Safety, pharmacokinetics and use of the novel NK-1 receptor antagonist maropitant (CereniaTM) for the prevention of emesis and motion sickness in cats
Abstract
The present study characterizes the safety, pharmacokinetics, and anti-emetic effects of the selective NK-1 receptor antagonist maropitant in the cat. Safety of maropitant was determined following 15 days of subcutaneous (SC) administration at 0.5–5 mg/kg. Maropitant was well tolerated in cats at doses that exceeded the efficacious anti-emetic dose range of the drug by at least a factor of 10 and adverse clinical signs or pathological safety findings were not noted at any dose.The pharmacokinetics of maropitant in cats were determined following single dose oral (PO), intravenous (IV) and SC administration. Maropitant had a terminal half-life of 13–17 h and a bioavailability of 50 and 117% when administered PO and SC, respectively. Efficacy was determined against emesis induced either by xylazine or by motion. A dosage of 1 mg/kg maropitant administered IV, SC or PO prevented emesis elicited by xylazine. The compound had good oral antiemetic activity and a long (24 h) duration of action. Maropitant (1.0 mg/kg) was highly effective in preventing motion-induced emesis in cats. These studies indicate that the NK-1 receptor antagonist maropitant is well tolerated, safe and has excellent anti-emetic properties in cats.
Introduction
Although substance P was identified in the 1930s from extracts of brain and intestine (Von Euler & Gaddum, 1931), it wasn’t until the early 1990s that its role in emesis became appreciated (Rupniak & Kramer, 1999). Substance P is a member of the tachykinin family of peptides, all of which share the common C-terminal amino acid sequence Phe-X-Gly-Leu-Met . NH2. Mammalian tachykinins also include neurokinin A and neurokinin B (McLean, 1996). Tachykinin receptors include tachykinin NK-1 receptor, tachykinin NK-2 receptor and tachykinin NK-3 receptor. Substance P is the most potent tachykinin at the NK-1 receptor whereas neurokinin A exhibits highest affinity for the tachykinin NK-2 receptor and neurokinin B has the greatest affinity at the tachykinin NK-3 receptor (Saria, 1999). Multiple lines of evidence indicate that substance P plays an important role in eliciting emesis. Substance P is found in high concentrations in areas of the brain stem involved in emesis including the nucleus tractus solitarius, the area postrema and the dorsal motor nucleus of the vagus (Ariumi et al., 2000; Hargreaves, 2002). Microinjection of substance P into the brain stem elicits an immediate emetic response (Gardner et al., 1994). Resinferatoxin, a compound that depletes substance P, was found to have broad spectrum anti-emetic effects in the ferret (Bhandari & Andrew, 1992; Andrew & Bhandari, 1993). These data suggested that an NK-1 receptor antagonist might have anti-emetic properties. The availability of the selective NK-1 receptor antagonist CP-99,994 allowed a test of this hypothesis. CP-99,994 blocked emesis produced by both peripheral and central emetogens (Watson et al., 1995). It is now well established that NK-1 receptor antagonists can be used to inhibit emesis produced by a wide variety of emetic stimuli, in contrast to the more limited efficacy observed with other commercially available anti-emetics such as 5-HT3 receptor antagonists (Lucot, 1989).
Motion sickness is a common condition is both dogs and cats. Pets afflicted with motion sickness display salivation and other signs of nausea/discomfort often leading to productive vomiting. Several anti-emetic or tranquilizing agents are commonly prescribed off-label for the treatment of motion sickness in pets. These include agents such as acepromazine, chlorpromazine, ondansetron, and metoclopramide. Non-prescription drugs such as diphenhydramine, meclizine, and dimenhydrinate are also used. The effectiveness of these medications in the treatment of motion sickness is often poor and all come with a variety of side effects which include sedation, ataxia, and hypotension (The Merck Veterinary Manual, 2005). Maropitant (CereniaTM) is a potent highly selective NK-1 receptor antagonist that was developed as a canine anti-emetic (De la Puente-Redondo et al., 2007a,c). Maropitant has been shown to be effective in preventing motion sickness in client-owned dogs with a previous history of vomiting during transportation (Benchaoui et al., 2007a; Ramsey et al., 2008). Maropitant administered 1, 2 or 10 h, before transportation reduced the incidence of motion induced emesis by 79, 92 and 82%, respectively compared to placebo treatment. The NK-1 receptor antagonist CP-99,994 has previously been shown to prevent motion-induced emesis in cats (Lucot et al., 1997).
The purpose of the present investigation was to characterize the safety, pharmacokinetics, and anti-emetic effects of maropitant in cats and to determine if this compound is also effective in treating motion induced emesis in the cat.
Materials and methods
Acute toleration and safety studies
Cats
Male and female Domestic Shorthair cats (2–8 kg) were used in all experiments. Cats were individually housed in standard feline cages at 21 °C ± 2 °C with a 12:12 h light:dark cycle and had free access to a standard commercial cat food (Iams Cat Food®, The Iams Company, Dayton, OH) and water ad libitum. All procedures were reviewed and approved by the Pfizer Institutional Animal Care and Use Committee.
Tolerability of maropitant
The acute tolerability of subcutaneously (SC) administered maropitant was evaluated in six cats. Maropitant was dissolved in 10% SBE cyclodextrin, filtered through a 0.22 μm Millipore syringe top filter and administered once only at doses of 1.0, 2.5 or 5.0 mg/kg (two cats/dose, one male and one female). Cats were observed continuously for 4 h following compound administration, and then hourly thereafter for an additional 4 h. Any signs of reaction to the drug were noted.
The tolerability of maropitant given SC once a day for four consecutive days in 10% SBE cyclodextrin at a dose of 0.5 mg/kg was then determined in five cats. All five cats were given the drug at a single site above the left shoulder each day. Cats were examined daily at 0, 2, 8 and 24 h after the injection for any adverse signs including reactions at the injection site (i.e. pain on injection, skin thickening, edema and pain on palpation).
Safety of maropitant
Study conduct
A maropitant safety study in cats was conducted by a commercial contract laboratory facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC). The study conduct was in general compliance with the US FDA Good Laboratory Practice Regulations currently in effect (21 CFR, Part 58), however, an independent quality assurance unit was not involved (e.g., no critical phase inspections or data/report audits).
Cats
Thirty domestic shorthair cats (15 male, 15 female) (Harlan Sprague Dawley, Madison, WI) were used in this study. Cats were approximately 6 months of age and had body weights ranging from 2.1 to 4.0 kg at time of first treatment. They were individually housed to prevent physical contact between animals. Food was provided daily in amounts appropriate for maintenance and water was available ad libitum.
Design
Cats were randomly assigned to five groups of six animals each (three male, three female). On study Day 0, the first day treatments were administered, cats received placebo or maropitant at 0.5, 1.5, 2.5 or 5.0 mg/kg SC based on Day-2 body weights. Dosing was repeated once daily for a total of fifteen days.
Treatments
Maropitant for SC injection was prepared daily as a 10 mg/mL solution by dissolving compound in 10% SBE cyclodextrin. Placebo consisted of a 10% SBE cyclodextrin solution. Prior to use, maropitant and placebo solutions were filtered through a 0.22 μm Millipore Stericup-GV filter unit. Maropitant treated cats received appropriate volumes of the 10 mg/mL maropitant solution to achieve daily doses of 0.5, 1.5, 2.5 or 5.0 mg/kg and placebo treated cats received 10% SBE cyclodextrin solution at a dose volume equivalent to that of the 5.0 mg/kg treated cats (0.5 mL/kg). Solutions were administered by SC injection dorsally between the shoulders at the same time each morning, approximately 1 h prior to feeding.
Procedure
All cats were weighed and had a physical examination on Day-2 and on Day 15 prior to euthanasia. Physical examinations were conducted by a veterinarian and included rectal temperature, heart rate, thoracic auscultation, and assessment of the general physical condition of each animal. Clinical observations of all cats were carried out by trained personnel approximately 1 h prior to each dosing, within 10 min immediately following treatment and at approximately 2, 4 and 8 h after treatment. Individuals performing physical exams and clinical observations were blinded to treatment assignment.
Following an overnight fast and prior to dosing, plasma samples were collected for drug analysis on Days 0, 1, 7, 14 and 15, and blood, serum, urine and fecal samples were collected for clinical pathology indices on Days-7, 0, 7 and 14. Serum chemistry analyses included amylase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, albumin, globulin, calcium, lactate dehydrogenase, chloride, total bilirubin, direct bilirubin, creatine phosphokinase, glucose, potassium, phosphorus, total protein, sodium, urea nitrogen, and creatinine. Hematology analyses included hematocrit, mean corpuscular volume, mean corpuscular hemoglobin concentration, erythrocytes, hemoglobin, mean corpuscular hemoglobin, platelet - and leukocyte counts (neutrophils, lymphocytes, monocytes, eosinophils, basophils). Coagulation analyses included prothrombin time and partial thromboplastin time. Urinalysis included color, pH, glucose, ketones, specific gravity, protein, occult blood, bilirubin, and microscopic examination of formed elements. Fecal analysis included parasites (gross and microscopic), consistency and blood or other signs of hemorrhage (visible and occult).
On Day 15, approximately 24 h after the last treatment, animals were euthanized by exsanguination following general anesthesia with a Telazol/Xylazine mixture and necropsied. Tissues were collected for histopathology evaluation. Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin, except for eyes which were fixed in 3% glutaraldehyde. All gross lesions were examined histologically together with tissue from the liver and kidneys of all animals receiving placebo and 5 mg/kg maropitant. If any abnormalities had been found in tissues from these dose groups, tissues from lower dose groups of maropitant were to have been examined.
Maropitant pharmacokinetic studies
Two pharmacokinetic studies were conducted with the citrate salt of maropitant citrate administered at 1 mg/kg PO, SC or IV. In each study four domestic mixed breed cats (two males and two females), received single doses of the test material followed by serial blood sampling via jugular or saphenous venipuncture for up to 120 h to assay plasma drug concentrations. Water was provided to the cats ad libitum during the studies, but food was withheld for 12 h prior to and 3–4 h after dosing. When intravenous doses were administered, the doses were infused into a cephalic vein over a 30–60 sec period. Plasma samples were assayed for parent drug by a sensitive and specific method involving liquid-liquid extraction and high pressure liquid chromatography with mass spectrometric detection. In this procedure, 100 μL of plasma fortified with internal standard, a chemical analogue of maropitant, was alkalinized with 100 μL of 0.1 N sodium hydroxide and extracted twice with 2 mL of methyl t-butylether. The organic extract was then evaporated, the residue reconstituted with 300 μL of mobile phase, and 10 μL injected onto the HPLC. Reverse phase chromatography was accomplished with a Keystone BDS C8 column (3 μ particle size, 4.6 × 30 mm) and a mobile phase of acetonitrile: 10 mmol/L ammonium formate pH 3.0 (80:20 v:v) at a flow rate of 0.5 mL/min. A Sciex API-150 was used for detection. Calibration standards ranged from 5 ng/mL (the lower limit of quantification, LLOQ) through 200 ng/mL. Each chromatographic run included quality control (QC) standards at three concentrations: a low concentration at or near the LLOQ (5 or 6 ng/mL), 80, and 160 ng/mL. Bias of the assay, expressed as the mean deviation from theory for recovery of the QC standards, was <20% at the LLOQ and <15% at 80 and 160 ng/mL. Precision of the assay, expressed as relative standard deviations of the QC standards, was <15% at the LLOQ and generally <10% at 80 and 160 ng/mL. No significant interferences were observed in the assay of blank plasma.
The concentration data were analyzed in a non-compartmental analysis with WinNonlin version 2.1 (Pharsight Corp). Pharmacokinetic parameters calculated in the non-compartmental analysis included the peak plasma concentration (Cmax), time of the peak concentration (tmax), area under the concentration time curve (AUC), the terminal disposition rate constant (kel), and half-life (t1/2). Total body plasma clearance (CL) was calculated following intravenous administration as the dose divided by total extrapolated AUC. The apparent volume of distribution (Vd(area)) was calculated from intravenous treatment data as CL/kel. Absolute bioavailability was calculated as the ratio of AUC from the SC or PO treatment to the AUC from an intravenous treatment.
The two pharmacokinetic studies are briefly described below.
Study 1
A two-period Latin-square crossover study design was utilised with maropitant administered either intravenously at a dose of 1 mg/kg as a 5 mg/mL solution in 0.9% saline or orally as a capsule formulation. For the intravenous treatment, venous blood samples for plasma analysis were obtained immediately prior to dosing, at 5, 10, 20, 30, and 45 min and 1, 2, 3, 6, 9, 12 and 24 h after dosing. A similar blood sampling scheme was used for the oral treatment except that a single sample was obtained at 15 min after dosing in place of the 5, 10, and 20 min samples.
Study 2
In this study a single-group of cats each received 1 mg/kg maropitant administered subcutaneously in a formulation containing 5 mg/mL maropitant in a vehicle of 20% sulfobutyl ether cyclodextrin. Blood samples were obtained immediately prior to dosing and at 30 min, 1, 1.5, 2, 3, 6, 9, 24, 36, 48 and 72 h after dosing.
Maropitant efficacy studies
Emetogen challenge
Several potential emetogens, which have been used successfully in other species, were tested for their ability to reliably reproduce emesis in domestic shorthair cats (four cats/emetogen). The emetogens tested were syrup of ipecac, apomorphine and xylazine.
Syrup of ipecac solution was used in its commercial form (SWAN Ipecac Syrup, Perrigo Allegan, MI) and cats were dosed orally via syringe at 0.5 mL/kg. Apomorphine hydochloride (Sigma Chemical Company) was dissolved in normal saline (0.9% NaCl) to produce a 0.1 mg/mL solution which was administered at a dose of 0.04 mg/kg, intravenously (IV). The solution was filtered through a 0.22 μm Millipore syringe top filter prior to administration. Xylazine (Rompun ®) was used in its commercial form (as a 20 mg/mL solution) and was administered intramuscularly (IM) into the hind leg at a dose of 0.44 mg/kg. Following administration of each emetogen, cats were monitored and the time, frequency and approximate volume of each emetic event were recorded by an observer blinded to the emetogen administered. A visual analog scale assessed by a single blinded observer was used to quantify clinical signs associated with nausea where 0 = no nausea and 10 = most severe nausea possible. Clinical signs of nausea included drooling, excessive licking, body posture, etc.
Efficacy of maropitant against xylazine challenge
Following characterization of the emetic response to syrup of ipecac, apomorphine and xylazine in cats, xylazine was selected as the most reliable emetogen. Maropitant (1 mg/kg) was then preadministered to determine if it would prevent the emesis produced by xylazine. In the first parallel design study, maropitant was given either orally in a capsule (PO), or by injection via the subcutaneous (SC) or intravenous (IV) routes (six cats/treatment route) and compared with six cats receiving no treatment except similar handling. The injectable formulation was a 5 mg/mL maropitant solution composed of 10% ethanol, 45% normal saline (0.9% NaCl) and 45% distilled water that was sonicated to ensure dissolution and filtered through a 0.22 μm Millipore syringe top filter. Capsules were custom made for each cat by weighing an appropriate amount of maropitant into a #4 gelatin capsule that was then back filled with dextrose. Cats were challenged with xylazine 2 h after maropitant administration and were observed for emetic events by an observer masked to treatment for a 2-h period thereafter. In a second study, maropitant at 1 mg/kg or placebo were administered as custom-made oral capsules to six cats in a cross-over design with a one week wash-out period. Cats were randomly assigned to treatment order. Cats were challenged with xylazine 24 h after maropitant or placebo administration and were observed for emetic events by a blinded observer for 2 h thereafter. The visual analog scale was used by a single blinded observer to quantify signs associated with nausea.
Motion sickness
Motion sickness was elicited by a motorized device resembling an amusement park Ferris wheel first developed by Crampton and Lucot (1985). Two ventilated Plexiglas boxes, each one large enough to accommodate and allow comfortable movement of a single cat within it, were suspended from the ends of a beam that rotated around a horizontal axle at 17 rpm. The boxes themselves were counter weighted in order to maintain a horizontal floor. Each test period lasted for 30 min of rotation and 1 min of additional observation following rotation. Tests were separated by at least two weeks in order to prevent habituation to the motion stimulus.
A total of 32 adult domestic shorthair cats were used in the motion sickness study. All cats were acclimated to the Plexiglas restraint box of the Ferris wheel prior to testing. Once cats were comfortable in the boxes, they were acclimated to rotation of the Ferris wheel device. Cats were considered acclimated when they were able to tolerate 5 min of rotation at 17 rpm and appeared calm and relaxed in the boxes. After acclimation, cats were screened to determine susceptibility to motion-induced emesis, by rotation in the wheel at 17 rpm for up to 30 min. Rotation was stopped prior to 30 min if a cat experienced an emetic event. A maximum of three screening trials for individual cats were conducted at least 14 days apart. Cats were identified as susceptible to motion-induced emesis if they experienced at least one emetic event in two consecutive screening trials.
A two treatment, two period crossover studies were performed in order to determine the effectiveness of maropitant against motion-induced emesis in the cat. As susceptible cats were identified, they were randomly assigned to two groups. One group of cats was first administered maropitant at a dose of 1 mg/kg, SC (10 mg/mL solution) 4 h prior to motion challenge and the second group of cats was administered the cyclodextrin vehicle. Four weeks later the treatments for the two groups were reversed. The time, number and approximate volume of each emetic event was recorded for each cat. A visual analog scale employed by a single blinded observer was used to quantify signs associated with nausea where 0 = no nausea and 10 = most severe nausea possible. Non-productive retching events were also recorded.
Statistical analysis
Mean ± SD values for emetic events and nausea score were calculated for emetogen challenge and motion sickness studies. Data were analyzed using a Signed Rank test. Nausea visual analog data were analyzed with a mixed linear model accounting for the effects of treametn order, period and treatment. Values of P < 0.05 were considered significant. Calculations were performed with the assistance of the SAS 9.1 statistical software package.
Results
Tolerability of maropitant in the cat
The tolerability of a subcutaneous injection of maropitant was evaluated in six cats at doses of 1.0, 2.5 and 5.0 mg/kg. Each dose was tested in one male and one female cat. Following injection, cats were observed for 8 h. No abnormal behavioral changes were noted in cats receiving either 1.0 or 2.5 mg/kg but the female cat treated with maropitant at 5.0 mg/kg developed mild tremors when sleeping. Trembling was not apparent when the cat was awake. The male cat treated at 5.0 mg/kg showed no signs of reaction to the administration of maropitant. In all cats, no abnormalities were noted in behavior, appetite or level of consciousness. Physical examination revealed no evidence of cardiovascular abnormalities.
The tolerability of repeated daily SC administrations of maropitant was evaluated in a second study where drug was administered for four consecutive days at a dosage of 0.5 mg/kg. No adverse effects, including injection site reactions, were noted in any of the five animals at any time during the course of the study.
Safety of maropitant over 15 days
The safety of maropitant at dosages of 0, 0.5, 1.5, 2.5 and 5.0 mg/kg, SC administered daily for 15 days was evaluated in 30 intact cats (six per treatment group). There were no mortalities prior to the planned study end point and maropitant was clinically well tolerated at all dosage levels. There were no abnormal physical examination findings on Day 15 and no adverse events observed on any day of the study with the exception of mild diarrhea which was recorded for one male cat in the placebo group on Day 0 and excessive salivation in one male cat receiving 0.5 mg/kg maropitant on Day 0 both pre-dose and approximately 10 min post-dose. Mean animal body weights increased slightly during the study in all treatment groups with similar magnitudes of change across treated and placebo groups (Table 1). There were no adverse effects on vital signs, serum chemistry, hematology, coagulation or urinalysis parameters. Mean serum chemistry values by treatment group on Day 0 and Day 14 are listed in Table 2.
| Dose (mg/kg) | Day 2 body weight (kg) | Day 15 body weight (kg) |
|---|---|---|
| Placebo | 2.85 ± 0.62 | 2.98 ± 0.70 |
| 0.5 | 2.62 ± 0.30 | 3.08 ± 0.46 |
| 1.5 | 2.62 ± 0.34 | 2.83 ± 0.37 |
| 2.5 | 2.85 ± 0.77 | 3.02 ± 0.84 |
| 5.0 | 2.55 ± 0.27 | 2.67 ± 0.31 |
| Maropitant dose (mg/kg) | Sodium (mmol/L) | Potassium (mmol/L) | Chloride (mmol/L) | Glucose (mg/dL) | BUN (mg/dL) | Creatinine (mg/dL) | Total protein (g/dL) | Albumin (g/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | |
| Placebo | 150.7 ± 1.6 | 150.2 ± 1.0 | 5.03 ± 0.53 | 4.95 ± 0.39 | 116.7 ± 2.2 | 116.7 ± 2.4 | 85.8 ± 9.2 | 81.7 ± 5.7 | 22.8 ± 3.2 | 23.0 ± 1.4 | 0.98 ± 0.12 | 1.05 ± 0.10 | 6.13 ± 0.18 | 6.03 ± 0.14 | 2.93 ± 0.10 | 2.82 ± 0.08 |
| 0.5 | 151.7 ± 3.3 | 151.3 ± 3.3 | 5.33 ± 1.13 | 5.45 ± 0.64 | 118.5 ± 1.4 | 117.8 ± 2.1 | 82.2 ± 4.1 | 88.5 ± 14.6 | 22.3 ± 2.0 | 23.3 ± 1.6 | 0.88 ± 0.12 | 0.97 ± 0.18 | 6.02 ± 0.26 | 5.97 ± 0.20 | 2.87 ± 0.15 | 2.78 ± 0.16 |
| 1.5 | 152.8 ± 5.3 | 151.7 ± 2.3 | 5.37 ± 1.21 | 5.63 ± 0.63 | 119.2 ± 4.0 | 118.2 ± 2.5 | 86.2 ± 8.1 | 80.3 ± 6.9 | 21.5 ± 2.7 | 23.3 ± 4.7 | 0.95 ± 0.14 | 1.00 ± 0.14 | 6.23 ± 0.33 | 5.95 ± 0.33 | 2.95 ± 0.24 | 2.78 ± 0.16 |
| 2.5 | 151.8 ± 4.4 | 151.5 ± 2.2 | 5.23 ± 1.07 | 5.30 ± 0.46 | 119.5 ± 2.4 | 118.2 ± 2.2 | 95.2 ± 18.2 | 89.3 ± 8.3 | 22.8 ± 4.8 | 20.8 ± 4.2 | 1.10 ± 0.28 | 1.10 ± 0.13 | 6.12 ± 0.36 | 6.27 ± 0.16 | 2.88 ± 0.30 | 2.88 ± 0.15 |
| 5.0 | 151.0 ± 1.3 | 149.8 ± 1.5 | 5.03 ± 0.40 | 5.32 ± 0.50 | 117.8 ± 1.2 | 117.0 ± 1.8 | 85.3 ± 9.2 | 90.8 ± 14.9 | 21.7 ± 1.0 | 18.7 ± 1.6 | 0.90 ± 0.13 | 0.97 ± 0.18 | 6.25 ± 0.16 | 6.17 ± 0.23 | 2.98 ± 0.08 | 2.83 ± 0.18 |
| Maropitant dose (mg/kg) | Total bilirubin (mg/dL) | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | Calcium (mg/dL) | Phosphorus (mg/dL) | Globulin (g/dL) | CK (IU/L) | ||||||||
| Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | |
| Placebo | 0.53 ± 0.15 | 0.43 ± 0.23 | 19.0 ± 2.6 | 24.0 ± 10.1 | 67.3 ± 18.3 | 83.3 ± 29.3 | 116.2 ± 13.6 | 105.3 ± 12.2 | 10.30 ± 0.19 | 10.37 ± 0.27 | 7.30 ± 0.36 | 7.02 ± 0.46 | 3.20 ± 0.09 | 3.22 ± 0.10 | 295.7 ± 154.5 | 619.3 ± 814.5 |
| 0.5 | 0.55 ± 0.10 | 0.55 ± 0.18 | 22.2 ± 3.6 | 23.7 ± 3.2 | 75.5 ± 22.5 | 75.3 ± 23.5 | 121.5 ± 27.8 | 112.2 ± 20.5 | 10.28 ± 0.40 | 10.50 ± 0.28 | 6.73 ± 0.97 | 6.87 ± 1.00 | 3.15 ± 0.14 | 3.18 ± 0.04 | 299.8 ± 217.1 | 373.3 ± 227.1 |
| 1.5 | 0.50 ± 0.09 | 0.47 ± 0.14 | 24.3 ± 4.3 | 22.5 ± 3.4 | 78.0 ± 15.0 | 72.3 ± 22.4 | 127.0 ± 30.8 | 116.8 ± 14.9 | 10.42 ± 0.50 | 10.35 ± 0.26 | 6.88 ± 0.42 | 6.75 ± 0.46 | 3.28 ± 0.12 | 3.17 ± 0.18 | 524.7 ± 378.2 | 265.8 ± 74.0 |
| 2.5 | 0.52 ± 0.15 | 0.55 ± 0.16 | 21.0 ± 3.8 | 24.8 ± 10.4 | 74.2 ± 20.0 | 74.3 ± 21.3 | 120.7 ± 62.0 | 115.5 ± 46.9 | 10.25 ± 0.62 | 10.35 ± 0.22 | 6.65 ± 0.81 | 6.57 ± 0.95 | 3.23 ± 0.22 | 3.38 ± 0.16 | 318.5 ± 233.3 | 424.7 ± 379.3 |
| 5.0 | 0.53 ± 0.10 | 0.47 ± 0.18 | 22.2 ± 4.6 | 22.7 ± 4.6 | 76.2 ± 22.9 | 68.3 ± 14.2 | 117.0 ± 28.2 | 104.2 ± 13.4 | 10.42 ± 0.23 | 10.35 ± 0.19 | 7.12 ± 0.53 | 7.12 ± 0.40 | 3.27 ± 0.12 | 3.33 ± 0.08 | 185.7 ± 65.5 | 264.8 ± 144.5 |
- AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; CK, creatine kinase.
On necropsy, the only significant treatment related gross observations were mild to moderate red foci present at the injection site. The incidence of the red foci increased with dose and was seen in both sexes. The only microscopic histopathology findings that were considered treatment-related were hemorrhage and inflammation of the subcutis in cats receiving ≥1.5 mg/kg maropitant, fibroplasia of the subcutis in cats receiving ≥2.5 mg/kg maropitant and mineralization of the infiltrate of the subcutis in females receiving 5.0 mg/kg maropitant. An incidental finding of hyperplasia, cysts and degeneration was noted in the follicular epithelium of the thyroid in one male cat receiving 2.5 mg/kg maropitant.
Trough maropitant concentrations for each dose were found to increase with repeated dosing, approaching steady state by day 7. This was not unexpected since steady state should be achieved within approximately four half-lives and the harmonic mean half lives in the two pharmacokinetic studies were approximately 13–17 h. Steady state trough plasma concentrations of maropitant were approximately proportional to dose over the range of doses administered. Trough plasma concentrations of maropitant from the safety study are shown in Fig. 1.
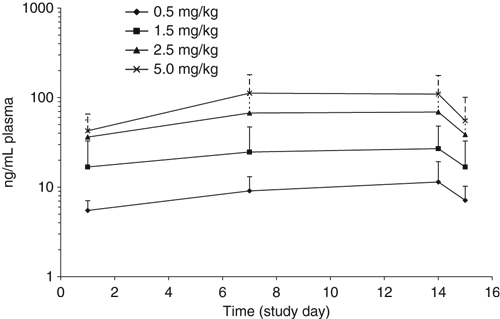
Mean (SD) trough plasma concentrations of maropitant from the safety study.
Pharmacokinetics of maropitant
Study 1: The total body plasma clearance of maropitant following intravenous administration at 1 mg/kg was 4.6 mL/min/kg (75% CV), the terminal rate constant was 0.042 L/h (21% CV), and the apparent volume of distribution was 6.2 L/kg (52% CV). When maropitant was administered orally as a capsule at 1 mg/kg, the absolute bioavailability was 50% (28% CV), the peak concentration (Cmax) was156 ng/mL (50% CV) and the time of the peak concentration (tmax) ranged from 2 to 3 h. The terminal rate constant from the capsule treatment was 0.050 L/h (50% CV), similar to the value from the intravenous treatment. The harmonic mean half-lives from the intravenous and oral treatments were 16.5 and 13.1 h, respectively. Mean plasma concentrations of maropitant from the study are shown in Fig. 2.
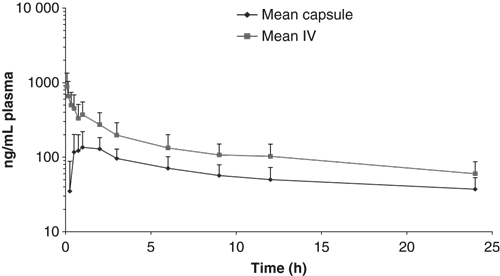
Mean (SD) plasma maropitant concentrations following intravenous and oral administration of maropitant, in tablet form, at a dose of 1 mg/kg (n = 4).
Study 2: Following subcutaneous administration of maropitant at 1 mg/kg, the peak maropitant concentration was 269 ng/mL (51% CV) with tmax values ranging from 0.5 to 2.0 h. The mean terminal rate constant was 0.040 L/h (25% CV), which corresponds to a harmonic mean t1/2 of 17.1 h. When the mean AUC over 24 h (AUC0-24) was compared to the AUC0-24 from the intravenous treatment of Study 1, the apparent absolute bioavailability from the subcutaneous treatment of Study 2 was estimated to be 117% and consistent with complete absorption.
Emetogen evaluation
There are few published reports of emetic models in the cat. Therefore, to test the anti-emetic efficacy of maropitant, several potential emetogens, which have been used successfully in other species10,20, were tested for their ability to reliably reproduce emesis in cats. The emetogens tested were syrup of ipecac, apomorphine and xylazine. Cats treated with 0.5 mL/kg syrup of ipecac PO became anorectic for several days and aversive to handling after a single dose, but did not vomit. Apomorphine (0.04 mg/kg, IV) failed to produce emesis in any cat at a dose that reliably causes emesis in the dog and other species12. In contrast, xylazine at 0.44 mg/kg, IM was found to be a reliable emetogen in cats and was selected to test the efficacy of maropitant.
Xylazine-induced emesis
Maropitant administered at 1 mg/kg PO or SC 2 h prior to administration of xylazine significantly reduced the mean number of emetic events when compared to untreated cats (P < 0.05, Table 3). When administered IV, maropitant completely prevented xylazine induced emesis in all cats (100% reduction in emetic events). In a second study, the duration of action of maropitant at 1 mg/kg PO was tested in a cross-over design study compared to placebo. Maropitant or placebo were administered 24 h prior to the xylazine challenge. Maropitant given 24 h prior to xylazine reduced the mean number of emetic events compared to placebo and also reduced the mean visual analogue nausea score (Table 4).
| Dose mg/kg | Route | Emetic events mean ± SD | Reduction in emesis vs. control |
|---|---|---|---|
| Untreated control | NA | 1.67 ± 1.21 | NA |
| 1.0 | SC | 0.42 ± 0.51* | 76.4% |
| 1.0 | IV | 0* | 100% |
| 1.0 | PO | 0.17 ± 0.41* | 90.0% |
- n = 6 per group; animals were observed for 24-h following the 0.44 mg/kg, SC dose of xylazine.
- *Denotes a significant reduction in emesis compared to control (P < 0.05).
| Dose mg/kg | Route | Emetic events mean ± SD | Reduction in emesis vs. control | Nausea Score (0–10) |
|---|---|---|---|---|
| Placebo | PO | 1.50 ± 0.55 | NA | 3.03 ± 1.72 |
| 1.0 | PO | 0.50 ± 0.55 | 66% | 1.58 ± 0.99 |
- n = 6 per group; animals were observed for 24-h following the 0.44 mg/kg, SC dose of xylazine.
Motion-induced emesis
The efficacy of maropitant in preventing motion-induced emesis was tested by placing susceptible cats in a motorized device resembling an amusement park Ferris wheel. Seven of the 32 adult cats initially tested, were found to be susceptible to motion sickness induced by the Ferris wheel device. These cats were used in a cross-over design study in which cats received either 1.0 mg/kg maropitant or an equivalent volume of vehicle SC, 4 h prior to a 30 min motion challenge Maropitant administered at 1 mg/kg SC effectively prevented motion-induced emesis (no emetic events were observed for any cat, Table 5) and reduced motion-induced nausea in susceptible cats. Maropitant prevented productive vomiting (no emetic events) and reduced total emetic events (emesis and retching) by 68% compared to vehicle treated animals (P < 0.05 for both results), although maropitant treated cats tended to retch more than vehicle animals. The motion induced nausea score was significantly less in maropitant treated animals vs. vehicle animals (5.0 ± 1.43 vs. 7.77 ± 0.83, P < 0.05). Treatment order was not significant in this study.
| Maropitant 1 mg/kg | Vehicle control | |||||
|---|---|---|---|---|---|---|
| Emetic events | Retching | Total events | Emetic events | Retching | Total events | |
| Mean ± SD | 0 | 0.57 ± 0.79 | 0.57 ± 0.79 | 1.57 ± 1.27 | 0.14 ± 0.38 | 1.71 ± 1.11 |
| Change vs. control | −100% | +307% | −68% | |||
- n = 7 cats in a crossover design.
Discussion
The purpose of the present investigations was to determine the pharmacokinetics, safety, tolerability and anti-emetic efficacy of maropitant in the cat. Maropitant has previously been shown to be a selective, potent NK-1 receptor antagonist with broad spectrum anti-emetic activity in the dog. It is safe and highly effective in preventing or treating apomorphine, ipecac and chemotherapy-induced emesis (e.g. cisplatin) and in treating vomiting in canine veterinary patients in the field, it also markedly reduces the incidence of motion sickness in dogs (De la Puente-Redondo et al., 2007a,b; Ramsey et al., 2008; Benchaoui et al., 2007a; Vail et al., 2007). The present study extends these findings to show that maropitant is safe, well tolerated and has potent anti-emetic activity in a second species, the cat. The data indicate that maropitant was effective in reducing both xylazine-induced and motion-induced emesis in the cat under laboratory conditions.
Maropitant was well tolerated in the cat, as demonstrated in acute toleration studies (single dose and 4 day administration) and during a 15 day safety study. Subcutaneous administrations of maropitant at up to 5.0 mg/kg produced no abnormalities in behavior, appetite or level of consciousness. Physical examination revealed no evidence of neurologic or cardiovascular abnormalities. One female cat treated with maropitant at 5.0 mg/kg in the acute single dose toleration study did develop mild tremors during sleep but appeared normal while awake making a connection between the drug and the behavior uncertain. Repeated administration of maropitant at a daily dose of 0.5 mg/kg for four consecutive days did not reveal any adverse clinical signs.
The safety of maropitant at dosages 0, 0.5, 1.5, 2.5 and 5.0 mg/kg, SC administered daily for 15 days was evaluated. Maropitant was well tolerated at all doses and no adverse effects on vital signs, serum chemistry, hematology, coagulation or urinalysis parameters were noted. On necropsy, the only significant treatment related gross observations were mild to moderate red foci present at the injection site. The incidence of the red foci increased with dose and was seen in both sexes. Histologic examination of the injection site revealed hemorrhage and inflammation of the subcutis in cats receiving ≥1.5 mg/kg maropitant, fibroplasia of the subcutis in cats receiving ≥2.5 mg/kg maropitant and mineralization of the infiltrate of the subcutis in females receiving 5.0 mg/kg maropitant. No other drug-related histological findings were noted in this study at any dose of maropitant. The present study suggests that maropitant produces anti-emetic effects at doses of 0.5–1.0 mg/kg in the cat suggesting that the drug can be used safely in this species to treat emesis.
A series of exploratory studies were performed to evaluate the pharmacokinetics of maropitant in the cat. The studies consistently indicated that the terminal half-life of maropitant is 13–17 h, regardless of the route of administration. This feature is clinically important to assure anti-emetic cover for at least 24 h and explains the persistent anti-emetic activity observed when the xylazine challenge was applied 24 h after oral dosing with maropitant. The pharmacokinetic and pharmacodynamic data derived from these studies support the recommendation of a once-daily treatment schedule in cats. The drug appears to be completely absorbed following subcutaneous administration and has 40–50% bioavailable after oral administration. The absorption kinetics of maropitant determine the onset of anti-emetic activity and underpin the robust efficacy observed when the xylazine emetic challenge was applied 2 h post-dosing. Total body plasma clearance of maropitant is relatively low, at 4.6 mL/min/kg, and inter-individual variability in clearance is relatively large. The clearance of maropitant in the cat is approximately four times lower than that observed in dogs (Benchaoui et al., 2007b) which accounts for the relatively long terminal half-life described in the present studies. The pharmacokinetics of maropitant are approximately proportional for subcutaneous doses ranging from 0.5 to 5 mg/kg.
In the evaluation of a potential antiemetic compound, it is of prime importance that a reliable form of experimentally induced emesis is identified. In the present study we evaluated the use of apomorphine, ipecac and xylazine as emetogens in the cat. Only xylazine was found to produce reliable emesis without serious behavioral side effects. Maropitant markedly reduced or prevented emesis induced by xylazine. Maropitant administered at a dosage of 1 mg/kg via the SC, IV or PO route 2 h before xylazine challenge reduced the mean number of emetic events by 76, 100 and 90%, respectively, compared to untreated cats. Maropitant also demonstrated a long duration of action when given orally, reducing mean emetic events by 66% compared to placebo, when the drug was given 24 h prior to the xylazine challenge. Maropitant has previously been shown to have a low affinity at adrenergic receptors including the α2−adrenergic receptor (IC50 > 10 υΜ; De la Puente-Redondo et al., 2007c). Thus maropitant did not prevent emesis by antagonizing the α2−adrenergic agonist activity of xylazine. Indeed, maropitant is highly selective for the NK-1 receptor (De la Puente-Redondo et al., 2007c). These data suggest that maropitant acts at the NK-1 receptor to produce a rapid onset and long duration anti-emetic effect. Maropitant was tested for efficacy against motion-induced emesis using a Ferris Wheel device previously described by Crampton and Lucot (1985). Cats susceptible to motion sickness were identified in preliminary screening if they had emetic events in two successive trials on the Ferris Wheel. We found that these cats were persistantly susceptible to motion sickness in subsequent trials as judged by the number of emetic events noted in the control group (Table 5). Maropitant administered at 1.0 mg/kg SC was 100% effective in preventing motion-induced emesis in susceptible cats in this laboratory model; no emesis was observed in maropitant treated animals during this study. The same cats when treated with placebo experienced between 1 and 4 motion-induced emetic events. Treatment order was not a significant factor in this experiment. The motion sickness nausea score was significantly reduced by administration of 1.0 mg/kg maropitant SC. There was a tendency towards more non-productive retching in maropitant treated cats vs. control, although this effect was not statistically significant. If retching is considered part of the continum which includes nausea and emesis, then the retching in maropitant treated cats may result from prevention of emesis by the drug while in untreated control animals motion results in an emetic event.
Motion sickness is a significant issue in a large number of cats. Pets afflicted with motion sickness display salivation and often develop vomiting or diarrhea. Several anti-emetic or tranquilizing agents are commonly prescribed off-label for the treatment of motion sickness. These include agents such as acepromazine, chlorpromazine, ondansetron, and metoclopramide. Non-prescription drugs such as diphenhydramine, meclizine, and dimenhydrinate are also used. These medications are associated with a variety of side effects which include sedation, ataxia, and hypotension (The Merck Veterinary Manual, 2005). In contrast, maropitant was found to be extremely effective and no behavioral side effects were noted during the course of the study. These data are in agreement with Lucot et al. (1997) who found that another NK-1 receptor antagonist, CP-99,994 produced a dose dependent decrease in motion-induced vomiting in cats in the absence of behavioral changes or obvious alterations in the autonomic nervous system. Furthermore, maropitant has previously been shown to decrease or prevent motion-induced emesis in the dog in the absence of side effects (Benchaoui et al., 2007a; Ramsey et al., 2008). In the dog, the dose of maropitant required to prevent motion-induced emesis is approximately four times greater than the dose needed to prevent emesis elicited by an emetogen (Benchaoui et al., 2007a; De la Puente-Redondo et al., 2007a; Ramsey et al., 2008). In the current study we found that the dose of maropitant required to prevent emesis induced by xylazine and motion was similar. However, a full maropitant dose-response curve against xylazine and motion is required before definitive conclusions can be drawn reqarding the relative potency of maropitant against xylazine- and motion-induced emesis in the cat. In addition it is possible that the Ferris Wheel model of motion sickness may not accurately reflect clinical motion sickness and therefore the effective dose of maropitant in treating emesis may be different in the laboratory model and the clinical situation.
Maropitant has previously been shown to markedly reduce nausea as well as emesis in the dog. De la Puente-Redondo et al. (2007b) infused a chemotherapeutic dose of cisplatin (70 mg/m2) in dogs and evaluated the ability of maropitant to reduce or prevent emesis and nausea. Nausea was measured using a VAS scale based on signs of salivation, exaggerated swallowing, lip licking, and behavior. In one study maropitant was given following the first cisplatin induced emetic event. Overall nausea was significantly reduced in maropitant treated dogs compared to control animals (P = 0.013). In a second arm of the study maropitant was given prior to cisplatin and prevented the emesis. In these animals maropitant again significantly reduced the nausea VAS score compared to control dogs (P < 0.001). In the present study we showed that maropitant at a dose of 1 mg/kg, SC significantly reduced both emesis and nausea 24 h after the administration of xylazine. Although additional work is required to extend these results, the data suggests that in the cat maropitant effectively reduces nausea as well as emesis. This is important as there are many disease states in the cat where nausea limits appetite which can lead to further metabolic complications.
Vomiting is one of the more common reasons why owners take their cats to the veterinarian. Isolated episodes of vomiting rarely are a cause for concern as long as the cat still has an appetite and is bright and alert. Most healthy cats will, on occasion, vomit whole or partially digested food, or foamy, clear liquid. On the other hand, excessive or chronic vomiting can be indicative of a more serious underlying condition that requires treatment. When a cat presents with emesis food is typically withheld for 24–36 h. If the vomiting persists, or if the animal is visibly debilitated, supportive care in the form of intravenous fluid therapy is used to prevent dehydration. There are numerous causes of vomiting in cats. Some examples include motion sickness, side effects of drugs, and overeating. Obstruction of the gastrointestinal tract by a swallowed piece of string or other foreign body will cause vomiting. Vomiting occurs in conjunction with parasitic infestation in organs such as the heart, kidney, liver, and pancreas. Emesis is asscociated in the cat with liver disease, pancreatitis, ulcers, intestinal lymphoma, inflammatory bowel disease and feline leukopenia. Emesis accompanies endocrinopathies such as hyperthyroidism and diabetes mellitus. Salmonella, giardia, and coccidial infections may also produce emesis in cats. Currently there are no drugs approved for the treatment of emesis in cats. Based on the broad spectrum of antiemetic activity of Cerenia in the dog and the fact that Cerenia prevents emetogen-induced and motion-induced emesis in the cat, it seems likely that Cerenia has the potential to treat emesis associated with all these feline conditions.
The site of the anti-emetic action of maropitant is most likely to be within the central nervous system. Provocative motion sickness requires the vestibular system, the signals from which ultimately activate brain stem areas involved in emesis including the nucleus tractus solitarius, the dorsal motor nucleus and an area adjacent to the area postremaBrizzee, 1990; Miller et al., 1994; Zajonc & Roland, 2006). Injection of emetogens into the cat increases c-fos immunoreactivity in the area postrema, the nucleus tractus solitarius, the lateral tegmental field and the ventrolateral medulla (Miller & Ruggiero, 1994). These areas are involved in both emetic and autonomic reflexes. They contain a dense concentration of substance P receptors (Brizzee, 1990). Unlike other anti-emetic agents, NK-1 receptor antagonists are effective against a wide range of emetogens including radiation, cisplatin, cyclophosphamide, copper sulfate and apomorphine and well as motion-induced emesis (Bountra et al., 1993; Patel & Lindley,2003; Benchaoui et al., 2007a; De la Puente-Redondo et al.,2007b; Ramsey et al., 2008; Vail et al., 2007). Such wide spectrum activity against peripheral and central emetogens suggests that NK-1 receptor antagonists must have a site of action in a final common pathway of the emetic reflex.
In conclusion, this investigation extends previous work in the dog (Ramsey et al., 2008; Benchaoui et al., 2007a; De la Puente-Redondo et al., 2007a,b,c; Vail et al., 2007; Benchaoui et al., 2007b) to show that the NK-1 receptor antagonist maropitant is an effective anti-emetic in the cat. Maropitant has excellent activity following oral and subcutaneous administration against xylazine-induced vomiting and it effectively prevented motion-induced vomiting following subcutaneous administration. Maropitant appears to be well tolerated in cats. The pharmacokinetic, efficacy and safety data generated support the use of maropitant as a once-daily anti-emetic treatment in this species.




