Prediction of xenobiotic clearance in avian species using mammalian or avian data: how accurate is the prediction?
The views expressed in this article are those of the authors and do not reflect the official policy of the FDA. No official support or endorsement by the FDA is intended or should be inferred.
Although the knowledge of pharmacokinetics in veterinary and avian medicine is important for dosage selection (Lees & AliAbadi, 2002) there are limited pharmacokinetic data in pet and nondomestic bird species. As a result, dosage regimens may be designed using extrapolation from doses approved in domestic avian species, such as chickens or turkeys (Baert & De Backer, 2003), and these proposed dosages are often reported to be problematic (Graham et al., 2005). Allometric scaling is an invaluable approach for dosage selection in the absence of either species-specific pharmacokinetic data or drug experience (Boxenbaum & Fertig, 1984; Boxenbaum & Dilea, 1995; Lave et al., 1997; Obach et al., 1997; Mahmood, 2005). Without an understanding of the factors that can influence the accuracy of these predictions, such extrapolations can lead to ineffective doses or excessively high doses that can cause serious toxicity (Cuthbert et al., 2007).
To date, the appropriateness of traditional allometric methods have not been carefully examined in avian species. Therefore, we explored whether or not we could apply allometric scaling to birds, using either data from other avian species or from mammalian species, to estimate the systemic clearance of xenobiotics.
 (1)
(1)Equation 1 was used to predict drug clearance in birds using at least three mammalian species. To predict the clearance from mammals to birds, seven drugs were selected based upon the availability of published reports of drug pharmacokinetic data following intravenous administration. The drugs employed are shown in Table 1.
| Compound | Primary clearance mechanism in mammals |
|---|---|
| Enrofloxacin | Hepatic‡ |
| Salicylic acid | Renal* |
| Meloxicam | Hepatic* |
| Flunixin | Renal and hepatic‡ |
| Gentamicin | Renal* |
| Chloramphenicol | Hepatic* |
| Sulphadimidine | Renal† |
The inclusion of drugs that are renally cleared and drugs cleared primarily by hepatic metabolism enabled an assessment of whether clearance mechanism biased the accuracy of the interspecies extrapolations. The following two methods were used to predict clearance in the birds.
- •
In the first method, the allometric Eqn (1) was developed using only mammalian data. The clearance was then predicted in the birds and the predicted clearance compared with the observed clearance.
- •
In the second method, clearance was predicted in the birds using only data from birds. There were five drugs for which clearance values were available in five bird species. At least three avian species were used for developing the allometric equation and then the clearance was predicted in the remaining two species.
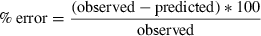
Table 2 summarizes the allometric exponents and the corresponding correlation coefficients (R) of the regression of log clearance vs. log body weight for seven drugs from mammals alone or five drugs from birds alone. In all cases, the correlation coefficient exceeded 0.88. The exponent of the allometry ranged from 0.497–1.577.
| Drugs | Species used | Coefficient | Exponent | R |
|---|---|---|---|---|
| From mammals | ||||
| Enrofloxacin | Mouse, rat, rabbit | 28.2 | 0.802 | 0.984 |
| Salicyclic acid | Rabbit, dog, goat | 0.33 | 1.577 | 0.983 |
| Meloxicam | Mouse, rat, minipig | 0.85 | 1.102 | 0.993 |
| Flunixin | Rabbit, cat, dog | 3.3 | 0.497 | 0.883 |
| Gentamicin | Rat, rabbit, dog | 4.2 | 0.668 | 0.944 |
| Chloramphenicol | Cat, dog, goat | 4.1 | 1.153 | 0.992 |
| Sulphadimidine | Rat, dog, goat | 0.53 | 1.015 | 0.989 |
| From birds | ||||
| Enrofloxacin | Hawk, rheas, ostrich | 8.6 | 1.659 | 0.942 |
| Salicyclic acid | Pigeon, chicken, ostrich | 1.9 | 1.253 | 0.986 |
| Meloxicam | Pigeon, chicken, ostrich | 0.52 | 1.843 | 0.983 |
| Flunixin | Pigeon, chicken, ostrich | 0.60 | 1.625 | 0.883 |
| Gentamicin | Owl, rooster, turkey | 1.3 | 0.720 | 0.925 |
Table 3 shows the observed vs. predicted clearance from mammalian data to avians. The percent error in predicted clearance values was less than 100% across all birds for both salicylic acid and chloramphenicol. For all other drugs, the percent error was largely species-specific. For example, the percent error for meloxicam ranged from 7% in the duck to 317% in chickens, despite their similar body weights.
| Drugs | Body weight (kg) | Observed Cl (mL/min) | Predicted Cl (mL/min) | % error |
|---|---|---|---|---|
| Enrofloxacin | ||||
| Turkey | 5.1 | 37 | 104 | 181 |
| Ostrich | 44 | 3268 | 576 | 82 |
| Broiler | 0.66 | 18 | 68 | 278 |
| Rheas | 3.0 | 179 | 68 | 62 |
| Red-tailed hawk | 1.3 | 5 | 34 | 580 |
| Salicylic acid | ||||
| Pigeon | 0.45 | 0.52 | 0.09 | 83 |
| Duck | 3.0 | 8.0 | 1.89 | 76 |
| Turkey | 8.0 | 61.3 | 8.8 | 86 |
| Ostrich | 19 | 60.2 | 34.3 | 43 |
| Chicken | 2.2 | 7.70 | 1.14 | 85 |
| Meloxicam | ||||
| Pigeon | 0.45 | 0.29 | 0.35 | 21 |
| Duck | 3.0 | 3.05 | 2.85 | 7 |
| Turkey | 8.0 | 7.3 | 8.4 | 15 |
| Ostrich | 19 | 228 | 21.8 | 90 |
| Chicken | 2.2 | 0.48 | 2 | 317 |
| Flunixin | ||||
| Pigeon | 0.45 | 0.48 | 2.22 | 363 |
| Duck | 3.0 | 7 | 5.70 | 19 |
| Turkey | 8.0 | 24 | 9.3 | 61 |
| Ostrich | 19 | 158 | 14.3 | 91 |
| Chicken | 2.2 | 0.33 | 4.9 | 1385 |
| Gentamicin | ||||
| Red-tailed hawk | 1.3 | 3.1 | 5.5 | 77 |
| Owl | 1.5 | 1.8 | 4.9 | 172 |
| Golden eagle | 3.6 | 5 | 12.3 | 146 |
| Roosters | 4.7 | 1.6 | 6.7 | 319 |
| Turkey | 11 | 5 | 13.9 | 178 |
| Cloramphenicol | ||||
| Pigeon | 0.45 | 11.7 | 1.84 | 84 |
| Duck | 0.98 | 40 | 14.6 | 64 |
| Turkey | 11 | 132 | 32.4 | 75 |
| Sulphadimidine | ||||
| Japanese quail | 0.13 | 0.077 | 0.064 | 17 |
| Pheasant | 1.2 | 0.11 | 0.64 | 482 |
| Hen | 1.6 | 0.18 | 0.85 | 372 |
Table 4 shows the observed vs. predicted clearance across avian species. In general, the percent error was substantially smaller when extrapolations were generated across birds using only data generated in birds. For example, unlike the 77 and 146% prediction error obtained when gentamicin clearance was estimated from mammalian data for Red-tailed hawks and Golden eagles, respectively, the corresponding error was only 45 and 18% when only avian data were used in these extrapolations. Markedly, larger extrapolation errors were observed for enrofloxacin and meloxicam, even when only the avian data are used.
| Drugs | Body weight | Observed Cl (mL/min) | Predicted Cl (mL/min) | % error |
|---|---|---|---|---|
| Enrofloxacin | ||||
| Turkey | 5.1 | 37 | 128 | 246 |
| Broiler | 0.66 | 18 | 53 | 194 |
| Salicylic acid | ||||
| Turkey | 8.0 | 61.3 | 24.8 | 60 |
| Duck | 3.0 | 8.0 | 7.4 | 8 |
| Meloxicam | ||||
| Turkey | 8.0 | 7.3 | 24.9 | 241 |
| Duck | 3.0 | 3.0 | 4.0 | 33 |
| Flunixin | ||||
| Turkey | 8.0 | 24.0 | 17.5 | 27 |
| Duck | 3.0 | 7.0 | 3.5 | 50 |
| Gentamicin | ||||
| Red-tailed hawk | 1.3 | 3.1 | 1.7 | 45 |
| Golden eagle | 3.6 | 5 | 4.1 | 18 |
Dosage regimens are often designed using linear extrapolation (i.e. based upon mg/kg dosages) from doses approved in domestic avian species, such as poultry (Baert & De Backer, 2003). Therefore, alternative methods of dosage predictions are needed. In the United States, under the Animal Medicinal Drug Use Clarification Act (AMDUCA), practitioners may take approved agents (veterinary or human) and extrapolate their use to nonapproved species, often with limited scientific data to support this decision. Because of the value of these animals or their status as threatened or endangered species, the traditional method of ‘trial and error’ for treatment selection is inappropriate.
Based upon our analysis, it is evident that allometric scaling between mammalian and avian species produces highly biased estimates of drug clearance, regardless of whether the drug is primarily cleared in mammalian species through renal or hepatic mechanisms. In contrast, markedly less error is achieved when the predictions are based solely upon data generated in other avian species. This is particularly true if the drug has a substantial renal component in the elimination process.
The question that follows from this observation is why drugs that are primarily cleared by renal excretion fail to scale between birds and mammals. One explanation for potential differences in clearance mechanisms is the anatomical differences between these two types of animals. The avian renal cortex is more similar to the reptile cortex than it is to the mammalian cortex. Glomerular filtration is not constant in birds, as it is in mammals, and likely has an impact on the pharmacokinetics in avian species (Frazier et al., 1995; Oaks et al., 2004). Renal function also varies within the reproductive cycle of birds, further complicating the dose selection process (Frazier et al., 1995).
Differences in hepatic metabolism are also documented. While avian cytochrome P450 activities have been reported, their expression and role in psittacine drug metabolism is unknown (Walker, 1998). Comparisons have been performed in cultured hepatocytes from poultry to fish eating bird species, and it was noted that expression, induction and stability of CYP1A isoforms is very different between chickens and herring gulls (Head & Kennedy, 2007).
Our findings suggest that better predictions are generated when using only avian data and that further improvements in prediction are seen in drugs that are renally cleared. However, such a caveat leaves us with the additional challenge of needing to presume that primary routes of elimination in avian and mammalian species will be and are similar. Unfortunately, this may not be the case. Biotransformation is reported to have a greater role in avian species than in mammals (Dorrestein et al., 1984). It should be noted, however, that hepatic vascularity appears to be similar between birds and mammals. Average portal blood flow is 32 mL/kg/min with an average hepatic blood flow of 42 L/kg/min. Mean cardiac output in the chicken is 218 mL/kg/min. This results in 6.7% of cardiac output to the liver in chickens. This is three times lower than the percent cardiac output in either rats or dogs (Purton, 1975). Species differences in mammalian p-glycoprotein-mediated transport have been identified (Suzuyama et al., 2007) and should be considered in the interpretation of the data presented in this paper.
All allometry methods assume that the routes of elimination for a particular agent are similar across all species used in the extrapolation. The reality is that most drugs, 75% in published data from Riviere et al. (1997), are not scalable across multiple species and that allometric scaling of pharmacokinetic parameters, while useful, has limitations (Mahmood et al., 2006; Martinez et al., 2006). Therefore, when considering a first-time dose to a rare, endangered or highly valuable bird species, the best therapeutic outcome will necessitate that pharmacokinetic and efficacy data is available from that species. Accordingly, additional pharmacokinetic studies in exotic birds should be strongly encouraged. However, in the absence of such data, allometric scaling can be used if the species included in the assessment are limited to avian. We have shown that such extrapolations can improve dose predictions, leading to more realistic and rational design of dosage regimens in avian medicine.




