Impact of the type of catheter on the absorption of tylvalosin (acetylvaleryltylosin) administered orally to broiler chickens
Pharmacokinetic and pharmacodynamic data are useful to interpret the efficacy and safety of a product. However, information on avian pharmacotherapy is scarce. Most of the pharmacokinetic studies on antimicrobials in chickens are based on drugs applied orally using ‘oral gavage’. However, it is very difficult to find a clear description of the type of catheter used for the administration of the antimicrobials.
Aivlosin® (ECO Animal Health, London, UK), a water-soluble powder for the treatment of mycoplasmosis and other diseases in poultry and swine production, contains the macrolide tylvalosin (acetylisovaleryltylosin) (Cerdáet al., 2002, 2006a). During pilot studies on the pharmacokinetics of tylvalosin in chickens, we found a high variation in the absorption profile not only between individuals but also within individuals when used on separate occasions. To investigate the cause of this variation, a study was designed with the objective of analysing the impact of the quality of the catheter used on the plasma kinetics of tylvalosin.
A total of 12, 2-week-old chickens were used. Group A (six chickens) received tylvalosin orally, through a flexible 25-cm-length catheter (polyvinyl chloride), at a dosage of 20 mg/kg bodyweight (b.w.); group B (six chickens) received tylvalosin orally, through a rigid 25-cm-length catheter (stainless steel cannula), at a dosage of 20 mg/kg b.w. Blood samples (0.5 ml) were collected from the wing vein in heparinized tubes at the following times: 0, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12 and 24 h postadministration.
Plasma was separated and frozen at −20°C until analysis. All samples were assayed on the week following collection. Tylvalosin plasma concentration was determined using a microbiological assay (Bennet et al., 1966) with Micrococcus luteus ATCC 9314 as test micro-organism (Cerdáet al., 2006b,c). The limit of quantification of the method was 0.05 μg/ml. The method was linear between 0.025 and 10 μg/ml (r = 0.9974). Inter- and intra-assay coefficients of variation were <10%.
Individual bird’s tylvalosin concentration vs. time curves were analysed by nonlinear least square regression analysis using pcnonlin (SCI Sofware, Lexington, KY, USA), applying a one-compartment open model with first order absorption and elimination. Initial estimates were determined using the residual method (Gibaldi & Perrier, 1982) and refitted by nonlinear regression. Most pharmacokinetic parameters were calculated using classic equations associated with compartmental analysis (Gibaldi & Perrier, 1982). Area under the plasma concentration–time curve [AUC(0–∞)] and mean residence time (MRT) were calculated by the trapezoidal rule (linear for the ascending and log-linear for the descending part of the curve).
Pharmacokinetic parameters are expressed as mean ± standard deviation, except for half-lives which are reported as harmonic mean ± pseudo-standard deviation. Main parameters for each animal were statistically compared for the two administration methods by applying Wilcoxon-signed rank test. Differences were considered statistically significant when P ≤ 0.05.
Figure 1 shows the tylvalosin plasma concentration vs. time curve after oral administration of tylvalosin to broilers using either a rigid or a flexible catheter. The results clearly show the impact of the type of the catheter on drug absorption.
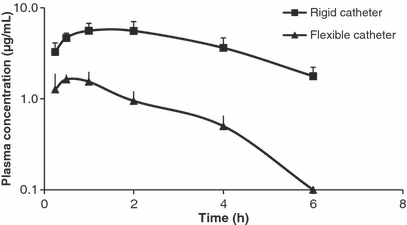
Mean (±SD) tylvalosin plasma concentrations as a function of time after oral administration to broilers at a dosage of 20 mg/kg bodyweight using a rigid catheter () or a flexible catheter () (n = 6).
Tylvalosin plasma concentrations determined after administration through the rigid catheter were higher and less variable than those determined through the flexible catheter. These differences are reflected in the estimated pharmacokinetic parameters (see Table 1). AUC for the rigid catheter is three times that of the flexible one. Moreover, statistically significant differences are observed for other parameters describing the absorption process. Cmax is almost six times higher when using a rigid catheter. Tmax and the absorption rate constant show a very important inter-subject variation. It is important to highlight the lack of differences in parameters reflecting elimination, such as λ (0.322 ± 0.153 and 0.418 ± 0.129 1/h, for the rigid and the flexible catheter respectively) and T½λ (2.474 ± 0.834 and 1.828 ± 0.604 h, for the rigid and the flexible catheter respectively).
| PK parameter | Rigid catheter | Flexible catheter |
|---|---|---|
| Vd(area)/F (l/kg) | 2.914 ± 0.768 | 9.067 ± 2.537* |
| λ1 (1/h) | 2.130 ± 1.272 | 9.226 ± 6.815* |
| λ (1/h) | 0.322 ± 0.153 | 0.418 ± 0.129 |
| AUC(0-∞) (μg·h/ml) | 17.060 ± 5.830 | 5.454 ± 1.856* |
| t ½λ1 (h)† | 0.471 ± 0.350 | 0.175 ± 0.122* |
| t ½λ (h)† | 2.474 ± 0.834 | 1.828 ± 0.604 |
| T max (h) | 1.202 ± 0.374 | 0.571 ± 0.203* |
| C max (μg/ml) | 6.104 ± 1.164 | 1.641 ± 0.389* |
- *Significantly different (P < 0.05).
- †Half-lives are expressed as harmonic mean ± pseudo-standard deviation.
- λ1 absorption rate constant; λ elimination rate constant; AUC(0–∞) area under the plasma concentration vs. time curve from 0 to infinite; Vd(area)/F volume of distribution calculated by area method; t½λ1 absorption half-life; t½λ elimination half-life; Tmax time of maximum concentration; Cmax maximum concentration; F, bioavailability (unknown).
These results suggest that the type of catheter used has a significant effect on the absorption of tylvalosin in broilers. Studies carried out in our laboratory, using dyes, have shown that when using a flexible catheter, the administered dose is deposited, most of the times, into the crop and not into the proventriculus.
The crop is an expansion of the cervical oesophagus and its main function is to store feed before it is delivered to the proventriculus. The crop is lined with incompletely keratinized stratified squamous epithelia (Hill, 1971; Sturkie, 1976) and absorption of drugs from the crop is thought to be minimal or absent (McLelland, 1979; Dorrestein, 1992). This lack of absorption may be a consequence of the presence of food in the crop, which would affect crop movements (Dorrestein et al., 1984; Dorrestein, 1992). Depending on the consistency of the feed, the emptying of the crop can be complete in 3–20 h (Dorrestein & van Miert, 1988). A second, and more plausible, hypothesis is related to the presence of Lactobacillus flora in the crop. The capacity of Lactobacillus to inactivate macrolides has been reported by Dutta and Devriese (1981) and Devriese and Dutta (1984) and could explain the differences observed when tylvalosin is deposited into the crop, in which the flora is almost 100% composed of Lactobacillus.
These results show the importance of pilot studies to investigate the impact of the type of catheter used for oral administration on the absorption of drugs in broiler chickens. Special attention should be given to antimicrobials such as macrolides as deposition of the drug into the crop may well affect absorption. In addition, it is important to highlight that other antimicrobials or formulations could also be affected by the type of catheter used to administer the drug.




