The effects of sleep and sleep deprivation on task-switching performance
Summary
Neural systems of the prefrontal cortex (PFC) involved in executive functions are particularly vulnerable to sleep deprivation (SD). In this study, we investigated whether SD selectively affects specific components of the executive control processes involved in task-switching performance. Two different tasks are performed in rapid and random succession in this procedure, so that the to-be-executed task may change from one trial to the next (switch trial), or may be repeated (repetition trial). Task-switches are usually slower than task repetitions, giving way to the ‘switch cost’. One hundred and eight university students were assigned randomly to the sleep (S) or the SD group. Each of them was tested on a task-switching paradigm before and after an experimental night (S or SD), and after one recovery night. SD impaired both task-switching accuracy and speed. A higher proportion of errors and increased switch costs after SD have been observed, compared to normal sleep. Control analyses on switch and repetition trials showed that the SD group was significantly worse only on the switch trials. The effects of SD are reverted by one night of recovery sleep. It is concluded that the ability to adjust behaviour rapidly and flexibly to changing environmental demands, which relies on the functional integrity of the PFC, is impacted negatively by sleep loss.
Introduction
Attention to sleep and sleeplessness-related problems has been growing in the last few years. This interest is due mainly to the awareness of the fact that sleepiness and fatigue are becoming endemic in the population, contributing to human error and, consequently, many accidents in industrialized societies (Ferrara and De Gennaro, 2001). Vigilance decreases during sustained wakefulness, often associated with performance impairment, are a serious problem for applied areas such as public health, medical care, transportation, road safety and military work. Indeed, injuries and deaths caused by sleepiness and/or fatigue involve enormous costs in terms of lives and money (e.g. Mitler et al., 1997).
For a long time, the prevailing view in sleep deprivation (SD) research was that high-level complex skills were relatively unaffected by SD because of the interest they generate and the implicit encouragement for participants to apply compensatory effort to overcome their sleepiness (Harrison and Horne, 2000). Real-world decision-making can occur in unique and unfamiliar circumstances, requiring a wide range of complex skills such as, for example, anticipating consequences, keeping track of events, being innovative, avoiding distractions and irrelevant stimuli (Jones and Harrison, 2001). Recent studies show that even one night of SD leads to significant deterioration in these skills, despite the individual’s best effort to perform well (Harrison and Horne, 2000).
Unlike rule-based, convergent and logical skills, the above-listed skills depend heavily upon the functional integrity of the prefrontal cortex (PFC). PFC is the hardest-working cortical region during wakefulness, sustaining a broad range of different cognitive demands (e.g. Duncan and Owen, 2000). The PFC networks show high levels of metabolic rate even during resting wakefulness, as already noted by Ingvar (1979) 30 years ago, and confirmed by more recent task-free analyses of brain activation during the resting state (e.g. Seeley et al., 2007). Sleep is thought to provide a crucial form of recovery for the frontal cortex. In fact, increases of electroencephalogram (EEG) power in the low-frequency range during prolonged wakefulness exhibit their maxima at more anterior regions (Cajochen et al., 2002; De Gennaro et al., 2007; Finelli et al., 2000; Tinguely et al., 2006), and the same areas increase low-frequency power drastically in recovery sleep (Cajochen et al., 1999; Ferrara et al., 2002; Finelli et al., 2001; Marzano et al., in press). Both phenomena suggest that higher-order (frontal) cortical areas may be particularly vulnerable to the negative effects of SD.
Several studies have demonstrated that neural systems of PFC involved in executive functions are particularly susceptible to SD (Curcio et al., 2006; Durmer and Dinges, 2005; Harrison and Horne, 2000). As a consequence, most neurocognitive functions are impaired by acute sleep loss and/or prolonged wakefulness, such as attention and divergent thinking (Wimmer et al., 1992), language (Drummond et al., 2000; Harrison and Horne, 1998, 2001), decision-making (Harrison and Horne, 1999), memory and response inhibition (Harrison et al., 2000) and serial subtraction (Drummond et al., 1999). Given the crucial cognitive skills subtended by the functional integrity of the PFC, and considering that this cortical region appears to be especially vulnerable to the effects of prolonged wakefulness, a further focus upon specific, higher-order executive functions is warranted.
The task-switching paradigm has been used widely to investigate the executive control of cognition (for a review see Logan, 2003; Monsell, 2003), and neuroimaging studies have demonstrated that the task-switching performance recruits various PFC regions (Braver et al., 2003; Collette et al., 2006; Sohn et al., 2000). In this type of procedure, two different tasks are performed in rapid succession and according to a random sequence of task presentation, so that the to-be-executed task can change from one trial to the next (‘switch’ trial), or can be repeated (‘repetition’ trial). Task-switches are usually slower and less accurate than task repetitions, and this difference is often referred to as the ‘task-switch cost’ (SC). This cost is thought to reflect the time needed for the executive control processes to reconfigure the cognitive system for the execution of a new task (Meiran, 1996; Rogers and Monsell, 1995; Rubinstein et al., 2001). Thus, the SC is considered an operational measure of the executive control.
In the present study, we evaluated the effects of sleep loss on the executive control processes assessed by a task-switching paradigm, with the aim of investigating whether one night of SD affects selectively the control processes involved in the task-switching performance. Given the well-known negative consequences of sleep loss on frontal lobe functions, we hypothesized that SCs would increase after SD. Moreover, we aimed to assess whether sleep loss exerts either a generalized effect on SCs or has a specific impact on one of the components of task-switching performance. Therefore, we investigated the specific effects of SD on the preparation and disengagement components of task-switching by varying systematically the intervals among subject’s response, cues and targets [response–target–intervals (RTIs) and cue–target–intervals (CTIs), see below for details].
Methods
Participants
A questionnaire and a clinical interview were used to select 108 university students (79 females, 29 males; mean age ± standard deviation: 20.9 ± 0.94 years; range 20–23), who participated in the experiment in order to earn university credits. None of the subjects had a history of medical, neurological or psychiatric disorders, nor of medication or drug intake. By self-report, all of them had a habitual sleep duration of 7–8 h per night, went to bed between 23:00 hours and midnight, and did not take naps during the day. Quality and quantity of participants’ usual sleep was assessed by a sleep log. Each of them also completed the Pittsburgh Sleep Quality Index (Buysse et al., 1989) and the Morningness–Eveningness Questionnaire (Horne and Östberg, 1976). Each subject was asked to maintain a regular sleep–wake cycle in the 3 days before the experiment: compliance was controlled by means of actigraphy (AMI MicroMini Motionlogger). The entire investigation was approved by the local Institutional Review Board and was conducted at the Laboratory of Sleep Psychophysiology and Cognitive Neurosciences, Department of Health Sciences of the University of L’Aquila, according to the principles established by the Declaration of Helsinki. Informed consent was obtained from all participants before the investigation.
Task-switching sessions: stimuli and tasks
Participants were tested individually in a dimly lit room. They were seated in front of a 15-inch computer monitor, at a distance of 50 cm. At the beginning of each session, task instructions were both displayed on the screen and explained verbally to participants, emphasizing the need for both accuracy and speed. SCs were assessed by means of a task-cueing procedure, which involved performing two tasks in rapid sequence according to a randomized order, and presenting a cue on each trial that indicated the task to perform on the subsequent target stimulus. The two tasks consisted of judging if a digit stimulus was odd or even (task A), or if it was larger or smaller than 5 (task B). Participants used their left and right index fingers for responding: odd digits and smaller-than-5 digits were mapped onto the left index finger response; even digits and larger-than-5 digits were mapped onto the right index finger response. The same two response keys on the computer keyboard (‘A’ for left and ‘L’ for right index finger) were used for both tasks. Stimuli presentation and responses recording were managed by means of a custom software (Superlab version 2.1 for Windows). A schematic illustration of the sequence of events in the present task-switching paradigm is reported in the lower section of Fig. 1.

Experimental protocol and task-switching paradigm. Upper section: schematic representation of the 4-day experimental protocol in the sleep group and sleep deprivation group. Lower section: on the left side, an example of a sequence of repetition and switch trials is reported. On the right side, the timing of the events within a single trial is specified. Target stimuli consisted of bold white digits from 1 to 9 (excluding 5), subtending approximately 3° × 5° of visual angle. Task cues stimuli consisted of outlined white squares or diamonds, indicating the A (even–odd) and B (smaller–larger-than-5) tasks, respectively; they subtended approximately 7° × 7° of visual angle. Each trial started with the presentation of a black background for 300–1000 ms, depending on the experimental condition. Then, task cue was presented at the centre of the screen. Finally, after 300–1000 ms, the target stimulus appeared inside the cue frame, and it remained visible together with the cue for 2500 ms or until the subject’s response. The same two response keys on the computer keyboard (‘A’ for left and ‘L’ for right index finger) were used for both tasks.
Experimental manipulation of response–cue–target (RCT) intervals
Several aspects of cognitive control are involved in task-switching, as suggested by the fact that switching costs consist of different components (Meiran, 1996). Specifically, task-switching not only involves preparation processes of task-set reconfiguration, necessary for the execution of the new task, it also involves processes related to the disengagement from the previously executed task. Accordingly, the SC is reduced when a cue is presented indicating which task participants have to carry out on a specific trial, allowing to prepare before the imperative stimulus is presented. Similarly, the cost is also reduced when the interval between the cue and the target presentation (CTI) increases; that is, when more time is available for task-set reconfiguration (Meiran, 2000; Rogers and Monsell, 1995). The SC is also reduced when the interval between the response on the previous trial and the presentation of the target stimulus for the new task is increased (RTI), suggesting that a disengagement process is also involved in task-switching (Meiran, 2000).
In principle, SD could affect the SC in (at least) two different ways: (i) reducing the ability to reconfigure the task-set for the execution of a new task before the perceptual information is specified (preparation component); and (ii) reducing the ability to disengage from the previous task set when a new task must be executed (disengagement component). In order to investigate the specific effects of SD on these components of task-switching, participants were assigned randomly to one of three different RCT interval conditions (18 subjects for each condition). The first experimental condition was designed so that subjects had more time available for task-set reconfiguration: RCI was set to 300 ms and CTI was set to 1000 ms (300–1000). In the second condition, subjects had less time to prepare for the task at hand, as RCI was 1000 ms and CTI was 300 ms (1000–300). By comparing subjects’ performance on these two conditions, we could evaluate independently the influence of the time available for the task-set reconfiguration from the effects of the disengagement component, because the overall time between the subject’s response and the next target was maintained constant (1300 ms). Finally, we designed a third RCT interval condition in which both RCI and CTI were set to 300 ms (300–300). In this manner, we could also evaluate the disengagement component independently from the effects of the preparation component, by comparing subjects’ performance on the second (1000–300) and third RCT interval conditions (300–300), in both of which subjects had the same time to prepare to the next task (300 ms).
Procedure
Half the subjects were randomly assigned to the sleep group (S), performing the task-switching sessions before and after one night of undisturbed nocturnal sleep. The remaining 54 subjects, assigned to the SD group, were tested before and after one night of total SD. Independently from this assignment, training and recovery sessions were administered to all subjects during the first and the fourth days of the experiment. Thus, all participants completed four task-switching sessions during four consequent days (training, baseline, experimental, recovery), always scheduled at 10:00 hours. A schematic representation of the protocol is reported in the upper section of Fig. 1.
Participants assigned to the S group always slept at home during the 4 days of the study. Subjects included in the SD group slept at home except for the second night, in which they remained awake in the laboratory under the supervision of two experimenters. During this night, lights were always switched on and subjects were not allowed to consume caffeinated beverages (i.e. coffee, energy drinks, cola) or to perform physical activity; however, they were allowed to listen to music, play cards, read, watch TV and use a PC.
On the first day (training), all subjects signed an informed consent before starting the experiment; subsequently, they were administered the training session, consisting of a first series of 12 blocks of trials, 1 h of resting and another 12 blocks of trials. The aim of this learning phase was to bring the subjects as closely as possible to their asymptotic performance. On each of the following days (baseline, experimental, recovery), subjects completed one task-switching session of four blocks of trials. Each task-switching trials block consisted of 40 trials that lasted about 5 min, including 1 min of pause. Consequently, the training session lasted about 3 h and the other sessions about 20 min.
Data analyses
Statistical analyses have been performed on the following dependent variables: SCs; median reaction times (in ms) to both repetition and switch trials; and angular transformations of the proportion of errors.
Switch costs were computed as the difference between median switch RT and median repetition RT. For each subject, proportions of errors were computed by including both wrong and missing responses. Before the statistical analysis, this variable was submitted to an angular transformation, y = arcsen[sqr(p)], where sqr(p) is the square root of the proportion.
Both median reaction times and angular transformations of the proportions of errors have been submitted to a mixed-model analysis of variance (anova) with group (sleep, deprivation) and RCT intervals (300–1000, 1000–300, 300–300) as between-factors, and day (baseline, experimental, recovery) and trial (repetition, switch) as within-factors. SCs were submitted to the same anova design, but omitting the trial factor.
For all the analyses, in case of significant effects planned comparisons were carried out and the level of significance was always set at P < 0.05.
Results
Switch costs (SC)
Three subjects were excluded from the analyses because of outliers (specifically, they made more than 9% of errors). The anova on SC showed significant main effects for group (F1,99 = 4.29; P < 0.05) and for day (F2,198 = 16.464; P < 0.00001); moreover, the interaction group × day was also significant (F2,198 = 7.24; P < 0.001). Planned comparisons revealed that SC of the deprivation and sleep groups differed only in the experimental day (F1,99 = 10.33; P < 0.01), due to higher SCs after one night of total sleep loss; this difference disappeared after one night of recovery sleep (Fig. 2, upper section). Moreover, mean SC on the baseline day were significantly larger than SC on recovery day in both conditions (both P < 0.001), suggesting that subjects continued to learn to switch between tasks even after the training session. Other interactions involving group and RCT intervals factors were not significant.
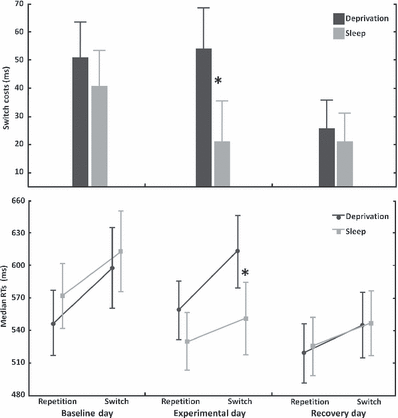
Switch costs and reaction times to the repetition and switch trials. Upper section: SCs (means and confidence intervals) as a function of the day (baseline, experimental, recovery) and of the group (deprivation, sleep). The asterisk indicates the significant difference between deprivation and sleep groups on the experimental day (P < 0.01). Lower section: means of median reaction times (and confidence intervals) on repetition and switch trials, as a function of the day (baseline, experimental, recovery) and of the group (sleep, deprivation). The asterisk indicates that, on the experimental day, the deprivation group showed slower reaction times on switch trials than the sleep group (P < 0.01).
Reaction times (RT)
In order to control for non-specific effects of arousal levels following SD on performance, median reaction times have been submitted to the above-specified mixed model anova. The analysis showed a significant main effect for RCT intervals (F2,99 = 3.32; P < 0.05), indicating that median RT in the 300–1000 condition were significantly slower than in the other conditions (P < 0.05 for both cases). The main effects for day (F2,198 = 25.51; P < 0.00001) and for trial (F1,99 = 88.32; P < 0.00001) were also significant. However, as they entered the three-way interaction, they are discussed below.
The following interactions were also significant: day × group (F2,198 = 12.58; P < 0.00001), trial × group (F1,99 = 4.30; P < 0.05) and group × day × trial (F2,198 = 7.24; P < 0.001).
With regard to the three-way interaction, RT on repetition trials were faster than those on switch trials, for all group and day conditions (P < 0.0001). More interestingly, the SD group showed slower RT on switch trials after the experimental night than the S group (P < 0.01), while RT on repetition trials of the two groups did not differ significantly.
This result indicates clearly that the slowing of the switch RT was particularly evident after one night of SD, and that it disappears after one recovery night (Fig. 2, lower section). It should also be noted that, with regard to the S group’s performance, baseline RT are slower than those recorded on the other 2 days (both P < 0.0001). On the other hand, experimental RT do not differ from recovery RT. With regard to the SD group’s performance, baseline RT to both repetition and switch trials are faster than experimental RT (P < 0.01), and slower than recovery RT (P < 0.00001); moreover, experimental RT are slower than recovery RT (P < 0.00001), again for both repetition and switch trials (Fig. 2, lower section).
Error proportion
In order to evaluate the speed/accuracy trade-off in the task execution, we analysed the error proportion (see above for design specifications). Table 1 summarizes means (and standard deviations) of this dependent variable.
| RCT interval (ms) | Group | n | Baseline day | Experimental day | Recovery day | |||
|---|---|---|---|---|---|---|---|---|
| Repetition | Switch | Repetition | Switch | Repetition | Switch | |||
| Mean ± standard deviation | Mean ± standard deviation | Mean ± standard deviation | Mean ± standard deviation | Mean ± standard deviation | Mean ± standard deviation | |||
| 300–1000 | S | 17 | 0.118 (0.080) | 0.128 (0.094) | 0.125 (0.086) | 0.161 (0.102) | 0.122 (0.095) | 0.151 (0.118) |
| 300–1000 | SD | 18 | 0.091 (0.054) | 0.117 (0.071) | 0.162 (0.075) | 0.227 (0.095) | 0.109 (0.062) | 0.154 (0.086) |
| 300–300 | S | 18 | 0.122 (0.060) | 0.114 (0.085) | 0.103 (0.071) | 0.144 (0.097) | 0.133 (0.088) | 0.158 (0.085) |
| 300–300 | SD | 17 | 0.106 (0.073) | 0.136 (0.089) | 0.144 (0.092) | 0.169 (0.096) | 0.132 (0.067) | 0.185 (0.072) |
| 1000–300 | S | 18 | 0.090 (0.060) | 0.126 (0.085) | 0.125 (0.081) | 0.123 (0.093) | 0.125 (0.079) | 0.138 (0.106) |
| 1000–300 | SD | 17 | 0.117 (0.068) | 0.109 (0.081) | 0.171 (0.099) | 0.199 (0.115) | 0.119 (0.073) | 0.139 (0.082) |
The anova showed significant main effects for day (F2,198 = 20.04, P < 0.00001) and for trial (F1,99 = 32.71; P < 0.00001). The latter effect indicated that subjects made more errors on switch trials than on repetition trials. Moreover, the interaction day × RCT intervals (F4,198 = 2.63, P < 0.05) was significant. Planned comparisons showed that subjects made more errors on the experimental day than on the other days, with both 1000–300 and 300–1000 ms RCT intervals (P < 0.05 for all cases). However, with 300–300 ms RCT intervals, subjects made more errors on the recovery day than on the other days (P < 0.05).
The group × day interaction (F2,198 = 10.29, P < 0.0001) was also significant. Planned comparisons on the means of the significant group × day interaction showed that the S group was more accurate (made fewer mistakes) than the SD group only on the experimental day (P < 0.001), regardless of the trial type, suggesting that SD affected participants’ accuracy non-specifically.
Discussion
In this study we have evaluated the effects of one night of total SD on the executive control processes, assessed by means of a task-switching procedure in a large sample of subjects. Results showed that the lack of sleep affects task-switching performance speed negatively, as indicated by the increased SCs after SD compared to those after normal sleep. We also ruled out the possibility that these results could be due to the non-specific effects of SD on the arousal levels of the participants. In fact, control analyses carried out separately on switch and repetition trials showed that the performance of the sleep-deprived group was significantly worse only on the switch trials. A generalized effect of the lower levels of alertness would have involved slower reaction times on both types of trials. In fact, such a non-specific effect was found only on the accuracy measure, as a higher proportion of errors was reported after SD, irrespective of the type of trial.
Therefore, SD, compared to one night of undisturbed sleep, affects negatively human ability to shift between different cognitive tasks, i.e. to adjust behaviour rapidly and flexibly to changing environmental demands. The present results extend previous findings (Drummond et al., 1999, 2000; Harrison and Horne, 1998, 1999, 2001; Harrison et al., 2000; Wimmer et al., 1992), supporting further the idea that the negative effects of SD are particularly evident with regard to PFC functionality. Our results are also in line with the hypothesis and the predictions of the local, use-dependent sleep theory (Krueger et al., 2008), according to which sleep is a fundamental property of neuronal networks and is dependent upon prior activity in each network. From this viewpoint, the peculiar susceptibility of the PFC to the effects of SD would derive from the hyperactivity of the frontal areas during waking and, consequently, from their higher sleep need (Cajochen et al., 1999; De Gennaro et al., 2007; Ferrara et al., 2002; Finelli et al., 2001).
The task-switching paradigm used in the present study, by manipulating RCT intervals, also allowed us to investigate the existence of possible specific effects of SD on the preparation and/or the disengagement components of the task. Nevertheless, the three different conditions (300–1000 ms, 1000–300 ms, 300–300 ms) did not result in any crucial interaction involving the experimental manipulation of sleep, suggesting that SD exerts a generalized negative impact on the executive control processes involved in task-switching performance, more than any particular effect on its specific components. With regard to the main effect of RCT interval, the 300–1000 ms condition produced the slowest RTs. This seems a counterintuitive effect, as in that condition participants had more time to prepare for the task at hand than in the other tasks. The effect can be attributed to a sort of proactive interference, because of incomplete disengagement from the previous task. It could be hypothesized that different preparation times affect participants’ arousal levels. When individuals have less time to prepare for a task, they may experience higher levels of psychophysiological activation. Thus, subjects in the 300–1000 ms interval condition might be less activated, with a reduced haste to respond. Only a within-subjects design, aimed directly at addressing this specific issue, could clarify this effect.
A functional magnetic resonance imaging (fMRI) study separated brain activity during task-switching into sustained and transient components (Braver et al., 2003). The authors proposed that sustained activation of the right anterior PFC may be important for maintaining a high level of cognitive control over an extended period in situations requiring rapid and flexible alternation between multiple different tasks. On the contrary, the left lateral PFC and left superior parietal cortex, respectively, would be activated transiently for the maintenance and for the reconfiguration of task-set information (Braver et al., 2003). Given the difficulty and the duration (about 20 min) of the testing session, the increases in SCs reported here could be due to the deactivating effects of SD on the right prefrontal brain regions, which are involved critically in sustained attentional functions (Posner and Petersen, 1990). Nevertheless, we cannot exclude that the specific brain regions showing sensitivity to transient aspects of task-switching (Braver et al., 2003) may also be affected negatively by sleep loss.
In conclusion, our results demonstrate that one night of sleep loss deteriorates the executive control processes involved in the task-switching performance, which depend upon the functional integrity of the PFC. Prefrontal cortical vulnerability to SD has been suggested previously by Horne (1993) and supported by several experimental findings (Blatter et al., 2005; Harrison and Horne, 1998, 1999, 2001; Harrison et al., 2000; Killgore et al., 2006). The present study indicates that another higher-order frontal function, namely the ability to adjust behaviour rapidly and flexibly to changing environmental demands, which represents one of the most sophisticated human abilities, needs sleep to be at its optimal levels. These results have some important practical implications: in fact, everyday life requires frequent shifts between different cognitive tasks. For example, medical decisions require the quick and flexible adaptation of behaviour to the changing characteristics and requests of the environment. Such crucial ability may be impacted negatively by sleep loss and fatigue.
Disclosure
None.
Acknowledgements
This work is dedicated to the memory of the students of the University of L’Aquila who died after the earthquake on 6 April 2009. We wish to thank Alessia Timperi, Valentina Petrangeli, Gabriella Catini, Arianna Valente, Elena Aloisi, Claudia Margarita, Monica Celeste and Pierangela Pellegrini for their help in data collection. We also wish to thank two anonymous reviewers for their helpful comments on a previous version of the manuscript. This work has been funded partly by a grant from the University of L’Aquila (Ricerche di Ateneo ex 60%) and by a grant from the Italian Ministry of the University and Research–MIUR–(PRIN 2007) to M. Ferrara.




