A dendritic cell-based vaccine for treating HIV infection: background and preliminary results
Abstract.
Antibody response against human immunodeficiency virus-1 (HIV) is ineffective and cellular immune response is not strong enough to achieve the complete suppression or at least a strong control of viral replication in HIV- infected patients. In 2001, we showed in vitro that dendritic cells (DCs) of HIV-infected patients loaded with autologous HIV chemically inactivated by aldrithiol-2 were capable of raising an HIV-specific cellular immune response powerful enough to allow the destruction of autologous HIV- infected CD4 T cells. In 2003, we showed that simian immunodeficiency virus (SIV)-infected macaques vaccinated with inactivated SIV-loaded autologous DCs raised a strong SIV-specific cellular response. Ten months after vaccination, plasma viral load of 7 out of the 10 vaccinated monkeys remained 1000-fold lower than initially.
In December 2004, we published results observed in 18 untreated HIV-infected patients vaccinated with autologous monocyte-derived DCs loaded with autologous inactivated HIV. A year following vaccination, 8 patients had a plasma viral load decrease >90%; among them, 4 had viral load <1000 copies mL−1. Moreover, by one year, the viral load decline of the 18 patients was significantly correlated with their percentage of HIV-1-gag-specific CD8+ T cells expressing perforin and that of HIV-1-specific CD4+ TH1 cells. This is the first demonstration of the capacity of a therapeutic vaccine to induce an effective HIV-specific T cell response associated with sustained viral suppression in untreated viremic patients. The manipulation of antigen presenting cells to elicit virus-specific cellular responses is a promising tool to control persistant viral infections.
Background
Viral infections
Viruses are obligate intracellular pathogens and therefore depend on living hosts for their propagation.
Transient viral infections caused by cytopathic viruses like smallpox or poliomyelitis generate the early production of large amounts of specific antibodies that allow the rapid eradication of the virus. The patients who survive these infections are immunized.
Persistent viral infections like the herpes simplex 1 and 2 (HHV1 and 2) or the varicella/zoster virus (HHV3) infections are caused by intermittent cytopathic viruses. After the primary infection, the replication of these viruses inside their target cells is strongly controlled by the permanent pressure of specific T cells which maintain them in a so-called state of latency. In fact, as soon as there is a transient or more prolonged weakness, disturbance or loss of anti-viral immune functions (whether provoked by ageing, immunosuppressive therapies or immunosuppressive infections), these viruses resume their replication with their associated cytopathic effects (before being controlled again or not depending on the level of impairment of the immune system).
Persistent viral infections caused by poorly/noncytopathic viruses include two devastating viruses: the hepatitis B virus (HBV) and the hepatitis C virus (HCV). The immune system exerts a strong control on viral replication in the majority of individuals who have acquired HBV. However, it is not effective enough in a minority of them and in almost all patients with HCV with the consequence that the viral replication in infected cells and measurable viral release in the extracellular milieu persist for life. This is also the case of the human immunodeficiency virus -1 (HIV-1) which is now more and more frequently viewed as a poorly cytopathic virus by many immunologists [1], although it has been considered for a long time as a cytopathic virus by virologists. Importantly, cellular damages provoked by these three persistent infections do not directly result from viral replication but from immunopathology associated with the chronic release of the virus and inadequate response of the immune system
HIV infection
Amongst the 65 million individuals who have been infected by HIV from the onset of the epidemics in the late 1970s, 25 million have so far died from AIDS and 38.6 million – including 25 million in Africa – are still presently living with HIV with an incidence of 4.1 million acquiring the virus and 2.8 million dying from AIDS every year [2].
The HIV-1 penetrates the body through sexual mucosa, blood and the oro-pharyngeal and/or digestive mucosa (at least in the newborn). Heterosexual transmission is presently the most frequent contamination modality in Africa. The rate of contamination from an infected patient to his/her heterosexual partner is significantly associated with the plasma viral load (PVL) level of the infected partner and PVLs <1500 HIV RNA copies mL−1 have been associated with the absence of contamination of the sexual partner [3].
An important characteristic of this virus is that its main target cell – inside which it replicates – is one of the cells governing the immune system, the CD4 lymphocyte. During the first phase of primary infection, HIV infects a small proportion of CD4 lymphocytes with a memory phenotype [4]. The antibodies targeted against the virus are not capable of neutralizing it [5] and the control of viral replication in CD4 lymphocytes relies therefore on the activity of specific cytotoxic/suppressive CD8 T lymphocytes [6] as shown by the dramatic increase in viraemia that occurs in animal models of AIDS virus infection after experimental removal of CD8 T cells [7]; however, this control is partial at best with a specific cytotoxic T-cell (CTL) activity variably impaired in different patients depending, at least to some extent, on the immune system characteristics of each infected patient [8]. Hence patients have a large range of HIV-infected CD4 lymphocytes and eventually of viral production by these cells. For reasons that are beginning to be understood [9, 10], the persistent release of HIV-1 provokes the progressive disappearance of (noninfected) CD4 T cells and finally the destruction of the architecture and functions of the lymphoid organs. PVL levels (which are representative of the global viral replication) observed after the completion of the phase of primary infection, at the beginning of the chronic phase of the infection are highly predictive of the rate of CD4 cell decrease and eventually of the time after which AIDS manifestations (such as certain virus-induced cancers and various types of infections which are normally controlled by the immune system) develop [11]. The median time of evolution from HIV contamination to AIDS manifestations is approximately 8–9 years in Western countries (and probably shorter in less developed areas of the world). However, there are great variations in the outcome. A small percentage of patients with high replication rates and high PVLs (above 200 000 HIV RNA copies mL−1) have their immune system destroyed within 3 years and are referred to as rapid progressors (Fig. 1, red lines). In contrast, the few patients with very slow rates of viral replication and low PVL levels (sometimes <1000 HIV RNA copies mL−1) have CD4 cell counts remaining almost stable ≥20 years after contamination. Such low PVL levels considerably limit their risk of contaminating others [2]. These few patients are referred to as long-term nonprogressors (Fig. 1, green lines).

Evolution of patients with high plasma viral load (PVL) levels and rapid rates of CD4 cells (red lines); evolution of patients with low PVL levels and very low rates of CD4 cells (green lines).
Anti-retroviral treatments (ARTs) are currently given relatively late (between 300 and 200 CD4 cells μL−1 depending on the different guidelines prevailing in each country) in the course of the infection, because it has been shown that their effectiveness to partially restore a competent immune system was almost the same whether ARTs were given earlier or later. Actually, ARTs (when they are working well and when they are taken daily for life) lead to a rapid and long-term decrease in viral replication, enabling the immune system to stabilize or regenerate sufficiently to avoid an evolution towards AIDS. At the same time, ARTs also lead to the lowering of virus concentration in sexual fluids, which lessens the risk of contaminating healthy subjects.
However, ARTs are still not available to a majority of patients living in underdeveloped countries where the number of infected patients is still rising and there is also a growing incidence of treated patients suffering from viral resistance and/or long-term side effects of ARTs. This has led national and international research institutions, and pharmaceutical firms to turn towards vaccines as a potential solution.
Definition of a vaccine
A vaccine against a viral infection is a pharmaceutical preparation containing an immunogen (which will provoke a specific response of the immune system against the wild virus); this immunogen can be the virus itself once it has been inactivated (so that it can no longer multiply in the body with its concomitant negative effects) or artificially-made virus-like particles; it can also be a weakened virus, i.e. it has lost its pathological power, but can multiply inside the body. Finally, the immunogen can also be made up of viral proteins or the nucleic acids which generate them. Vaccine preparations always include, in association with the immunogen, one or more mineral, chemical or biological adjuvants. Their role is to stimulate the transformation/presentation of the immunogen by specialized antigen presenting cells [such as B cells, dendritic cells (DCs) or Langerhans cells], the production of CTLs capable of destroying the cells within which the viruses multiply or the activity of B lymphocytes involved in the production of antibodies capable of neutralizing circulating viruses.
Preventive vaccines
To date, almost all existing preventive vaccines were developed towards cytopathic viruses that caused transient infections (like poliomyelitis). These vaccines protect people very effectively against viruses that the immune system would have actually eradicated spontaneously (once the virus had produced its harmful clinical effects). The administration of such preventive vaccines to healthy persons provokes the rapid production of large amounts of antibodies directed against the virus. For months or years after the vaccination, even low levels of specific circulating antibodies can neutralize/eliminate the virus as it enters the body of vaccinated individuals. Vaccines against poliomyelitis in their Salk version (subcutaneously injected inactivated virus) or in their Sabin version (orally administered weakened virus) are excellent examples of preventive vaccines. They have succeeded in entirely eradicating poliomyelitis in developed countries. For two decades, a preventive vaccine against HBV, has been in existence, protecting the large majority of vaccinated people not to be infected.
So far there are no preventive vaccines against HIV, and the various prototypes that have been tried out on thousands of high-risk individuals have not provided them with any protection [12]. However, the fact that some highly exposed female sex workers remain free of HIV infection with increased levels of anti-HIV CTLs suggests that a purely cellular immune response could be protective in certain situations [13]. We are currently trying to reproduce this phenomenon by immunizing (by the vaginal route) female macaques with inactivated simian immunodeficiency virus (SIV).
Therapeutic vaccines
The concept of therapeutic vaccines applies to diseases which are chronic (due to a lack or an insufficient spontaneous response of the immune system). In fact, most prototypes of therapeutic vaccines that were developed in recent years were aimed at certain types of cancer with disappointing results. However, some new developments seem promising. Concerning therapeutic vaccines against chronic viral infections, research is just beginning.
The goal of a therapeutic vaccine against HIV is to help the immune system of chronically infected patients to produce antibodies capable of neutralizing the virus and/or killer T lymphocytes capable of destroying HIV-infected CD4 lymphocytes. The administration of an efficient therapeutic vaccine (probably to be repeated every year or every 2 years) to HIV-infected patients should (if successful) lead to a decrease in viral replication and release. This would result in the stabilization/partial reconstitution of the immune system allowing vaccinated patients to avoid or postpone ARTs; a successful therapeutic vaccine would also result in the decline of HIV concentrations in sexual fluids which should diminish the risk of vaccinated patients contaminating others. In short, the best possibility for a therapeutic vaccine against HIV would be to transform vaccinated patients into long-term nonprogressors (Fig. 2).

Theoretical role of a therapeutic vaccination in the course of HIV infection.
Experimental results
In vitro studies
The insufficient activity of CTLs towards HIV-infected CD4 lymphocytes, and the subsequent impossibility of spontaneously controlling HIV replication (which leads to the persistence of infection) was the basis on which we developed our research. In 2000, we hypothesized that an inadequate or inappropriate signalling virus-specific antigen presentation might contribute to the persistent failure to mount efficient anti-HIV immunity in HIV-infected patients (except in the few with long-term nonprogression who had retained this capacity).
We first conducted an in vitro study with 10 untreated and 20 ART-treated patients. The virus of each patient, once it had been cultivated in sufficient amount, was inactivated by aldrithiol-2 (AT-2), a chemical compound which covalently modifies zinc-finger cysteines of the nucleocapsid without affecting the conformation of the envelope glycoproteins [14].
The inactivated virus was then loaded in autologous monocyte-derived DCs which allow them to present HIV antigens to autologous CD8 T cells in association with major histocompatibility complex class I molecules. The final result was the expansion and maturation/terminal differentiation of virus-specific CD8 CTLs which became capable of killing HIV-infected cells and eradicating the virus from the culture of patients’ peripheral mononuclear cells independently of patients’ disease stages and ART response status. However, following a 2-day treatment with a culture supernatant derived from immune-activated T cells (to mimic the in vitro environment of HIV-disseminated and immune-activated lymphoid tissues), DCs lost their capacity to present de novo inactivated virus-derived antigens [15]. Results of these experiments provided us knowledge of the interactions of DCs with T cells in the chronic phase of HIV-1 infection and opened up the possibility of an in vivo restoration of anti-HIV immunity in infected individuals.
Vaccination of infected macaques
In 2001, we carried out a therapeutic vaccine trial in SIV-infected Chinese rhesus macaques which are the best animal model of HIV-1 infection [16]. Two months after having been infected by SIV 251, 10 monkeys received a vaccine made of autologous monocyte-derived DCs loaded with AT-2-inactivated SIV. This vaccine was given subcutaneously five times at 2-week intervals; four other monkeys received unloaded DCs as control. One year after the therapeutic vaccination, plasma SIV load of the 10 vaccinated monkeys decreased by more than 99% (P < 0.001) whilst it remained stable in the four control monkeys (Fig. 3). Moreover, the analysis of lymph node biopsies showed a strong decline in cell-associated SIV DNA or RNA burden which inversely correlated with the frequency of SIV-specific γ-interferon-expressing T cells (measured by an ELISPOT assay). The follicular DC network and the germinal centres of the 10 vaccinated monkeys were well preserved in contrast to the four control monkeys [17].

Evolution of plasma viral loads in 10 vaccinated (green lines) and four control (red lines) SIV251-infected Chinese macaques.
Clinical trial
In September 2002, after receiving approval from the National Ethics Committee of Brazil, we launched a phase I/II clinical trial in Recife. From September 2002 to January 2003, 18 HIV-infected untreated patients were included in the trial. They were aged between 18 and 41 years; they were HIV-positive for a median duration of 2 years; they had not received ARTs; their CD4 cell counts ranged from 270 to 1009 cells μL−1 (median 523) and their PVL ranged from 11 000 to 300 000 viral RNA copies mL−1 (median 48 000). The preparation of this ‘auto-vaccine’ was complex and expensive; it included several steps. First, 1010 mononuclear cells were collected by a 3-h leukapheresis. Approximately 108 monocytes were then isolated by plastic adhesion. After 5 days of culture with interleukin (IL) 4 and granulocyte monocyte colony stimulating factor (GM-CSF), monocytes were transformed into immature DCs. Monocyte-derived immature DCs were then put in contact with each autologous virus for 2 h (that had been previously grown and chemically inactivated by AT-2); inactivated HIV-1-loaded-immature DCs were then cultivated with GM-CSF, IL-4, IL-1β, IL-6 and tumour necrosis factor-α for another 2 days. Finally this vaccine preparation made of 3 × 107 autologous mature DCs loaded with autologous inactivated HIV-1 was subcutaneously injected at the root of both arms and both thighs (0.25 mL/site) of each patient. Two further injections of the same number of inactivated HIV-1-loaded DCs were given at 2-week intervals. All patients were followed up for a year thereafter without ART.
The only clinical manifestation associated with the vaccine was a slight but significant increase in the size of peripheral lymph nodes which still persisted at 1 year. No clinical AIDS or milder immunodeficiency symptoms developed during the study period. Four months after the first vaccination, the median PVL of the 18 patients decreased by 80% (P < 0.01) and their CD4 lymphocytes count stabilized. A year after the first vaccination, viral concentration decreased by more than 90% in 8 of the 18 patients. They were all still free of ART.
In four patients the viral load was below 1000 particles mL−1, meaning that they were most likely noncontaminating (Fig. 4). Two years after the vaccination, PVLs of two of these eight patients remained below 1000 particles mL−1 whilst the viral loads of the others started to reincrease.
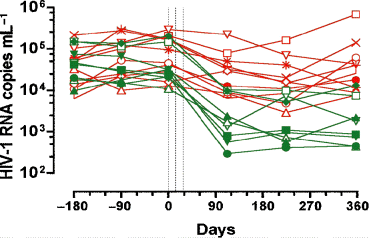
Evolution of plasma viral loads of the 18 patients before and after the three vaccinations (dotted lines). One-year viral load decline <90% (red lines) and >90% (green lines).
Over the year following vaccination, the percentage of HIV-1-specific γ-interferon-expressing CD4 cells significantly increased and by 1 year, it highly correlated with the decrease in PVL. Similar was the case for HIV-1-specific IL-2-expressing CD4 cells (Fig. 5).

Evolution of the percentage of HIV-1-specific interleukin-2 (IL-2)-expressing CD4 T cells after vaccination (dotted lines); 1-year viral load decline <90% red lines and >90% green lines (left). Significant correlation (r2 = 0.455, P = 0.002) at 1 year between percentages of HIV-1-specific IL-2-expressing CD4 T cells and plasma viral load change from baseline (right). Intracellular IL2-expressing CD4 cells following ex vivo stimulation with autologous DCs loaded with inactivated autologous virus were detected by an intracytoplasmic cytokine assay.
On the other hand, the percentage of γ-interferon-expressing HIV-1-specific CD8 cells increased only marginally over the year of the study. Similar was the case for the percentage of γ-interferon-expressing HIV-1 gag-tetramer-specific CD8 cells in the 10 HLA-A*0201-positive patients; moreover, at 1 year, the correlation of these percentages with PVL change was not significant. In contrast, amongst the 10 patients where perforin expression was tested, the percentage of HIV-1 gag-specific CD8 T cells expressing perforin clearly increased over the year of the study (Fig. 6 left), and by 1 year, it strongly correlated with the change in PVL (Fig. 6 right).

Evolution of the percentage of perforin-expressing cells in gag-tetramer specific CD8T cells after vaccination (dotted lines); 1-year viral load decline <90% red lines and >90% green lines (left). Significant correlation at 1 year between percentages of perforin-expressing cells in gag-tetramer CD8T cells and plasma viral load change from baseline (right).
We then wanted to find whether any initial biological parameter predicted the 1-year PVL responses and we discovered that the only initial variables which correlated positively with 1 year decrease in PVL were the CD4 cell count (P = 0.029) and marginally, the percentage HIV-1-specific IL-2-expressing CD4+ T cells [18].
Conclusion
This is the first demonstration in untreated viraemic patients that a therapeutic vaccine is capable of inducing an effective HIV-1-specific T-cell response associated with sustained viral suppression. The impairment of DC functions associated with HIV-1 infection, which resulted in the lack of efficiency of killer lymphocytes vis-à-vis HIV-infected CD4 lymphocytes, was partly restored by the therapeutic vaccine. This DC-based vaccine provoked the proliferation and maturation of CTLs which had now acquired the capacity to destroy the HIV-infected CD4 lymphocytes in vaccinated patients. A year after the vaccination, the therapeutic vaccine remained effective in close to half of the vaccinated patients.
The fact that, 1 year after vaccination, the percentage of HIV-1-gag-specific CD8+ T cells expressing perforin was positively correlated with the decline in PVL underscores the major role of perforin-expressing effectors in controlling HIV-1 replication in vivo. In addition, the significant correlation between viral suppression and durable increase in the percentage of HIV-1-specific CD4+ TH1 cells (represented by HIV-1-specific γ-interferon and IL-2-expressing CD4+ T cells) observed in vaccinated patients favours the notion that a strong virus-specific CD4+ TH1-cell response is required to enable virus-specific CD8+ effectors to contain HIV-1 replication in vivo. This is in keeping with the correlation observed between high levels of virus-specific CD4+T cells and the control of viral replication observed in the few HIV-1-infected patients with long-term nonprogression [19].
Given that stronger 1 year PVL decline following DC vaccination is associated with higher baseline CD4 cell counts or HIV-1-specific IL-2-expressing CD4+T cells (both of which decline progressively along the natural course of the infection), it is most likely that an early therapeutic vaccine intervention could increase the probability of achieving sustained viral suppression. This notion is in keeping with the dramatic viral suppression that we observed in SIV-infected macaques which were vaccinated early in the course of their chronic infection [17].
On the other hand, our results suggest that inactivated whole virus-pulsed DCs could be a better antigenic preparation than a simple protein such as Gag protein to expand and activate in vivo virus-specific CTLs because it allows DCs to present a wider range of epitopes and because AT-2-inactivated SIV/HIV enters the DCs through a receptor-mediated mechanism [20] eliciting a potent HLA-1-restricted CTL response [15, 21] whereas recombinant viral proteins enter DCs through nonspecific endocytosis inducing preferentially humoral (antibody) responses.
Future developments
We are now preparing a randomized trial on 100 patients to confirm our findings. By selecting the patients with CD4 cells >450 cells mm−3, by increasing the number of DCs per vaccination and by adding several booster injections, we will have conclusive evidence whether DC-based vaccines can effectively increase the percentage of responding patients, as well as the depth and the duration of response. If it is the case, our therapeutic vaccine should next be tested on patients who are resistant to ART as well as on the large number who would like to stop ART. However, this first-generation vaccine will not be easily applicable to patients of developing countries because it is a patient-based preparation which requires specifically equipped facilities (P3/class D) and costly reagents and devices.
Conflict of interest statement
No conflict of interest was declared.
Acknowledgements
We thank all the Brazilian, Chinese and French technicians and scientists who participated in these studies. This work was supported by ‘Institut de Recherche sur les vaccins et l'immunothérapie des cancers et du SIDA’, Paris, and Biovaxim Ltd, London.
We express our gratitude to Air France for assistance with travel.




