Blood p50 evaluation enhances diagnostic definition of isolated erythrocytosis
Abstract.
Background. High oxygen-affinity haemoglobin variants and 2,3-diphosphoglycerate (2,3-DPG) deficiency are inherited diseases generating low tissue oxygen tension and erythropoietin-driven erythrocytosis, that characterizes the clinical phenotype of patients. Level of blood p50 (the oxygen tension at which haemoglobin is 50% saturated) is used to recognize these conditions.
Objectives. To define the clinical utility of blood p50 measurement in the diagnosis of isolated erythrocytosis.
Subjects and design. Venous blood p50 measurement was included in the diagnostic work-up of 102 consecutive patients with isolated erythrocytosis besides blood cell count, arterial oxygen saturation, serum erythropoietin measurement and screening for JAK2 mutations.
Setting. Haematological Outpatient Section at University Hospital.
Results. Seven patients had relative erythrocytosis. Among 95 patients with absolute erythrocytosis, 4 (4.2%) had decreased p50 level. The extended study of family members revealed a familial inheritance. Two families had haemoglobin variants already described as Haemoglobin Malmö and Haemoglobin San Diego. In one family, the proband had a new high oxygen-affinity haemoglobin variant (Haemoglobin Safi) resulting from the transversion C→A at codon 81 of the α2-globin gene. In the last family, a deficiency of 2,3-DPG was found. Within the 91 patients with normal p50 values, 46 (51%) had secondary erythrocytosis, 13 (14%) polycythemia vera and 32 (35%) idiopathic erythrocytosis.
Conclusions. This study suggests that the investigation of blood p50 level may be useful to define diagnosis in patients with isolated erythrocytosis.
Introduction
High oxygen-affinity haemoglobin variants and 2,3-diphosphoglycerate (2,3-DPG) deficiency are numbered within causes of polycythemia [1] and are considered rare disorders. To date, 91 high oxygen-affinity haemoglobin variants have been described worldwide (database of Human Haemoglobin Variants, http://globin.bx.psu.edu) [2]. These conditions are inherited with an autosomal dominant pattern and are caused by mutations of α- or β-globin chain [3]. Concerning 2,3-DPG deficiency, rare cases have been described including different types of enzymatic abnormalities such as diphosphoglycerate mutase deficiency, phosphofructokinase deficiency and piruvate kinase increase [4–10]. As a consequence of the enzymatic abnormality, the affinity of the haemoglobin for oxygen is increased. In most instances the pattern of inheritance is autosomal dominant [7–9], but it may be also recessive [6]. High oxygen-affinity haemoglobin variants and 2,3-DPG deficiencies result in low tissue oxygen tension and a left shift of the oxygen dissociation curve, with reduction of the p50 (the oxygen tension at which haemoglobin is 50% saturated) [11]. Therefore, the level of p50 is currently used to recognize these conditions [12]. Finally, the hypoxia is compensated by an erythropoietin-driven erythrocytosis.
The clinical phenotype of patients with high oxygen-affinity haemoglobin variants and 2,3-DPG deficiency is dominated by isolated erythrocytosis. In most instances, patients are asymptomatic or with mild symptoms of blood hyperviscosity [13]. Especially when the family history is unknown or uncertain, these conditions might remain underestimated. In the setting of isolated erythrocytosis, other conditions such as secondary erythrocytosis (SE), polycythemia vera (PV) [14, 15] with well defined somatic mutations [16, 17], idiopathic erythrocytosis (IE) [18] and familial erythrocytosis [19–23] should be taken into account.
Starting from January 2005, we routinely included venous blood p50 assessment in the diagnostic work-up of patients presenting with isolated erythrocytosis besides recommended investigations. Among 95 patients with absolute erythrocytosis, four (4.2%) showed decreased p50 values and were further investigated for haemoglobin variants and 2,3-DPG deficiency.
Materials and methods
Patients
From January 2005 to February 2008, we evaluated 102 consecutive patients with isolated erythrocytosis observed at the Division of Hematology of the Fondazione Policlinico San Matteo, University of Pavia. Isolated erythrocytosis was defined as the presence of haematocrit over 51% for males and over 48% for females, leucocyte and platelet count within normal range (leucocyte count <10 × 109 L−1 and platelet count <400 × 109 L−1). Initial diagnostic work-up included: clinical evaluation and family history, blood cell count, p50 level measurement, arterial oxygen saturation, serum erythropoietin (normal range 3.7–31 mU mL−1), and screening for JAK2 mutations in circulating granulocytes. Further investigations, such as chest X-ray, abdominal ultrasound, echocardiography, high-resolution CT scan, lung function test and bone marrow biopsy were performed when needed.
Absolute erythrocytosis included acquired SE, PV and IE. Relative erythrocytosis has been excluded on the basis of red cell mass measurement. Diagnosis of PV was made according to the WHO criteria [24]. Diagnosis of IE implied the exclusion of SE and of PV [18]. The study was approved by the institutional ethics committee of Pavia and the procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000. Samples for molecular analysis were obtained after patient provided written informed consent.
P50 measurement
Venous blood was collected in vacutainer tubes and placed on ice. Samples were processed within 10 min after drawing. The blood gas analyzer used (Radiometer ABL 800 Flex; Radiometer America, Westlake, OH, USA) was equipped with the FLEXQ module to automatically identify p50 value (normal range: 25–29 mmHg). An equation converts venous oxygen tension and oxygen saturation to the p50 [12].
JAK2 mutational analysis
Granulocytes were obtained from the neutrophil fraction by osmotic lysis of red cells. Genomic DNA was obtained by using the Puregene Blood DNA isolation kit (Gentra Systems, Minneapolis, MN, USA). A quantitative real-time polymerase chain reaction-based allelic discrimination assay was used to detect the JAK2 (V617F) mutation [25]. Exon 12 of JAK2 gene was also screened [26].
Structural characterization of the variant haemoglobin and DNA analysis
The different components of haemoglobin were separated and measured by cation exchange high-performance liquid chromatography using Variant II™β-Thalassemia Short Program (Bio-Rad Laboratories, Hercules, CA, USA). Red cell lysates were analysed by electrophoretic techniques at alkaline pH (on cellulose acetate) and acidic pH (on agar citrate). The isopropanol precipitation test was carried out as described [27]. Haemoglobin variants were characterized at both the protein and the DNA level. The amino acid replacement within the globin sequence was assessed by a combination of matrix-assisted laser desorption ionization and tandem liquid chromatography-mass spectrometry (LC-MS/MS), as previously described [28]. To assess DNA mutations, genomic DNA was extracted from peripheral blood leucocytes by the salting-out method. In family 1 (patient II-1), the mutation was assessed by restriction fragment analysis of amplified β-globin gene. The PCR product was then digested with PstI restriction enzyme [13]. In family 2 (patient III-1), an amplification refractory mutation system polymerase chain reaction (ARMS-PCR) technique was established using appropriate primers (5′-TTATCTTCCTCCCACAGCTCCTGGGGAACA-3′ and 5′-GAGTCAAGGCTGAGAGATGCAGGA-3′) for the detection of the variant. In family 3 (patient II-2), amplification of individual α1- and α2-globin genes was performed using specific primers for each gene [29]. Sequence analyses of the amplified fragments were carried out on gel purified sample (Qiagen Gel Purification Kit; Qiagen Inc., Valencia, CA, USA) on an Applied BioSystems 3100 automated sequencer using the PRISM™ Dye Terminator Cycle Sequencing Kit (Applied BioSystems, Norwalk, CT, USA).
Measurement of diphosphoglycerate mutase and 2,3-diphosphoglycerate
For diphosphoglycerate mutase (DPGM) measurement, heparinized whole blood was collected and filtered with a mixture of equal weights of dry-cellulose and dry microcrystalline cellulose (Sigma Cell Type 50; Sigma Chemical Company, St Louis, MO, USA). Erythrocyte DPGM activity was measured by spectrophotometry at 340 nm as the rate of reduction of nicotinamide adenine dinucleotide (NAD) to NADH, as previously described [30]. Regarding 2,3-DPG, fresh blood was collected in EDTA and deproteinized extract was prepared. 2,3-DPG was assayed by 2,3-diphosphoglycerate kit (Roche Diagnostic, Mannheim, Germany).
Statistical analysis
The nonparametric Kruskall–Wallis test was adopted to compare numerical variables among different groups.
Results
P50 value in patients with isolated erythrocytosis
Of 102 patients presenting with isolated erythrocytosis, seven had relative erythrocytosis (Fig. 1). Of 95 patients with absolute erythrocytosis, four (4.2%) had p50 values below the normal range (11, 15.7, 21 and 21.3 mmHg, respectively). This prompted us to perform specific investigations in relatives available for study. The remaining 91 patients had normal level of p50 (median 26.3, range 25.8–28.9 mmHg). Among these, 46 (51%) had secondary erythrocytosis caused by pulmonary diseases in 16 (35%), smoking in 15 (33%), sleep apnoea in 10 (22%), renal diseases in four (8%) and anabolic steroids assumption in one (2%). All patients with secondary erythrocytosis did not display the JAK2 mutations and endogenous erythroid colony. Thirteen (14%) patients had polycythemia vera: 11 carried the JAK2 (V617F) mutation, one had exon 12 mutation of JAK2 gene (E543-D544del) and one did not carry any JAK2 mutation. All patients with polycythemia vera displayed endogenous erythroid colonies. Thirty-two (35%) patients had idiopathic erythrocytosis and were JAK2 wild-type.
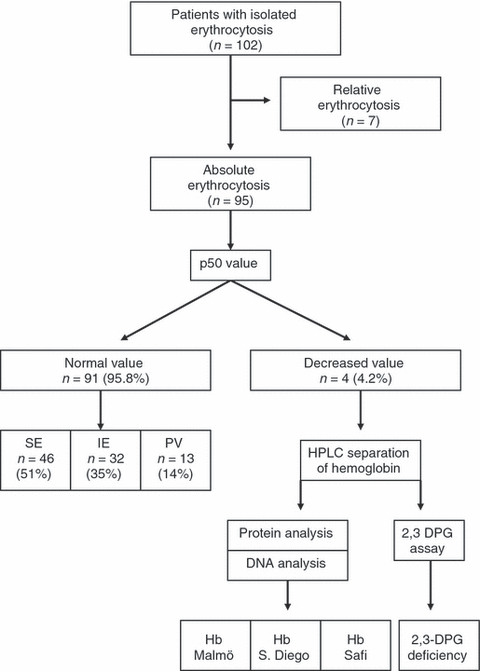
Flow chart of the p50 study. 102 patients with isolated erythrocytosis have been studied, of whom seven had relative erythrocytosis. Within 95 patients with absolute erythrocytosis, four had decreased p50 levels and carried haemoglobin variants or 2,3-diphosphoglycerate deficiency. The remaining 91 patients had polycythemia vera (PV), secondary erythrocytosis (SE) or idiopathic erythrocytosis (IE).
We compared the p50 value among patients with PV, SE and IE. Kruskall–Wallis test did not show significant differences among groups.
To evaluate whether haematological parameters differ among these conditions (low-p50 group, SE, PV, IE), we evaluated haemoglobin level, leucocyte and platelet count, and serum erythropoietin at diagnosis. Kruskall–Wallis test showed that haemoglobin was statistically higher in PV than in SE (P = 0.02), while did not differ among other conditions. Concerning platelet count, the value was significantly higher in PV than in low-p50 group (P = 0.01), SE (P < 0.001) and IE (P < 0.001). Leucocyte count was similar in all conditions, while serum erythropoietin was significantly lower in PV than in low-p50 group (P = 0.005), SE (P < 0.001) and IE (P = 0.04).
Clinical history and diagnosis in the cases with decreased p50
The pedigree of the four patients with decreased p50 is reported in Fig. 2. Demographic and haematological characteristics of probands and affected relatives are listed in Table 1. Patients with a low level of p50 were studied for high oxygen-affinity haemoglobin variants and 2,3-DPG deficiency.
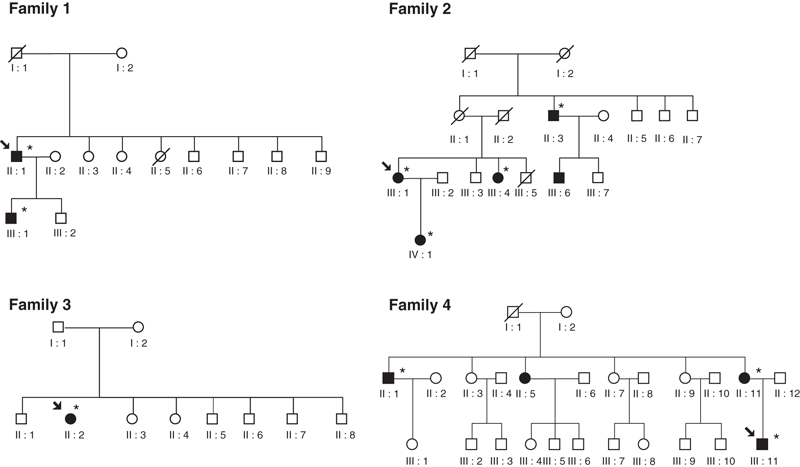
Pedigrees of families with high oxygen-affinity haemoglobinopathy [1–3] and 2,3-DPG deficiency [4]. Each family member is identified by a pedigree number. Circles represent female, squares male; filled symbols patients with abnormal haemoglobin; slashes dead relatives. Arrow indicates the proband; star key indicates patients evaluated at our Department.
| Single patients with low p50 (n = 10) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age (years) | Sex | WBC (×109 L−1) | Hct (%) | Hb (g dL−1) | PLT (×109 L−1) | Epo (mU mL−1) | p50 (mmHg) | |
| Family 1 | ||||||||
| II-1 | 43 | M | 7.2 | 59.4 | 20.4 | 159 | 9.5 | 11.0 |
| III-1 | 23 | M | 6.7 | 56.4 | 18.6 | 199 | 11.0 | 15.6 |
| Family 2 | ||||||||
| III-1 | 55 | F | 5.0 | 51.9 | 17.7 | 137 | 12.8 | 15.7 |
| III-4 | 63 | F | 5.0 | 51.0 | 17.0 | 224 | 6.0 | 17.8 |
| IV-1 | 27 | F | 7.0 | 50.3 | 16.5 | 195 | 6.0 | 16.0 |
| II-3 | 67 | M | 10.5 | 60.0 | 20.2 | 250 | 83.4 | 19.0 |
| Family 3 | ||||||||
| II-2 | 43 | F | 7.6 | 52.0 | 17.2 | 201 | 5.1 | 21.0 |
| Family 4 | ||||||||
| III-11 | 24 | M | 5.8 | 51.4 | 18.4 | 214 | 9.1 | 21.3 |
| II-11 | 42 | F | 8.5 | 49.8 | 17.2 | 156 | 4.4 | 23.0 |
| II-1 | 38 | M | 7.6 | 55.0 | 18.0 | 192 | 11.2 | 22.5 |
| Patients with normal p50 (n = 91), median value (range) | ||||||||
| 55 | 72 M | 7.3 | 54.0 | 18.6 | 205 | 8.1 | 26.9 | |
| (18–78) | 19 F | (3.4–11.6) | (48.6–69.9) | (16.7–23.4) | (173–391) | (0.1–52.0) | (25.8–31.2) | |
- 2,3-DPG, 2,3-diphosphoglycerate; M, male; F, female; WBC, white blood cell; Hct, haematocrit; Hb, haemoglobin; PLT, platelet count; Epo, serum erythropoietin level; EEC, endogenous erythroid colonies; JAK2 (V617F) %, proportion of JAK2 (V617F) mutant alleles; SO2, arterial oxygen saturation; p50, oxygen tension at which Hb is 50% saturated.
In family 1, the propositus (II-1), a 43-year-old man of Italian origin, had haemoglobin value of 20.4 g dL−1 and complained of headache. The level of p50 was 11 mmHg. Although the patient did not report a family history for this condition, we tested the son (III-1), who was asymptomatic. We found he had erythrocytosis (haemoglobin 18.6 g dL−1) with low p50 value (15.6 mmHg). No other family members were available for testing. The propositus was heterozygous for a C→G transversion (CAC-CAG) at codon 97 of the β-globin gene, which corresponded to the His→Gln amino acid substitution, previously described as Haemoglobin Malmö [13, 31–33].
In family 2, the propositus (III-1) was a 55-year-old woman of Italian origin with a 7-year history of erythrocytosis treated with phlebotomy. At the first interview, she reported that a maternal uncle (II-3) and his son (III-6) had PV diagnosed in another institution: relative II-3 was receiving hydroxyurea (an anti-metabolite cytotoxic drug) and relative III-6 was treated with phlebotomy. The propositus had a p50 value of 15.7 mmHg. Her sister (III-4) and daughter (IV-1) were investigated: they displayed isolated erythrocytosis with a low p50. Afterwards, we studied one of the relatives with the diagnosis of PV (II-3) and we found decreased p50 level. In this case we modified diagnosis, stopped hydroxyurea and planned a treatment with phlebotomy. The propositus, her sister and her daughter were heterozygous for a G→A (GTG-ATG) mutation at codon 109 of β-globin gene resulting in the Val→Met substitution, previously described as Haemoglobin San Diego [34, 35].
In family 3, the propositus (II-2), a 43-year-old woman of Moroccan origin, had mild erythrocytosis recognized at the time of spontaneous abortion. Venous blood gas analysis revealed a slight decrease of p50 (21 mmHg). As all the relatives lived abroad, the extended study of the family was not possible. Haemoglobin and DNA analysis identified a new haemoglobin variant, that we named Haemoglobin Safi (the birthplace of the proband). This resulted from a mutation in the α-chain: the transversion C→A (TCC-TAC) at codon 81 of the α2-globin gene results in the Ser→Tyr substitution (Haemoglobin Safi α2 81 Ser→Tyr).
In family 4, the propositus (III-11) was a 24-year-old man of Italian origin, who found by chance isolated erythrocytosis (haemoglobin 18.4 g dL−1). He reported a family history of PV: II-1 received hydroxyurea for 7 years and II-5 was treated with phlebotomy for 14 years. The propositus had a low p50 value (21.3 mmHg). We studied his mother (II-11) and one of the relatives diagnosed as PV (II-1). Both displayed erythrocytosis with low p50 value. After these findings, hydroxyurea was stopped. Proband of family 4 did not show high oxygen-affinity haemoglobin variants at the protein level. So, we studied 2,3-DPG deficiency. We found a partial deficiency of 2,3 diphosphoglycerate mutase (2.5 IU g−1 of haemoglobin; reference value: 4.64–6.46 IU g−1 of haemoglobin), determining a decreased level of 2,3-DPG (6.2 IU g−1 of haemoglobin; reference value 8.82–12.26 IU g−1 of haemoglobin) and an elevation of red cell ATP (5621 nmol g−1 of haemoglobin; reference value 3601–4861). Reticulocyte count and serum lactate dehydrogenase level were within normal range in all evaluated patients. To date, no patient within these four families developed vascular complications.
Discussion
In this study, we systematically evaluated blood p50 level in 102 patients with isolated erythrocytosis to estimate whether this approach may enhance diagnostic definition of this condition.
The diagnostic tools in cases presenting with isolated erythrocytosis have been recently improved by the discovery of mutations of the JAK2 gene, that identify more than 95% of patients with PV [16, 17, 25]. Nevertheless, PV represents only a small portion of patients with isolated erythrocytosis [36] and other disorders display this clinical phenotype. High-affinity haemoglobinopathies [3, 37] and 2,3-DPG deficiencies [4–10] are considered rare causes of congenital erythrocytosis. In a cohort of 95 consecutive patients with absolute erythrocytosis, four (4.2%) showed low level of p50. Three patients had high oxygen-affinity haemoglobinopathies (one had a new variant resulting from the transversion C→A at codon 81 of the α2-globin gene) and one had 2,3-DPG deficiency. Although our Department is not a referral Center for the study of haemoglobin variants, the frequency of decreased p50 seems unexpectedly high. The nature of the study (case-series study) does not allow to apply the 4.2% prevalence of high-affinity haemoglobin variant/2,3-DPG deficiency to the entire population of isolated erythrocytosis. On the other hand, a population-based study to ascertain the real prevalence of these conditions seems difficult to be conducted.
Within the other 91 patients with normal p50, the final diagnosis was secondary erythrocytosis in 51%, idiopathic erythrocytosis in 35%, polycythemia vera in 14%. These data confirm that secondary erythrocytosis is the more frequent cause of isolated erythrocytosis [36], while PV is a much lesser frequent condition [38, 39].
This study also indicates that misdiagnosing this condition may lead to inadequate treatments. Regarding this point, clinical histories of family 2 and family 4 are particularly instructive. In fact, two members of family 2 were diagnosed as PV and one of them received chemotherapy for a long period of time. The same occurs in family 4 including two patients diagnosed as PV: one of them received chemotherapy with hydroxyurea for 7 years.
The occurrence of polycythemia in patients with these haemoglobin variants is explained by the intrinsic property of the abnormal haemoglobin. The high oxygen affinity of this haemoglobin reduces tissue oxygen delivery, leading to hypoxia and a left shift of the oxygen dissociation curve with a reduction of the p50 level. This mechanism induces an increase of erythropoietin production and finally polycythemia [40]. The pathogenesis of erythrocytosis is similar in 2,3-DPG deficiency. The physiological function of 2,3-DPG is to bind deoxygenated haemoglobin decreasing its oxygen affinity and shifting the oxygen dissociation curve to the right. As a consequence, the 2,3-DPG deficiency produces an increase in the affinity of haemoglobin for oxygen and a left shift of the oxygen dissociation curve [9]. Blood p50 is a cheap test to recognize both conditions and is automatically assessed by blood gas analyzer or easily calculated on the basis of a mathematical formula [37]. A caveat on the interpretation of decreased p50 value should be taken into account in smokers as haemoglobin has 250-fold higher affinity for carbonmonoxy than for oxygen [3]. In this event, repeated p50 measurement after giving up smoking is indicated.
The pattern of inheritance in the four families is consistent with an autosomal dominant trait as reported for all high-affinity haemoglobin variants [3, 37] and for the majority of 2,3-DPG deficiencies [7–9]. Then, a thorough investigation of family members is mandatory once such a diagnosis is performed.
Clinical phenotype of patients with these conditions is dominated by erythrocytosis [4, 6, 7, 37] and in a few cases by headache [41]. Patients with 2,3-DPG deficiency may show jaundice and haemolysis instead of erythrocytosis [9, 42]. Erythrocytosis and laboratory evidence of haemolysis may also coexist in the same patient [10]. In our series, patients with 2,3-DPG deficiency had isolated erythrocytosis without signs of haemolysis.
Differently from patients with polycythemia vera [43], members of the four families did not develop over time leucocytosis, thrombocytosis and splenomegaly.
Regarding treatment of these conditions, the role of phlebotomy is controversial [13, 37]. Reduction of red cell mass with phlebotomy may stimulate erythropoiesis. On the other hand, this implies a predominant production of young 2,3-DPG-rich red cells, that may enhance oxygen release to the tissues [13]. In addition, in some patients phlebotomy may induce improvement of symptoms [13], as happens in patient II-1 of family 1.
In conclusion, investigation of blood p50 levels enhances diagnostic definition of patients with isolated erythrocytosis. The evaluation of p50 should be routinely included in the diagnostic work-up of these patients, as we demonstrated that high-affinity haemoglobinopathies and 2,3-DPG deficiencies are rare but existing causes of erythrocytosis.
Conflict of interest statement
No conflict of interest was declared.
Acknowledgements
This study was supported by grants from Fondazione Cariplo, Milan, Italy; Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy; Fondazione Ferrata Storti, Pavia, Italy; Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; Ministry of University and Research, Rome, Italy.