Serum pharmacokinetics of oxytetracycline in sheep and calves and tissue residues in sheep following a single intramuscular injection of a long-acting preparation
Abstract
The pharmacokinetics of a long-acting oxytetracycline (OTC) formulation (Liquamycin® LA-200®) injected intramuscularly (i.m.) at a dose of 20 mg/kg were determined in four calves and 24 sheep to determine if the approved label dose for cattle provided a similar serum time/concentration profile in sheep. The AUC for the calves was 168±14.6 (μg ? h/mL) and was significantly less than the AUC for sheep (209±43 μg ? h/mL). Using the standard two-stage approach and a one-compartment model, the mean Cmax for the calves was 5.2±0.8 μg/mL, and for the sheep was 6.1±1.3 μg/mL. The mean terminal phase rate constants were 0.031 and 0.033 h, and the Vdss were 3.3 and 3.08 L/kg for the calves and sheep respectively. Analysis of the data using the standard two-stage approach, the naive pooled-data approach and a population model gave very similar results for both the cattle and sheep data. Sheep tissue residues of OTC in serum, liver, kidney, fat, muscle and injection site were measured at 1, 2, 3, 5, 7 and 14 days after a single i.m. injection of 20 mg/kg OTC. Half-lives of OTC residues in the tissues were 38.6, 33.4, 28.6, 25.4, 21.3, and 19.9 h for injection site, kidney, muscle, liver, mesenteric fat and renal fat, respectively. The ratio of tissue to serum concentration was fairly consistent at all slaughter times, except for the fat and injection sites. The mean ratios were 1.72, 4.19, 0.11, 0.061, 0.84 and 827 for the liver, kidney, renal fat, mesenteric fat, muscle and injection sites, respectively. The tissue concentrations of OTC residues were below the established cattle tolerances for OTC in liver (6 p.p.m.), muscle (2 p.p.m.) and kidney (12 p.p.m.) by 48 h, and in injection site muscle by 14 days after the single i.m. injection of 20 mg/kg.
INTRODUCTION
Oxytetracycline (OTC) is a broad-spectrum antibiotic that inhibits the growth of many pathogenic organisms, including bacteria, mycoplasma, and some protozoa (Baxter & McKellar, 1995), which is approved for use in the USA in cattle for the treatment of bacterial pneumonia.
There have been numerous studies carried out on the pharmacokinetics of OTC in cattle to examine the effects of formulation, animal age and disease status. There have been several published studies on OTC pharmacokinetics in sheep (Ziv & Sulman, 1974; Immelman & Ziv, 1982; Immelman & Van Rensburg, 1983; Ponferrada et al., 1988a,b; Nouws et al., 1990; Burrows, 1992; Elsheikh et al., 1997). In addition, there have been several papers published on OTC residues found in tissues in cattle (Pakkala et al., 1976; Nouws & Ziv, 1978; Masera et al., 1980; Bretzlaff et al., 1982; Schifferli et al., 1982Ames et al., 1983; Bretzlaff et al., 1983a,b; Ames & Patterson, 1985; George et al., 1985; Maritim et al., 1986; MacNeil et al., 1989; TerHune & Upson, 1989; Mawhinney et al., 1996) but only two limited residue studies have been published for sheep. Baxter and McKellar (1990) looked at the concentration of OTC in lung tissue of sheep 1 h after an i.v. dose of OTC, and Nouws et al. (1990) reported on residues in various tissues of sheep, calves and pigs 10 days after i.m. injection with five different OTC formulations.
Because a long-acting injectable OTC formulation has not been approved for use in sheep in the USA, these studies were carried out to produce data necessary to establish the human food safety of the long acting preparation in sheep. Tissue and serum residues of OTC were measured in sheep and serum residues measured in cattle were treated with a long-acting formulation of OTC (Liquamycin® LA-200®) in order to provide information that could be used by the US FDA Center for Veterinary Medicine (CVM) to fulfill the registration requirement for efficacy and human food safety (drug residues). In addition, a comparison of a microbiological assay, and a high-pressure liquid chromatography (HPLC) assay for OTC residues in tissues was carried out for both spiked tissues and incurred residues in sheep.
MATERIALS AND METHODS
Animals
Seventeen wether (one used as control) and eight ewe mixed breed sheep which ranged in weight from 45.5 to 61.5 kg at the time of initial injection were used. The animals were procured from the Michigan State University Sheep Teaching and Research Farm and all were previously vaccinated and dewormed. Four castrated male Angus crossbred calves weighing from 185–300 kg (mean 242 kg) were purchased from a local supplier.
Drug treatment
Calves
All calves were dosed at 20 mg/kg body weight via i.m. administration in the cervical muscle. Blood samples for serum residue data were collected by jugular venipuncture just prior to dosing and at 0.5, 1.0, 1.5, 2.0, 4.0, 6.0, 8.0, 10.0, 12.0, 16.0 and 24.0 h, and every 12 h thereafter until 336 h after injection.
Sheep
Residues and kinetics: Sheep were weighed and divided into six treatment groups. All animals (excluding control) were dosed at 20 mg/kg body weight via i.m. administration in the cervical muscle. Treatment groups corresponded to six different slaughter times for tissue residue data: 1, 2, 3, 5, 7 and 14 days after cessation of OTC administration. Blood samples for serum residue data were collected by jugular venipuncture just prior to dosing and at 0.5, 1.0, 1.5, 2.0, 4.0, 6.0, 8.0, 10.0, 12.0, 16.0 and 24.0 h, and every 12 h thereafter, before the animals were sacrificed. Samples of the following tissues were taken from each slaughtered animal: skeletal muscle and injection site muscle, liver, kidney, mesenteric and renal fat. Tissue samples were stored at a minimum of −20 °C. The animals were killed by injection of Fatal Plus®. This study was performed in complete accordance with all local, state and federal laws, ordinances, rules and regulations applicable to animal welfare.
Sample handling: blood samples were allowed to clot for at least 1 h at room temperature, then centrifuged at 1000×g for 10 min and the serum separated and frozen as two aliquots at −70 °C immediately. All samples were kept frozen at less than −20 °C until assay. One aliquot of each serum sample was shipped frozen to the University of California NRSP-7 laboratory for assay, and all samples arrived frozen and in good condition. The tissue samples were immediately frozen in separate containers. All samples were kept frozen at less than −20 °C until assay. All the tissue samples were shipped frozen to the University of California NRSP-7 laboratory for assay, and all the samples arrived frozen and in good condition. Standards were collected from control cattle and sheep serum and control sheep tissues, and frozen to provide stability data.
Serum assay
Quantitation of OTC in serum samples was accomplished using a modification of the microbial inhibition assay of Bennett et al. (1966). Bioassay plates were prepared by placing 9.5 g Mueller Hinton medium and 250 mL H2O into 500 mL Erlenmeyer flask and autoclaved for 20 min. The resulting solution was cooled to 50 °C in a waterbath, and 0.4 mL of spore suspension (1 mL Bacillus cereus spore plus 50 mL sterile saline) added. After solidification of the media, 90-μL wells were cut into the solidified bioassay plates. Serum samples were prepared for the wells by adding 20 μL of TCA (30%) to 400 μL serum, vortexed, then centrifuged for 10 min at 1000 x g. Triplicate 90 μL aliquots of the supernatant from each sample were pipetted into individual wells. Standards collected from the control serum were also added to each plate, and the plates were incubated overnight at room temperature (23 °C). Zones of inhibition were measured using micrometres and the results from the standards used to calculate the OTC concentration in each of the samples. The limit of quantitation for the calf and sheep sera was 0.125 μg/mL. The recoveries (comparison of zones produced by standards in buffer to standards in serum) were 99 and 92% for sheep and calves, respectively, and the relative standard deviations (SD) (between all assays) were 14 and 7% for sheep and calves, respectively.
Tissue analysis
The microbial inhibition assay used was the same as that used for the serum except for the well size and the extraction method. For tissues after solidification of the media, 500 μL-wells were cut into the solidified bioassay plates. Ten-gram tissue samples were homogenized in 20 mL of a potassium phosphate buffer (0.1 m, pH=4.5) by a Brinkman Polytron (Westbury, NY, USA) for 30 sec (power setting=5). The homogenates were centrifuged for 15 min at 2500 r.p.m. Duplicate 500 μL aliquots of the supernatant from each sample were pipetted into individual wells. Standards prepared in phosphate buffer were also added to each plate, and the plates were incubated overnight at room temperature (23 °C). Zones of inhibition were measured using micrometres and the results from the standards were used to calculate the OTC concentration in each of the samples. The limit of quantitation for all tissues was 150 p.p.b. The recoveries for fat, liver, muscle and kidney were 117, 95, 72 and 51%, respectively. The relative SD for all assays was 17, 15, 22 and 8% for fat, liver, muscle and kidney, respectively.
Tissues were also analysed for OTC residues using an FDA HPLC method (Hadley et al., 1995) developed for detection of OTC residues in shrimp with minor modifications including the exclusion of dry ice homogenization used for shrimp tissues, and a scaling down of the extraction and sample sizes. Samples were homogenized in 1 N HCl, suspended in acetonitrile and filtered through glass wool. The filtrate was further cleaned using liquid–liquid extraction with methylene chloride and petroleum ether. The aqueous phase was filtered through a nylon syringe filter and injected on the HPLC. HPLC detection was by fluorescence after post column derivatization. The column was a Vydac (Hesperia, CA, USA), 250×4.6 mm, 5 μm, 300 Å, C18 operated at 40 °C. The gradient was 100% water with 0.1% TFA to 75% acetonitrile with 0.1% TFA over 20 min. Post column derivatization was achieved with a 50 μm solution of europium(III) ion in 0.2 m CAPS buffer. The excitation wavelength was 395 nm and the emission wavelength was 615 nm. The recoveries from fortified samples were 86, 81, 80 and 78% for fat, liver, kidney and muscle, respectively. The limits of quantitation (LOQ) were 42, 85, 45 and 46 p.p.b. for fat, liver, kidney and muscle, respectively. The inter-assay precisions (relative standard deviations) were 7, 10, 7 and 8% for fat, liver, kidney and muscle, respectively.
A comparison of the two methods was carried out using aliquots of identical OTC fortified (300 and 1500 p.p.b.) control tissue samples, and three incurred residue samples of each tissue from animals dosed with OTC during the residue study. Ratios of the HPLC method results to the microbiological inhibition assay results were calculated.
Pharmacokinetic data and statistical analysis
The data for the serum concentrations of OTC in calves and sheep were analysed using the standard two-stage approach (STS) and the Naive-Pooled data method (NPD) with WinNONLIN® (Pharsight Corporation, Palo Alto, CA, USA), and population pharmacokinetic modeling (POP) with WinNONMIX® (Pharsight Corporation). A one compartment open model with first order absorption was fit to all data, and the data were not weighted. The linear regression algorithm in Microsoft Excel 97® was used to determine slope, intercept and half-lives (t1/2) of the tissue residue data. Pharmacokinetic parameters estimated for sheep and cattle were compared statistically using a single-factor analysis of variance and unpaired Student's t-test.
RESULTS
Table 1 shows the results of the pharmacokinetic analysis of the cattle and sheep serum data. The STS analysis of the data showed that a one compartment open model with first order absorption provided the optimum fit to the data for both calves and sheep. This model was also used for the NPD and POP data analyses. There were no significant differences between cattle and sheep for pharmacokinetic parameters calculated using the STS approach. The three different approaches to modelling the data gave very similar results for these two data sets. The parameters for the sheep and cattle are very similar using the NPD and the POP approaches; however, the apparent Vd and clearance (Cl) values calculated were higher in cattle than in sheep.
| K01 (1/h) | K10 (1/h) | AUC (μg ? h/mL) | Cl ((mL/min)/kg) | Vd (L/kg) | Cmax (μg/mL) | tmax (h) | |
|---|---|---|---|---|---|---|---|
| Calves (n=4) | |||||||
| STS | |||||||
| Mean | 1.43 | 0.031 | 168 | 1.88 | 3.30 | 5.20 | 2.83 |
| SD | 2.21 | 0.032 | 14.6 | 0.122 | 0.499 | 0.798 | 1.43 |
| NDP | 1.74 | 0.0296 | 182.7 | 1.82 | 3.69 | 5.04 | 2.38 |
| POP | 1.78 | 0.036 | – | – | 3.45 | – | – |
| CV% | 24.3 | 6.3 | – | – | 7.60 | – | – |
| Sheep (n=24) | |||||||
| STS | |||||||
| Mean | 1.23 | 0.033 | 209.5 | 1.65 | 3.08 | 6.09 | 3.5 |
| SD | 0.69 | 0.0069 | 43.1 | 0.30 | 0.82 | 1.27 | 1.2 |
| NPD | 1.07 | 0.0322 | 209 | 1.59 | 2.97 | 6.04 | 3.40 |
| POP | 1.04 | 0.033 | – | – | 2.97 | – | – |
| CV% | 4.200 | 4.0 | – | – | 4.20 | – | – |
1, 2 show the time/concentration plot of the data points and the line predicted by the POP model for the cattle and sheep, respectively.
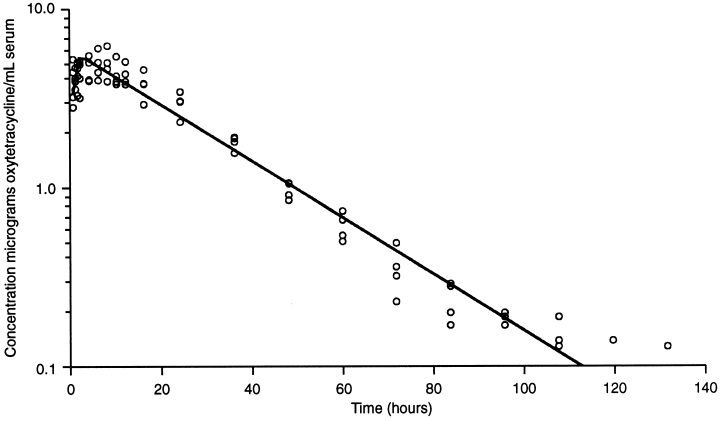
OTC concentration in cattle serum following a single i.m. injection of 20 mg OTC (Liquamycin® LA-200®) per kilogram body weight (n=4). The line represents the time/concentration profile using the calculated population parameters.
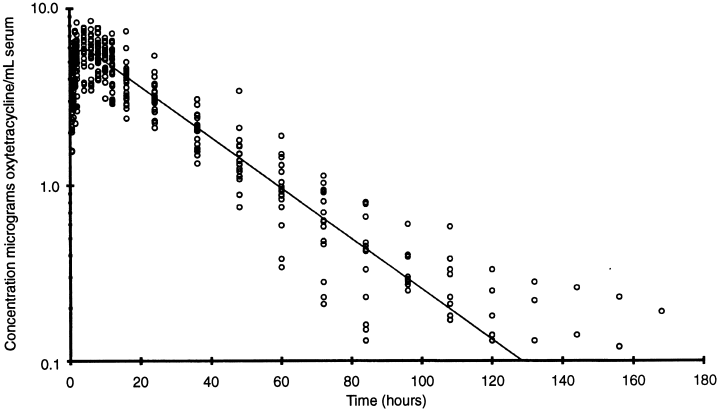
OTC concentration in sheep serum following a single i.m. injection of 20 mg OTC (Liquamycin® LA-200®) per kilogram body weight (n=24). The line represents the time/concentration profile using the calculated population parameters.
The results of the sheep tissue residue determinations are presented in Table 2. A graphic presentation of all the data is shown in Fig. 3. The kidney and the injection site were the tissues with the highest concentrations of OTC residues, and also had the longest elimination half-lives. The elimination t1/2 for the tissues were: injection site, 38.6 h; kidney, 33.4 h; muscle, 28.6 h; liver, 25.4 h; mesenteric fat, 21.3 h; and renal fat, 19.9 h.
| Sheep no. | Time (h) | Liver | Kidney | Renal fat | Mesenteric fat | Muscle | Injection site | Serum |
| 427 | 24 | 6028 | 17 008 | 1192* | 327 | 2966 | 1 390 500 | 3180 |
| 442 | 24 | 7623 | 15 254 | 408 | 191 | 3106 | 813 500 3650 | 3650 |
| 468 | 24 | 5760 | 14 726 | 291 | 182 | 3249 | 51 000 | 3060 |
| 518 | 24 | 8462 | 11 056 | 315 | 137 | 2469 | 1 904 500 | 3130 |
| 418 | 48 | 2359 | 5521 | 129 | 62 | 1028 | 520 500 | 1510 |
| 456 | 48 | 1777 | 3744 | 85 | 81 | 964 | 925 000 | 1310 |
| 512-48 | 48 | 2837 | 7253 | 190 | 125 | 1457 | 198 500 | 1380 |
| 529 | 48 | 2266 | 5932 | 100 | 56 | 902 | 16 285 000 | 1370 |
| 421 | 72 | 1063 | 3061 | 64 | - | 607 | 473 500 | 940 |
| 490 | 72 | 1204 | 3880 | - | 52 | 649 | 670 000 | 580 |
| 558 | 72 | 1505 | 2770 | 91 | 59 | 813 | 382 000 | 930 |
| 561 | 72 | 706 | 2105 | 88 | 64 | 468 | 414 000 | 700 |
| 425 | 120 | 501 | 1260 | – | – | 259 | 767 000 | 330 |
| 443 | 120 | 271 | 624 | – | – | 121 | 146 000 | 140 |
| 448 | 120 | 65 | 247 | – | – | – | 43 | – |
| 537 | 120 | 238 | 714 | – | – | 152 | 41 500 | 140 |
| 474 | 168 | – | – | – | – | – | 358 | – |
| 480 | 168 | 245 | 728 | – | – | 126 | 614 500 | 190 |
| 485 | 168 | 180 | 345 | – | – | 80 | 101 000 | – |
| 512 | 168 | 52 | 125 | – | – | 49 | 545 | – |
| 430 | 336 | – | 65 | – | – | – | 196 | – |
| 466 | 336 | – | – | – | – | – | 53 | – |
| 510 | 336 | – | – | – | – | – | 182 | – |
| 511 | 336 | – | – | – | – | – | 59 | – |
- *Sample noted to be bloody and not composed entirely of fat.
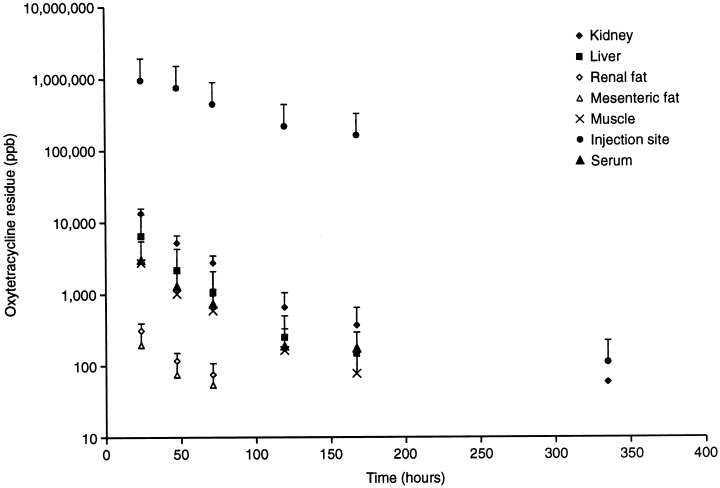
The mean±SD of concentrations of OTC in tissues of sheep dosed with 20 mg OTC per kilogram body weight prior to being killed (n=4 sheep per data point).
The ratios of the tissue OTC concentrations to the serum OTC concentrations at each slaughter point are presented in Table 3. The ratio was highest for the injection sites, followed in decreasing order by the kidney, liver, muscle and fat. The coefficients of variation were greatest for the injection sites and fat samples, and less than 25% for the muscle, liver and kidney samples.
| Time (h) | Liver | Kidney | Renal fat | Mesenteric fat | Muscle | Injection site |
|---|---|---|---|---|---|---|
| 24 (n=4) | 2.14 | 4.47 | 0.17 | 0.06 | 0.91 | 321 |
| 48 (n=4) | 1.66 | 4.02 | 0.09 | 0.06 | 0.78 | 596 |
| 72 (n=4) | 1.46 | 3.98 | 0.07 | 0.06 | 0.83 | 665 |
| 120 (n=3) | 1.72 | 4.46 | 0.91 | 1221 | ||
| 168 (n=1) | 1.29 | 3.83 | 0.661 | 3234 | ||
| Mean | 1.72 | 4.19 | 0.111 | 0.061 | 0.841 | 827 |
| SD | 0.42 | 1.05 | 0.090 | 0.029 | 0.167 | 851 |
| %CV | 24.7 | 25.0 | 81.1 | 47.3 | 19.9 | 103 |
The results of the comparison of the microbiological assay with the HPLC assay for OTC residues in tissues is presented in Table 4. The samples were essentially equivalent when measuring spiked tissue samples; however, for incurred residues in tissues with the exception of fat, the HPLC assay consistently measured higher concentrations than the bioassay. In fat samples, the microbiological assay consistently gave higher values for incurred residues than the HPLC assay.
| Fortified samples | Liver | Kidney | Muscle | Fat |
| 300 p.p.b. | 0.96 | 1.25 | 1.04 | 0.81 |
| 1500 p.p.b. | 0.91 | 1.67 | 1.23 | 0.79 |
| Average of all fortified samples=1.08 | ||||
| Incurred residue (range in p.p.b.) | Liver (501–5760) | Kidney (624–5521) | Muscle (468–3106) | Fat (327–1193) |
| 1.21 | 1.62 | 1.76 | 0.57 | |
| 1.16 | 1.49 | 1.31 | 0.97 | |
| 1.17 | 1.20 | 1.45 | 0.55 | |
| Incurred mean | 1.18 | 1.44 | 1.51 | 0.70 |
| Average of all incurred residue samples=1.21 | ||||
DISCUSSION
The pharmacokinetic parameters measured for the calves in this study were similar to those found following i.m. injection of long acting preparations and reported in other studies. For cattle, Kumar and Malik (1998) reported values for the Vd and apparent Cl of 0.86±0.07 L/kg and 76.1±3.3 (mL/h)/kg (equal to 1.3 (mL/min)/kg)), respectively. Clarke et al. (1999) reported mean Cmax values of 7.25 and 6.97 μg/mL, and mean AUC values of 260 and 253 μg ? h/mL for two different long-acting OTC preparations given i.m. at a dose of 20 mg/kg. The main purpose of the current study was to compare the time/concentration profiles in the sheep and calves and to measure the tissue residues in sheep. Therefore, an exhaustive review of the numerous previously published parameters in cattle will not be included, and this discussion will focus on the results in sheep, for which there are far fewer published data.
Table 5 presents a summary of the published pharmacokinetic parameters of OTC in sheep. There are considerable differences in the parameters found which can be attributed to the use of different formulations, different breeds, etc. Ziv and Sulman (1974) published the first report on serum pharmacokinetics of OTC in sheep and cattle. They noted that the concentration/time profiles for the sheep and cattle were so similar that they pooled them for data analysis. Immelman and Van Rensburg (1983) studied plasma concentrations of OTC in sheep after dosage with 10 and 20 mg/kg of a 10% long acting (polyvinylpyrrolidine) preparation. Using their graphic data to calculate time/concentration points with a commercial software program which translates graph data points into xy coordinates (Ungraph, Professional Software, Inc., Ferguson, MO, USA) and WinNONLIN to calculate pharmacokinetic parameters, a one-compartment model gave AUCs of 69.8 and 143 μg ? h/mL, beta t1/2 of 9.3 and 15 h, and Vd of 1.9 and 1.5 L/kg, and apparent Cl values of 0.14 and 0.07 (L/h)/kg for the 10 and 20 mg/kg doses, respectively. Burrows (1992) reported that acute and chronic, induced fascioliasis had no effect on the pharmacokinetics of OTC after i.v. dosage in sheep. Escudero et al. (1996) reported that a 20 mg/kg dose of a long-acting OTC formulation (Terramicina/LA; Pfizer, Madrid, Spain) gave mean values of: AUC=112 μg/h/mL, Vd area=7.01 L/kg, Cmax=3.47 μg/mL, terminal elimination rate constant (λz)=0.03 h−1, and Cl=0.183 (L/h)/kg (3.05 (mL/min)/kg. The Cmax, and AUC values they report are almost one-half of those found in this study, and their clearance and Vd values are about double those found in this study. The sheep they used were all lactating females and also a different breed than those used in this study, which could account for some of the difference. Elsheikh et al. (1997) reported that for i.v. OTC (5 mg/kg), the beta t1/2 in sheep was 6.30 h and the Cl was 281 (mL/h)/kg (4.7 (mL/min)/kg. Moreno et al. (1998) found a volume of distribution of 0.78 L/kg after i.v. dosage, and t1/2 of 14.1 and 58.2 h in sheep after i.m. dosage (20 mg/kg) with a conventional preparation and lidocaine containing preparation, respectively. Overall, the published parameters are most consistent for clearance and AUCs, although the AUCs calculated in this study are higher than those reported in the other studies. The Vd values are difficult to compare because the method of calculation for each was often not presented in the papers.
| Study | Dose (mg/kg) | Route | Formulation | AUC/dose (μg ? h/mL) | Cl ((mL/min)/kg) | Vd (L/kg) | t1/2 a (h) | t1/2 b (h) |
|---|---|---|---|---|---|---|---|---|
| Ziv and Sulman (1974) | 20 | i.v. | 4.1 | |||||
| Immelman and Van Rensburg (1983) | 10 | i.m. | LA | 7.0 | 2.3 | 1.9 | 9.3 | |
| 20 | i.m. | LA | 7.2 | 1.1 | 1.5 | 15 | ||
| Ponferrada et al. (1988a) | 10 | i.v. | Conventional | 2.7 | 0.52 | 6.1 | ||
| Ponferrada et al. (1988b) | 250 | i.m. | LA | 5.7 | 29.6 | |||
| Burrows (1992) (1.5 years old) | 10 | i.v. | 2.0–2.3 | 0.079–0.84 | 4.1–4.6 | |||
| Burrows (1992) (2–5 years old) | 10 | i.v. | 1.4 | 0.58–0.65 | 5.2–5.7 | |||
| Escudero et al. (1996) | 20 | i.m. | LA | 5.6 | 3.1 | 7.01 | 23.1 | |
| Elsheikh et al. (1997) | 5 | i.v. | Conventional | 4.7 | 6.3 | |||
| Moreno et al. (1998) | 20 | i.v. | Conventional | 6.5 | 2.6 | 0.78 (i.v.) | 0.12 | 3.3 |
| 20 | i.m. | Conventional | 5.7 | 14.1 | ||||
| 20 | i.m. | With lidocaine | 6.3 | 58.2 | ||||
| Craigmill et al. (2000) (this paper) | 20 | i.m. | LA | 10.5 | 1.7 | 3.08 | 20.9 |
Only limited data have been published on tissue residues of OTC in sheep. Nouws et al. (1990) reported concentrations of OTC in tissues taken from sheep treated with five different formulations of OTC at 10 days post i.m. injection. Residues were undetectable (LOD 0.08 p.p.m.) in muscle, and ranged from undetectable to 0.22 p.p.m. in kidney, and from 0.27 to 1.75 p.p.m. in urine. Baxter and McKellar (1990) published data on residues of OTC in lung after dosing sheep with 10 mg/kg i.v. They reported that 1 h after a single dose, the plasma, normal lung and consolidated lung concentrations were 9.5, 7.0 and 8.1 μg/g.
In contrast, there have been numerous published papers on OTC residues in cattle (Pakkala et al., 1976; Nouws & Ziv, 1978; Maritim et al., 1986; Guillot et al., 1989; TerHune & Upson, 1989; Nouws et al., 1990; Landoni & Errecalde, 1992; Meijer et al., 1993; Mawhinney et al., 1996). Of particular interest is the paper of Meijer et al. (1993) which presented the results of a longitudinal slaughter study with four time points out to 12 days after the last injection of five daily i.m. doses of 20 mg/kg of a 10% preparation. They reported a serum t1/2 of 98.5 h. Using their tissue data, FARAD (Food Animal Residue Avoidance Databank) calculated tissue t1/2 of 81.1, 61.4 and 55.9 for kidney, liver and muscle, respectively. They also reported tissue/serum ratios of 2.6, 7.4 and 0.7 for liver, kidney and muscle, respectively, which compared with published ranges of 2.4 to 2.9, 5.5 to 9.9, and 0.53 to 0.86 for liver, kidney and muscle, respectively. Landoni and Errecalde (1992) reported t1/2 of 39.11, 22, 24.7 and 52.6 h for serum, liver, kidney and muscle, respectively after giving a single i.m. dose of 20 mg/kg of a long-acting OTC preparation. It should be noted that their study was only carried out for 72 h after dosing. Tissue/serum ratios calculated from their data show mean values of 6.0, 6.5, 0.7 and 0.65 for liver, kidney, muscle and fat, respectively. TerHune and Upson (1989) calculated the tissue/serum ratios of OTC after dosing cattle with a single 40 mg/kg dose of long-acting OTC. They reported ratios at 12 and 48 h after injection of 6.5 and 9.8, 2.3 and 3.5, 0.72 and 1.3, and 0.55 and 1.0 for kidney, liver, muscle and lung, respectively. Mean tissue/serum ratios were also calculated from the data of Nouws and Ziv (1978) and were 5.9, 3.8, 3.8 and 0.5 for kidney cortex, kidney medulla, liver and muscle, respectively at times up to 118 h after a single 10 mg/kg dose of two different OTC preparations. These data for cattle are very similar to the ratios found for sheep tissues in this study. Although, the mean liver and kidney ratios are slightly lower than those for cattle, and the value found in this study for sheep fat is considerably lower than that calculated from the data of Landoni and Errecalde (1992).
The comparison of the microbial inhibition bioassay to the HPLC procedure shows that for fortified samples the assays give equivalent results; however, for the incurred samples, the HPLC assay consistently measured higher concentrations for all tissues except fat. Thus, the HPLC assay would provide a greater margin of safety when measuring residues of OTC for the establishment of withdrawal times. The relatively consistent relationship between OTC concentration in serum and tissues between cattle and sheep suggests that monitoring of serum could be a useful method for predicting tissue concentrations and could be used as an aid in determining when animals could be slaughtered after high-dose extra-label OTC use. The data produced in this study shows that sheep tissues are below the cattle tissue tolerances for OTC of 2 p.p.m. for muscle, 6 p.p.m. for liver and 12 p.p.m. for kidney at 2 days for muscle, liver and kidney. The injection sites, however, are not below the tissue tolerance for muscle until 14 days after dosing.
Acknowledgement
This study was funded primarily by the USDA CSREES National Research Support Project Number 7 (NRSP-7), the Minor Use Animal Drug Program. The authors are grateful and indebted to Ms Sandra Ogletree, the Western Region NRSP-7 Administrative Assistant, for her outstanding technical assistance in the conduct and coordination of the research, and editorial assistance in the preparation of this manuscript.




