the drug is present in higher amounts and persist during longer time in mastitic quarters than in healthy ones. At the same time, the Fmilk of azithromycin was higher in the mastitic quarters indicating a different PK profile of azithromycin depending on the quarter status. The previous data, reported after a single 10 mg/kg i.m. dose of azithromycin to lactating Holstein cows, support our observations (Turic et al., 2003a). The Clmam/F (P ≤ 0.05) showed that azithromycin elimination was faster in healthy quarters than in mastitic quarters.
| Plasma | Mastitic milk | Healthy milk | |
|---|---|---|---|
| pH | 7.40 | 7.13 | 6.90 |
| Dissociated/non dissociated molecules | 21.88/1 | 40.74/1 | 69.18/1 |
| Theoretical ratio of dissociated molecules | 1 | 1.86 | 3.16 |
| Experimental Milk/plasma AUC | – | 743.60 | 482.72 |
After an intramammary azithromycin syringe containing 125 mg in each mammary quarter of lactating Holstein cows, difference between the AUC in milk of mastitic and healthy cows resulted statistically significant. The AUC of azithromycin in mastitic cows was 1336.82 μg·h/mL and in healthy cows was 458.02 μg·h/mL (Errecalde et al., 2003). Showing a similar pattern, after an intramammary azithromycin syringe containing 500 mg in each mammary quarter of Holstein cows at drying off, the Fmilk in mastitic cows was higher than the Fmilk in healthy cows (Turic et al., 2003b).
As expected, the variability of the concentrations in mastitic quarters was higher than that in healthy quarters. Variability of the concentrations in healthy quarters is inherent to the animal physiology and the quarters themselves. In mastitic quarters, there is an extra factor related to the pathological status. Therefore, in addition to the physiological variability there are effects related to infection and inflammation levels, the somatic cell count, the presence of abscesses and/or fibrosis in glandular tissue, milk compositional changes and other circumstances related to mastitis.
As a consequence of the animal selection criterion, the productive levels of both groups were not extremely different. However, separating the groups according to median production allowed us to identify observations that may be extrapolated to situations of major differences in productive levels. The AUC0-∞ was higher in quarters of low-producing cows than in quarters of high-producing cows. It is possible that a lower milk production causes a slower antibiotic elimination, with a lower Clmam/F value and more prolonged T½λ2. On the basis of these results, we could suggest that low-producing cows have a high tendency to exceed LMR in milk while high-producing cows can eliminate (and dilute) the drug fast enough so as to diminish ‘contact time’ and clinical efficacy possibilities.
According to the excellent availability obtained in milk and a relatively low MIC determined in vitro, we consider azithromycin as a potential antimastitic drug and especially an alternative for the treatment of S. aureus mastitis. Nevertheless, before its recommendation, the withdrawal time must be carefully calculated, especially in consideration of its long persistence. This long persistence is a disadvantage of AZT, although its use in dry cow therapy might be an interesting alternative. Nonetheless, clinical studies are required to establish the optimal PK/PD ratio of azithromycin in milk and mammary tissue. Additionally, we consider that therapeutic protocols should be adjusted according to the level of production of the animals to be treated, because optimal concentrations must be reached avoiding cow under/over dose. That would result in public health benefits, as it is a way to avoid the presence of chemical contaminating agents in milk. Moreover, unwanted exposure of bacteria to sub-optimal antimicrobial concentrations, with the risk of resistant strains emergence, would be minimized.
Acknowledgments
The authors thank CONICET for its collaboration by granting 2004–2009 doctoral scholarship. Research at the Pharmacology Department, Faculty of Veterinary Science, Universidad Nacional de La Plata (Buenos Aires, Argentina) is partially supported by the Agencia Nacional de Promoción Científica y Tecnológica (PICT N°975).
The milk SCC was carried out by ‘La Serenísima Laboratory’, Ranchos, Buenos Aires, Argentina.
Field work was done thanks to the collaboration of Roberto Vaca, María V. Lucas, Juan M. Rodríguez Persico and Florencia Rigally.
Bárbara Huber, Andrea Lambertini and María L. Marchetti are also acknowledged for their lab work collaboration.




