The effects of sleep deprivation in humans: topographical electroencephalogram changes in non-rapid eye movement (NREM) sleep versus REM sleep
Summary
Studies on homeostatic aspects of sleep regulation have been focussed upon non-rapid eye movement (NREM) sleep, and direct comparisons with regional changes in rapid eye movement (REM) sleep are sparse. To this end, evaluation of electroencephalogram (EEG) changes in recovery sleep after extended waking is the classical approach for increasing homeostatic need. Here, we studied a large sample of 40 healthy subjects, considering a full-scalp EEG topography during baseline (BSL) and recovery sleep following 40 h of wakefulness (REC). In NREM sleep, the statistical maps of REC versus BSL differences revealed significant fronto-central increases of power from 0.5 to 11 Hz and decreases from 13 to 15 Hz. In REM sleep, REC versus BSL differences pointed to significant fronto-central increases in the 0.5–7 Hz and decreases in the 8–11 Hz bands. Moreover, the 12–15 Hz band showed a fronto-parietal increase and that at 22–24 Hz exhibited a fronto-central decrease. Hence, the 1–7 Hz range showed significant increases in both NREM sleep and REM sleep, with similar topography. The parallel change of NREM sleep and REM sleep EEG power is related, as confirmed by a correlational analysis, indicating that the increase in frequency of 2–7 Hz possibly subtends a state-aspecific homeostatic response. On the contrary, sleep deprivation has opposite effects on alpha and sigma activity in both states. In particular, this analysis points to the presence of state-specific homeostatic mechanisms for NREM sleep, limited to <2 Hz frequencies. In conclusion, REM sleep and NREM sleep seem to share some homeostatic mechanisms in response to sleep deprivation, as indicated mainly by the similar direction and topography of changes in low-frequency activity.
Introduction
The current models of sleep regulation indicate that the electroencephalogram (EEG) power in the slow-frequency range represents a reliable and strong measure of a homeostatic process. Both the two-process model (Borbély, 1982) and the synaptic homeostasis hypothesis (Tononi and Cirelli, 2006) assume slow wave activity (SWA) during non-rapid eye movement (NREM) sleep as the biological marker of sleep pressure. Although the evaluation of EEG changes induced in recovery sleep by extended waking or by selective sleep deprivations is a consolidated experimental paradigm, empirical observations on REM sleep and comparisons between changes in NREM sleep versus REM sleep are sparse. Indeed, results seem consistent, as it has been demonstrated repeatedly that slow-frequency EEG activity in REM sleep increases as a consequence of sleep deprivation (Borbély et al., 1981; Brunner et al., 1990, 1993; Endo et al., 1998). It has been confirmed recently by Tinguely et al. (2006), who showed an increased delta and theta power in recovery after sleep deprivation. Interestingly, the increase in EEG power in frequencies up to 8 Hz has been also demonstrated without manipulating time spent awake (i.e. without deprivation of sleep time) by using a procedure of selective slow wave sleep (SWS) suppression that did not affect sleep time and architecture (Ferrara et al., 2002). In rats, during selective REM sleep deprivation, SWA also increased during the residual REM sleep episodes (Shea et al., 2008).
As in NREM sleep, regional modulation has been also suggested for changes in REM sleep. A general increase of EEG power within the 0.5–8.0 Hz range is predominant at the fronto-central derivations after SWS deprivation (Ferrara et al., 2002). Alpha activity decreased during recovery REM sleep after three nights of selective REM sleep deprivation (Endo et al., 1998; Roth et al., 1999), again with an antero-posterior gradient (Roth et al., 1999). This deprivation procedure also determined a decrease in the beta power range in recovery sleep (Endo et al., 1998).
Hence, the very few empirical findings on topographical changes in REM sleep after manipulations of sleep pressure seem to suggest that recovery sleep is characterized by largely similar EEG power changes in REM sleep and NREM sleep. These findings are consistent with the idea that the regulation of REM sleep EEG power content is dependent upon the homeostatic regulation of NREM sleep (Benington, 2002; Benington and Heller, 1994a,b).
In this study, we focussed upon the description of temporal and spatial changes of EEG frequency bands both in REM sleep and NREM sleep after 40 h of sleep deprivation in a large sample of healthy subjects, selected within a database of full-scalp recordings (De Gennaro et al., 2008). Spatial changes were investigated by comparing maps of Hz × Hz EEG power values before versus after sleep deprivation, during both NREM and REM sleep. Temporal changes in REM sleep and NREM sleep were studied by comparing maps of EEG power values of the first versus the second halves of the night. Finally, we correlated the magnitude of the relative EEG sleep changes after 40 h sleep deprivation in REM sleep with those in NREM sleep.
Methods
Subjects
Forty right-handed healthy experimental subjects (20 males and 20 females; age range 18–29 years, mean age 23.8 ± 2.88 years) were selected from a university student population. The inclusion criteria were: normal sleep duration and schedule (habitual sleep time: midnight–08:00 ± 1 hours), no daytime nap habits, no excessive daytime sleepiness, no other sleep, medical, neurological or psychiatric disorder, as assessed by a 1 week sleep log and by clinical interview. Participants were required to avoid napping throughout the experiment; compliance was controlled by actigraphic recordings (AMI Mini motion logger; AMI Inc., Ardsley, NY, USA). Drugs and caffeinated beverages were not permitted (for more details, see De Gennaro et al., 2008).
All subjects gave their written informed consent. The study was approved by the Institutional Ethics Committee of the Department of Psychology of University of Rome ‘Sapienza’ and was conducted in accordance with the Declaration of Helsinki.
Procedure
Each subject participated in a sleep deprivation (SD) study across four consecutive days and nights. The sleep recordings, carried out in a soundproof, temperature-controlled room, were scheduled into the first night (adaptation), the second night (baseline sleep, BSL) and the fourth night (recovery sleep, REC).
The subject’s sleep was undisturbed on all three nights, beginning at midnight, and ending after 7.5 h of accumulated sleep (as checked online visually by expert sleep researchers). After awakening from baseline sleep, a protocol of 40 h sleep deprivation with 36 EEG recording sessions at 1 h intervals started at 10:00 hours (data not shown).
When not involved in testing sessions, subjects were allowed to carry out their own preferred activities, such as reading, writing, listening to music, watching TV or playing games, always under the direct supervision of at least one experimenter. Lying down, sleeping and vigorous physical activity were not permitted. Meals were provided to subjects at 08:30 hours, 14:30 hours and 19:30 hours. Non-scheduled light snacks were permitted, while caffeinated beverages, chocolate, alcohol and medications that can influence sleepiness were not allowed during the deprivation protocol. Time information was available to subjects, and light exposure was not strictly controlled for (although the laboratory was constantly illuminated by four neon lamps; blinds only partly attenuated the light coming from outside). The scheduled waking EEG recording sessions ended at 21:00 hours and the SD period terminated around midnight, in accordance with the lights-off time of the baseline night.
Polysomnographic recordings
An Esaote Biomedica VEGA 24 polygraph (Esaote Biomedica, Florence, Italy) was used for polygraphic recordings. EEG signals were filtered analogically [(high-pass filter at 0.50 Hz and anti-aliasing low-pass at 30 Hz (−30 dB octave−1)]. The 19 unipolar EEG derivations of the international 10–20 system (Fp1, Fp2, F7, F8, F3, F4, Fz, C3, C4, Cz, P3, P4, Pz, T3, T4, T5, T6, O1, O2) were recorded from scalp electrodes with averaged mastoid reference.
The submental electromyogram (EMG) was recorded with a time constant of 0.03 s. Bipolar horizontal eye movements were recorded with a time constant of 1 s. The bipolar horizontal electro-oculogram (EOG) was recorded from electrodes placed about 1 cm from the medial and lateral canthi of the dominant eye. Impedance of these electrodes was kept below 5 kOhm.
Data analysis
Sleep measures
Sleep stages of BSL and REC nights were scored visually in 20 s epochs, according to standard criteria (Rechtschaffen and Kales, 1968), and SWS scoring followed the >75 μV amplitude criterion strictly.
The following were considered dependent variables: (i) Stage 1 latency; (ii) Stage 2 latency; (iii) REM latency; (iv) total sleep time (TST), defined as the sum of time spent in Stage 1, Stage 2, SWS and REM; (v) total bedtime (TBT); (vi) sleep efficiency index (SEI = TST/TBT × 100); (vii) percentage of each sleep stage (time spent in a sleep stage TST−1); (viii) wakefulness after sleep onset (WASO), expressed as the intra-sleep time (min) spent awake; (ix) number of awakenings (the number of >10 s episodes of WASO); and (x) number of arousals (the number of <10 s episodes of WASO). The polysomnographic EEG measures were submitted to one-way repeated-measure analyses of variance (ranovas), comparing BSL and REC nights.
Quantitative analysis of sleep EEG
The polygraphic signals (19 EEG channels, EOG and EMG) were converted analogue to digital online with a sampling rate of 128 Hz and stored on the disk of a personal computer. Ocular and muscle artefacts were excluded offline by visual inspection. We investigated the 0.50–25.00 Hz frequency range, computing power spectra by a fast-Fourier transform routine for 4 s periodgrams. Before conducting statistical analysis, the data were reduced to a 1 Hz bin width by collapsing four adjacent 0.25 Hz bins. The only exception was the 0.5–1.0 Hz bin, for which two adjacent 0.25 Hz bins were collapsed.
A further data reduction of power spectra was achieved by averaging 15 consecutive 4 s epochs to yield a 60 s spectrum. As a result, this spectrum included three consecutive 20 s visually scored epochs. Power spectra were calculated separately for NREM sleep (Stages 2 + 3 + 4) and REM sleep. When an NREM–REM or an REM–NREM transition occurred within the same 1 min epoch, this epoch was not considered for the subsequent analyses. Similarly, a 1 min epoch was discarded when Stage 1 NREM epochs intruded within epochs of Stages 2–4.
The bins are referred to and plotted in this study by the lowest frequency included (e.g. the 2 Hz bin refers to the averaged values of the following bin intervals: 2.00–2.25, 2.25–2.50, 2.50–2.75 and 2.75–3.00 Hz).
Electroencephalogram power values for each 1 Hz frequency bin were considered as dependent measures. Values were log-transformed, colour-coded, plotted at the corresponding position on the planar projection of the scalp surface and interpolated (biharmonic spline) between electrodes.
Electroencephalogram power maps were computed separately for the BSL and REC nights, and separately for NREM sleep and REM sleep. In a similar manner, the decline of sleep pressure during sleep has been expressed by power statistical maps of the first versus the second halves of both BSL and REC nights for the following frequency EEG band: delta (0.50–4.75 Hz), theta (5.00–7.75 Hz), alpha (8.00–11.75 Hz) and sigma (12.00–14.75 Hz).
Bonferroni’s correction for multiple comparisons was applied. Considering the mean correlation between the variables (r = 0.61), the alpha level was then adjusted to ≤0.003 (t ≥ 3.13).
Finally, Pearson’s correlations between the relative changes of EEG power spectra (power in the recovery compared to baseline night) in NREM sleep and in REM sleep were calculated. Mean correlation values over all 19 scalp locations were calculated by averaging these values after Fisher’s Z transformation; averaged Z-values were then retransformed to r-values. Significance was estimated on the average Fisher’s Z values with an alpha level adjusted to ≤0.0043 (r ≥ 0.44), considering the mean correlation between these variables (r = 0.48; d.f. = 38) and the number of statistical comparisons (98 correlations).
Results
Polysomnography
Table 1 reports the results of the analyses of variance on polysomnographic variables. Macrostructural variables of sleep point to differences between baseline and recovery nights that represent the typical consequences of a night of sleep deprivation. Recovery after sleep deprivation is characterized by drastic shortening in the latency of NREM sleep stages, by a large increase of SWS and a decrease of Stage 1, by a decreased percentage of WASO and arousals and of the number of awakenings, and by higher sleep efficiency. TBT also decreased slightly. With respect to REM sleep, its latency did not change while its amount during recovery sleep shows an approximately 4% decrease compared to baseline.
| Variables | Baseline | Recovery | F (1,39) | P | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | K–S d | Mean | SD | K–S d | |||
| Stage 1 latency (min) | 6.58 | 5.82 | 0.19 | 1.89 | 2.08 | 0.21 | 32.98 | <0.0001 |
| Stage 2 latency (min) | 11.15 | 11.43 | 0.20 | 3.20 | 2.38 | 0.21 | 22.99 | <0.0001 |
| SWS latency (min) | 28.45 | 17.43 | 0.20 | 11.25 | 5.45 | 0.10 | 51.47 | <0.0001 |
| REM latency (min) | 85.05 | 30.07 | 0.19 | 84.98 | 44.27 | 0.20 | 0.0001 | 0.99 |
| Stage 1 (%) | 6.31 | 2.92 | 0.12 | 2.84 | 1.69 | 0.15 | 79.25 | <0.0001 |
| Stage 2 (%) | 59.56 | 6.75 | 0.07 | 59.08 | 8.53 | 0.08 | 0.42 | 0.52 |
| Stage 3 (%) | 7.90 | 3.84 | 0.09 | 11.96 | 3.88 | 0.09 | 60.26 | <0.0001 |
| Stage 4 (%) | 2.36 | 3.37 | 0.21 | 6.20 | 6.26 | 0.20 | 42.49 | <0.0001 |
| SWS (%) | 10.27 | 6.29 | 0.11 | 18.16 | 8.02 | 0.10 | 150.22 | <0.0001 |
| REM (%) | 24.12 | 4.77 | 0.12 | 20.33 | 6.23 | 0.11 | 18.89 | <0.0001 |
| WASO (min) | 26.07 | 19.19 | 0.19 | 11.68 | 7.66 | 0.16 | 24.26 | <0.0001 |
| Awakenings (#) | 28.52 | 10.64 | 0.12 | 20.45 | 7.52 | 0.10 | 32.35 | <0.0001 |
| Arousals (#) | 35.40 | 17.59 | 0.13 | 26.15 | 18.49 | 0.15 | 10.27 | 0.003 |
| TST (min) | 441.4 | 38.65 | 0.09 | 449.2 | 20.01 | 0.11 | 2.07 | 0.16 |
| TBT (min) | 484.8 | 63.93 | 0.17 | 470.8 | 50.59 | 0.25 | 5.57 | <0.05 |
| SEI% (TST/TBT) | 91.65 | 6.84 | 0.20 | 95.99 | 6.12 | 0.22 | 42.66 | <0.0001 |
- SWS, slow wave sleep; REM, rapid eye movement; WASO, wake after sleep onset; TST, total sleep time; TBT, total bed time; SEI, sleep efficiency index; K–S d, Kolmogorov–Smirnov distance; SD, standard deviation.
Macrostructural variables of sleep also appear distributed normally, as in most cases Kolmogorov–Smirnov (K–S) tests are not significant (P < 0.05 corresponds to K–S ≥ 0.21 and P < 0.01 corresponds to K–S ≥ 0.28; Table 1).
Baseline versus recovery sleep
Fig. 1a shows EEG activity in NREM sleep and REM sleep, averaged over all 19 scalp locations during baseline and recovery sleep. A profound difference between NREM sleep and REM sleep is visible in terms of asbsolute values. There is a prevalence of EEG power in NREM sleep from 0.5 to 15.50 Hz. The extent of this predominance ranges from maxima, which were found below 2 Hz (from 4 to 11 times higher in NREM sleep than in REM sleep), to minima for the theta and alpha activity (about two times higher in NREM sleep than in REM sleep), without substantial differences between baseline and recovery nights.

Electroencephalogram (EEG) power spectra (mean over all 19 scalp locations) of EEG activity in non-rapid eye movement (NREM) sleep and in REM sleep. (a) Absolute values of EEG activity in NREM sleep and REM sleep in the whole night. Power spectra (mean over all 19 scalp locations) of NREM sleep and REM sleep were recorded in the frequency range from 0.50 to 24.75 Hz (expressed in logarithmic scale) during baseline and recovery sleep. Mean values [±standard error of the mean (SEM)] are plotted for 0.25 Hz bins. Standard errors refer to interlocation variability. (b) Effect of the decline of sleep pressure during the sleep nights. Power spectra (mean over all 19 scalp locations) of NREM sleep and REM sleep in the first half of both the baseline and the recovery nights are expressed as a percentage of the second half of the night (100%). Mean values (±SEM) are plotted in the frequency range from 0.50 to 24.75 Hz for 0.25 Hz bins. Standard errors refer to inter-location variability.
Power maps of recovery and baseline nights (data not shown) reveal stable patterns within different frequency ranges, and the data of maxima and minima exhibited the typical features of power spectra during NREM sleep. The delta and alpha bands exhibit a frontal midline predominance and minimum values over the temporal regions. In the theta band, the highest values are at the fronto-central midline areas, while the sigma band shows centro-parietal maxima. The same stable patterns within different frequency bands are maintained roughly in REM sleep, with the notable exception of the 8–15 Hz range. Within this frequency range, REM sleep shows parieto-occipital midline maxima for the alpha activity (i.e. more posterior than in NREM sleep) and central midline maxima for the sigma activity (i.e. more anterior than in NREM sleep). The quantitative evaluation of the differences between recovery and baseline nights in NREM sleep and in REM sleep is illustrated by 1 Hz maps of statistical comparisons (Fig. 2).

Topographic statistical distribution of the change in electroencephalogram (EEG) power, assessed by comparing (t-tests) recovery and baseline nights separately for non-rapid eye movement (NREM) sleep (upper panel) and REM sleep (lower panel). Values are expressed in terms of t-values: positive t-values indicate a prevalence of the recovery over the baseline night. The two-tailed level of significance (P = 0.003 after the Bonferroni correction, corresponding to a t = 3.13) is indicated by the arrows in correspondence of the t-values colour bar. Average values are normalized by total power, colour-coded, plotted at the corresponding position on the planar projection of the scalp surface and interpolated (biharmonic spline) between electrodes. The maps are based on the 19 unipolar EEG derivations of the international 10–20 system with averaged mastoid reference. Maps are plotted for 1 Hz bins, with the exception of the frequency range from 0.50 to 1.00 Hz that reports maps for a 0.5 Hz bin.
The largest significant increase in NREM sleep after sleep deprivation has been found in low frequencies (below 8 Hz). Maxima are located at the fronto-central midline sites, and minima at the temporal sites that did not change significantly from baseline to recovery. Within this range of slow frequencies, the <1 Hz activity shows relatively smaller increases, and the fronto-central gradient is less prominent. The 8–11 Hz range shows similar regional changes as a consequence of sleep deprivation, although to a lesser extent. Notably, coexistence of significant increases and decreases is visible at 12 Hz along the midline, where power decreases significantly over the anterior areas and increases over the parietal lead, compared to baseline sleep. The sigma range shows decreases that are significant at centro-parietal midline sites (14–15 Hz) and at frontal sites (13 Hz). There are no significant changes above 15 Hz.
Changes in recovery after sleep deprivation also show a mainly similar topographical pattern in REM sleep. Again, the largest significant increases have been found below 8 Hz, with maxima at the fronto-central midline sites and minima at the temporal sites. On the contrary, the topography of alpha and sigma activity changes in REM sleep differs from NREM sleep. The 8–11 Hz activity decreases generally during REM sleep that follows sleep deprivation; the decrease is significant in some central derivations at 9 Hz. Conversely, the 12–15 Hz activity increases in some frontal leads and at Pz. The beta activity shows a significant decrease only at Fz and Cz in correspondence with the 22–24 Hz range.
In synthesis, topography and significance of changes after sleep deprivation are shared mainly by NREM sleep and REM sleep, at least in the EEG spectrum of <8 Hz low frequencies. On the contrary, the range of frequencies encompassing the alpha and sigma activity shows a clear dissociation between the ways the two states respond to sleep deprivation: in REM sleep, the alpha activity declines non-significantly compared to baseline night; in NREM sleep, it follows the homeostatic increase of lower frequencies. Conversely, sigma activity declines in NREM sleep while it shows increases in REM sleep. Independent from the direction, changes of sigma activity in both REM sleep and NREM sleep have been found at parietal and more anterior frontal areas, while changes of alpha activity show an antero-posterior gradient in NREM sleep and a postero-anterior gradient in REM sleep. Finally, beta activity shows a general non-significant increase in both NREM sleep and REM sleep, with a local decrease at Fz and Cz at the 22–24 Hz range only in REM sleep.
The relations between changes in NREM sleep and REM sleep
Although the data reported in Fig. 1a show very different absolute power values in the two states, at least until 15 Hz, the relative magnitude of their changes after sleep deprivation segments the EEG spectrum differently. The upper panel of Fig. 3 shows these changes after sleep deprivation, expressed as percentages of baseline values and averaged over all derivations. Considering the full-scalp EEG activity, the <1 Hz and the 3–7 Hz ranges show very similar relative changes, with 25–30% increases in both REM sleep and NREM sleep. A remarkably higher increase in NREM sleep than in REM sleep is restricted to the 1–2 Hz activity. We have thus assessed the extent of the association by calculating for each derivation the correlation between EEG power changes in NREM sleep and REM sleep. Mean correlations averaged over the 19 derivations are significant from 2.00 to 12.50 Hz and from 14.25 to 18.00 Hz (lower section of Fig. 3).
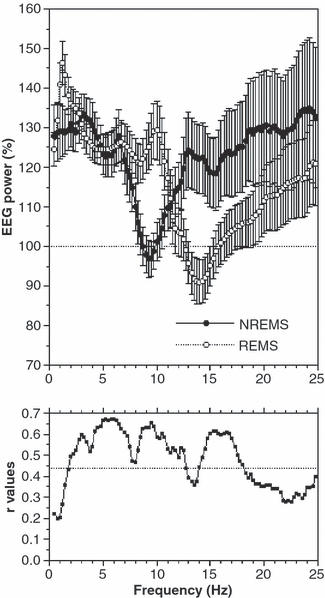
Effect of sleep deprivation on non-rapid eye movement (NREM) sleep and REM sleep in the 0.50–24.75 Hz frequency range, and correlations between their relative changes. Upper panel: electroencephalogram (EEG) power spectra (mean over all 19 scalp locations) of NREM sleep and REM sleep are expressed as a percentage of baseline (100%). Mean values [±standard error of the mean (SEM)] are plotted for 0.25 Hz bins. Values of SEM show inter-individual variability (mean over all 19 scalp locations). Lower panel: values of the correlations (Pearson’s r) between the relative changes in the recovery compared to baseline night. The r-values of all 19 scalp locations have been averaged after Fisher’s Z transformation, and then retransformed to r-values. Mean r-coefficients are plotted for 0.25 Hz bins. The horizontal dotted line points to the significance level, adjusted to ≤0.0043 (r ≥ 0.44) with the Bonferroni correction [considering the mean correlation between these variables (r = 0.48; d.f. = 38) and the number of statistical comparisons (98 correlations)].
First versus second halves of the night
Because the decline of delta activity during the sleep period is another well-recognized index of sleep homeostasis during NREM sleep, we compared changes in EEG power of NREM sleep and REM sleep in the two halves of the night. Fig. 1b plots relative changes for NREM sleep and REM sleep in the second half of the baseline and recovery nights, expressed as percentages of values of the respective first half of the night and averaged over all derivations. What is immediately evident from inspection of the figures is the larger magnitude of changes after sleep deprivation (Fig. 3, upper panel) compared to the changes as a function of time spent in sleep (Fig. 1b). The full-scalp EEG activity of NREM sleep in the delta and theta ranges shows a remarkably higher increase in the comparison between the first versus second halves than in the comparison between recovery versus baseline sleep. Homeostatic pressure, which declines during NREM sleep of the second half of the night until reaching values of about one-third of the EEG power of the first half, decreases only slightly during REM sleep. These relative differences are maintained substantially in the recovery night.
Independent of the magnitude of changes across the night, regional changes point to very different effects on NREM sleep and REM sleep. The maps of the statistical comparisons between the first and second halves of the baseline night show a different topography in NREM sleep and REM sleep (Fig. 4, upper panel). In NREM sleep, regional changes after the first half of the night resemble mainly those found after sleep deprivation (Fig. 4, lower panel). The delta, theta and alpha bands confirm the frontal maxima and the temporo-occipital minima, although the midline derivations do not peak as after sleep deprivation, showing a less circumscribed effect of the decline of homeostatic pressure. As an expression of this phenomenon, the temporal and occipital areas are also affected by significant decreases in the second half of the baseline night, while their increase after sleep deprivation is generally not significant. Changes in sigma activity also resemble those found after sleep deprivation, although to a smaller extent.

Topographic statistical distribution of the change in electroencephalogram (EEG) power by comparing the first and second halves of baseline night (top panel) and the recovery and baseline nights (bottom panel). In each panel, maps of non-rapid eye movement (NREM) sleep (upper row) and REM sleep (lower row) are reported. Average values are normalized by total power, colour-coded, plotted at the corresponding position on the planar projection of the scalp surface and interpolated (biharmonic spline) between electrodes. The maps are based on the 19 unipolar EEG derivations of the international 10–20 system with averaged mastoid reference. Maps are plotted for the following EEG bands: delta (0.50–4.75 Hz), theta (5.00–7.75 Hz), alpha (8.00–11.75 Hz) and sigma (12.00–14.75 Hz). Values are expressed in terms of t-values, and positive t-values indicate a prevalence of the first over the second halves of the baseline night in the top panel, and of the recovery over the baseline night in the bottom panel. The two-tailed level of significance of (P = 0.003, after the Bonferroni correction, corresponds to a t = 3.13) is indicated by the arrows in correspondence of the t-values colour bar.
On the contrary, REM sleep exhibits a peculiar pattern of EEG power changes across the night. Delta power does not decrease significantly from the first to the second halves of the night. Theta power shows significant differences, with largest decreases peaking on parietal areas, while the corresponding maxima after sleep deprivation were found on frontal areas. The alpha and sigma ranges are affected by widespread significant decreases in the second half of the night, higher on more posterior sites, clearly dissociated from those found after sleep deprivation.
Discussion
The main result of this study indicates that a night of sleep deprivation affects EEG power spectra of both NREM sleep and REM sleep in the subsequent recovery night. Topography- and frequency-specific changes in NREM sleep and REM sleep are coherent with the existence of shared (state-aspecific) homeostatic responses. EEG changes in the low-frequency range (<8 Hz) in REM sleep resemble strictly, in fact, those in NREM sleep. On the contrary, changes in alpha activity are characterized by a clear dissociation after sleep deprivation in REM sleep and NREM sleep with respect to both topography (showing an antero-posterior gradient in NREM sleep and a postero-anterior gradient in REM sleep) and direction (with increased power in NREM sleep and decreased power in REM sleep). Conversely, during recovery sleep sigma activity shows decreased power in NREM sleep and increased power in REM sleep.
Hence, the main biological marker of sleep homeostasis in NREM sleep (i.e. low-frequency EEG activity) also changes consistently in REM sleep as a consequence of sleep deprivation, although with a reduced magnitude compared to NREM sleep. Specific dissociations for alpha and sigma power prevent an interpretation in terms of a simple intrusion/contamination of NREM sleep power into REM sleep.
The existence of common EEG power responses to sleep deprivation is strengthened by correlational analysis. Even though the existence of an intrinsic correlation between adjacent frequency bins should be taken into account, a robust association involves a large portion of the EEG spectrum encompassing theta, alpha, beta and part of the delta bands. Thus, most of the EEG changes in response to sleep deprivation show a between-states intercorrelation, indicating that REM sleep and NREM sleep share some homeostatic mechanism in response to sleep deprivation. In particular, EEG power in most of the delta/theta range and, although not significantly, also in the beta (>16 Hz) range increases in both states during recovery sleep. On the contrary, alpha activity increases in NREM sleep and decreases in REM sleep after sleep deprivation. Although the latter changes go in an opposite direction, the intrinsic physiological mechanisms underlying these responses do not seem to be independent, because larger increases of alpha activity in NREM sleep are associated with smaller decreases in REM sleep and vice versa.
Nevertheless, the correlational approach also indicates the presence of state-specific homeostatic mechanisms for NREM sleep during recovery sleep, limited to the very low frequencies (below 2 Hz) and to sigma activity. The peculiar behaviour of these frequencies in NREM sleep suggests their functional differentiation between sleep states. In other words, an increase in very low frequencies (i.e. slow oscillations and K complexes) and a decrease in sigma activity can be considered the NREM sleep-specific changes after sleep deprivation, as reported repeatedly by previous research (e.g. Achermann and Borbély, 1997; Borbély et al., 1981). However, results in this frequency range should be considered with caution, because of the potential distortion by the analogue filters on EEG power close to the cut-off frequency. In fact, previous studies on the effects of sleep deprivation on very low frequencies provided mixed findings: power below 2 Hz did not increase after sleep deprivation (Borbély et al., 1981), power in the 1 Hz bin increased as a function of the duration of prior wakefulness (Campbell et al., 2006) and power below 1 Hz did not increase after sleep deprivation (Bersagliere and Achermann, in press).
Results on the comparisons between the first versus the second halves of the night do not seem coherent with the existence of shared homeostatic mechanisms. With regard to NREM sleep, comparisons between the first versus the second halves of the night grossly parallel the differences between baseline and recovery sleep, showing EEG changes within similar frequencies and with comparable topography. EEG power in the delta and sigma bands shows the well-known complementary behaviour, decreasing and increasing, respectively, during the course of the night. These results are coherent with previous findings by Finelli et al. (2001) in NREM sleep. On the contrary, REM sleep exhibits a very different pattern. In fact, the decline of delta power during the night, a well-recognized index of sleep homeostasis, is not evident in REM sleep. On the contrary, theta, alpha and sigma power in REM sleep show decreases throughout the night, mainly on posterior sites.
The comparisons between the first versus the second halves of the night (see maps in Fig. 4) suggest that EEG frequency changes in NREM sleep and REM sleep are characterized by opposite power gradients. While the largest EEG power changes in NREM sleep involve the lowest frequencies, with a decreasing power gradient across the frequency spectrum, REM sleep shows an opposite gradient, with the smallest changes for SWA and the largest for sigma. Similar opposite power gradients have also been found in the recovery night (data not shown). Finally, NREM sleep changes between the first and the second halves of the night follow a mainly antero-posterior gradient, while REM sleep shows a postero-anterior gradient (Fig. 4).
The rationale for comparing the first and second halves of the night assumes a decline of sleep pressure (and, consequently, of EEG power in the slow frequencies) across the sleep episode, considering it as a further expression of sleep homeostasis (Finelli et al., 2001). This is undoubtedly true for NREM sleep, at least for the frequency range between 0.5 and 11.5 Hz. It seems that, in normal conditions, when the homeostatic mechanism is not challenged by strong manipulations of wake duration, the most peculiar EEG features of NREM sleep (delta and sigma power) are specific homeostatic markers of NREM sleep. From this viewpoint, the homeostatic mechanisms influencing the EEG power spectrum become aspecific only after sleep deprivation, and are thus shared by both sleep states, at least for the frequencies below 8 Hz.
A further interpretation of this result takes into account that both REM sleep pressure and alpha power in REM sleep display a circadian rhythm with, respectively, a peak and a trough in correspondence of the second half of the night (Dijk, 1999). It could thus be hypothesized that a greater strength of the circadian than the homeostatic regulation of REM sleep precludes the expected differences between the first versus the second halves of the night, explaining the actual decrease of alpha and sigma power during the night. A stronger influence of the chronobiological regulation in REM sleep is confirmed by changes in sleep stage latency as a consequence of sleep deprivation. Shortening of the latency of each NREM sleep stage is accompanied by a practically identical REM sleep latency. This finding has already been reported (e.g. Borbély et al., 1981), and is coherent with the robust circadian regulation of REM sleep timing (Czeisler et al., 1980). Overall, these results suggest that circadian factors are predominant in the regulation of REM sleep, reinforcing the notion of a weak homeostatic drive proposed by Endo et al. (1998) to explain the rising trend in the number of interventions requested during the night to induce a selective REM sleep deprivation.
Conclusions
To the best of our knowledge, the current study is based upon the largest data set collected after total sleep deprivation and using a full-scalp quantitative EEG analysis. The effects of sleep deprivation on REM sleep EEG power provide some support for the existence of a general, state-aspecific homeostatic mechanism reflected by an increase of the >2- and <8 Hz EEG activity, more pronounced over the anterior cortical regions. On the contrary, the decrease of alpha activity is a distinctive EEG response of REM sleep following sleep deprivation, whose biological meaning is still unknown. In this respect, the parallelism with the topography of waking alpha EEG activity proposed by Tinguely et al. (2006) and confirmed partially by another study (De Gennaro et al., 2007) remains only suggestive. Another distinctive specific response in REM sleep to increased homeostatic sleep pressure is the decreased power within a segment of beta activity, circumscribed at more anterior areas. This also confirms preliminary observations by Roth et al. (1999) and again seems suggestive of a parallelism with the decreased beta activity that has been observed during prolonged waking (De Gennaro et al., 2007).
Disclosure
We have no financial interest to disclose and no conflicts of interest.




