Microfauna associated with amoebic gill disease in sea-farmed Atlantic salmon, Salmo salar L., smolts
Abstract
A study of microfauna, associated with pathological changes in the gills of Atlantic salmon, Salmo salar L., was conducted over 2001–2002. Monthly samples of 1+ salmon smolts were taken, protozoan populations were quantified and gill health was assessed histologically. Protozoan densities were correlated with pathological changes, in order to determine their possible role in lesions in the gills. The most severe gill tissue changes were observed in summer/autumn and the least in spring. A diverse polyphyletic protozoan community was observed colonizing the gills, including Neoparamoeba sp., other amoebae, scuticociliates, Ichthyobodo-like flagellates, trichodinid ciliates and prostomatean ciliates. The earlier gill tissue changes in the gill were not always associated with the presence of these microorganisms, whereas amoebae (other than Neoparamoeba sp.), Ichthyobodo-like flagellates and trichodinid ciliates correlated with augmenting gill lesions. Neoparamoeba sp. was present, but its abundance did not correlate with the disease. This study suggests that a diversity of protozoans including Ichthyobodo-like flagellates, trichodinid ciliates and amoebae other than Neoparamoeba sp. are involved in the aetiology of amoebic gill disease in the Irish situation.
Introduction
Amoebic gill disease (AGD) is characterized by focal epithelial hyperplasia of the gills, resulting in fusion of the lamellae, as well as extensive mucus secretion coinciding with an increase in the number of mucous cells (Munday, Foster, Roubal & Lester 1990; Zilberg & Munday 2000; Bermingham & Mulcahy 2004), a decrease in chloride cell densities (Munday et al. 1990; Bermingham & Mulcahy 2004), and the presence of Neoparamoeba sp. (Zilberg & Munday 2000).
Neoparamoeba pemaquidensis (Page) (formerly Paramoeba pemaquidensis, Dyková, Figueras & Peric 2000), subclass Gymnamoebae (Page 1970), is an endemic, facultative, amphizoic protozoan (Dykováet al. 2000) with a worldwide distribution (Cann & Page 1982). The amoeba is exclusively marine and is found in a free-living, as well as in a parasitic form, in fish (Tan, Nowak & Hodson 2002). In Ireland amoebae in gill sections from AGD affected gills have been shown to give a positive reaction with antiserum specific to Neoparamoeba sp. (Palmer, Carson, Ruttledge, Drinan & Wagner 1997; Bermingham & Mulcahy 2004). However, in a study in Ireland, Neoparamoeba sp. appeared to be out-competed by other amoebae colonizing the gill, and a negative correlation with gill lesions was confirmed (Bermingham & Mulcahy 2004). Furthermore, simultaneous isolation of Neoparamoeba sp. and other amoebae from the gills of clinically diseased fish has raised the question of the involvement of free-living amoebae other than Neoparamoeba sp. in gill disease (Howard & Carson 1992; Dyková & Novoa 2001; Bermingham & Mulcahy 2004). In addition many other accompanying organisms have been isolated from affected gills, the most important being histophagous scuticociliates (Dyková & Novoa 2001; Bermingham & Mulcahy 2004).
This study was undertaken in 2001–2002 to determine the roles played by the various microfauna, including Neoparamoeba sp., in AGD in Ireland.
Materials and methods
Study site
Marine Harvest Ireland (Marine Harvest) is based in Kindrum, Fanad, Co. Donegal. Marine Harvest has eight operating sites within Mulroy Bay, and production levels within these sites has remained fairly constant at 800–900 t per annum over the last 5–10 years. AGD problems have been experienced annually since the 1995 outbreak, when mortalities reached 60% in their Cranford TC site. The Cranford TC site was not in operation during the 2001–2002 study period, consequently the adjoining Cranford TA site, an enclosed sheltered site, was selected for study (Fig. 1).
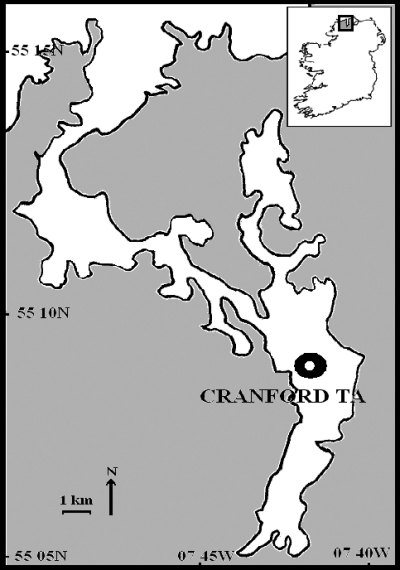
Marine Harvest Ireland's Cranford TA cage site, situated in the fully marine fjordic Mulroy Bay adjacent to the Fanad peninsula on the northern coast of Donegal, Ireland.
Atlantic salmon smolts
Smolts (S1, 66–72 g) were transferred to sea to the Cranford TA site in October 2001 and 2002. Monthly smolt samples (n = 10) from April to September 2001 (sample dates: 20 April, 22 May, 7 June, 12 June, 5 July, 1 August and 24 September 2001) and monthly smolt samples (n = 10) from May to September 2002 (sample dates: 22 May, 30 May, 24 June, 24 July, 19 August and 25 September) were collected, measured, weighed and the second gill arch was excised from the right hand side of the fish and placed in Davidson's fixative.
Histological examination
Fixed tissue was sectioned (4 μm thick), stained with haematoxylin-eosin (H&E) and examined under a light microscope. In this study histopathological changes were recorded, based on proportional morphometry of the branchial tissue (Spear, Arsenault, MacNair & Powell 1997), which readily allows for the integration of histopathological findings with other work. The average phase of AGD in 10 lamellar units, and the number of micronodules per filament (Table 1), the average number of mucous cells, chloride cells, amoebae and other protozoans in 10 lamellar units were recorded. Neoparamoeba sp., other amoebae, scuticociliates, Ichthyobodo-like flagellates, trichodinid and prostomatean ciliates were identified in tissue sections using key features (Bruno, Nowak & Elliott 2001; D. Bruno personal communication) and Neoparamoeba sp. verified in all sections in which amoebae were detected by specific immunofluorescence (Howard 2001). Protozoans may have been lost from gill sections during histological processing, however the aforementioned methodology still provides a relative means of quantification of gill microfaunal populations within the gills.
| The phase of amoebic gill disease was defined by seven phases (Bermingham & Mulcahy, 2004) | |
| Phase 1 | Healthy gill |
| Phase 2 | Increased mucous cells and decreased chloride cell numbers |
| Phase 3 | Thickening of the individual lamellae |
| Phase 4 | Fusion of two lamellae |
| Phase 5 | Fusion of less than five lamellae |
| Phase 6 | Fusion of five or more lamellae |
| Phase 7 | Structural reorganization of gill tissue |
| Lamellar unit (LU) | Area between two lamellae tips (i.e. one complete lamella; M. Powell personal communication) |
| Micronodule | One or more fused lamellae (Bermingham & Mulcahy 2004) |
| Micronodules/filament | Total number of micronodules observed in a section divided by the total numbers of filaments (B. Nowak personal communication) |
Statistics
Data were analysed using one way ANOVA, followed by a Tukey–Kramer multiple comparisons test, to determine whether the gill measurements and protozoan populations varied significantly over the seasons or between the two sample years; and simple/multivariate (stepwise) regression analysis, to detect associations between the gill measurements and protozoan populations over 2001–2002. Results are given at the 5% level of significance. The seasons were divided evenly among the months: spring: February to April; summer: May to July; autumn: August to October and winter: November to January. All tests were performed using the SPSS 11.0 statistical package (SPSS Inc., Chicago, IL, USA).
Results
Over the 2-year period of study (2001–2002) significant AGD lesions were observed in the gills. Gill tissue changes commenced in the secondary lamellae, with an increase in mucous cell numbers, which coincided with a reduction in chloride cell densities (Fig. 2a), followed by hyperplastic thickening of the secondary lamellae (Fig. 2b). Subsequently neighbouring lamellae attached to one another, forming micronodules (Fig. 2c), then fused entirely, forming plaques, followed by necrosis and structural reorganization of gill tissue (Fig. 2d). The observed lesions were not always associated with the presence of amoebae or other gill parasites (Fig. 2a–d).

(a–d) Photomicrographs of pathology observed in the absence of protozoan and bacterial colonisation of the gill. (a) Phase 2, high mucous cell densities. (b) Phase 3, hyperplastic thickening of the primary lamellae. (c) Phase 4, fusion of two primary lamellae (i.e. a micronodule). (d). Phase 7, structural reorganization of gill tissue.
Phase of the disease
All phases of the disease were observed over the study period. The fish sampled in summer–autumn manifested the most severe lesions, and those in spring the least (Table 2). In addition, gill tissue changes were more severe in 2002 than in 2001 (Table 3). Amoebae were seen in all phases of the disease, however, their densities increased with advancing gill lesions (Table 4). Neoparamoeba sp. was also observed in all phases of the disease, but failed to correlate with advancing gill lesions. The association of protozoans with gill lesions is shown in Table 4. Ichthyobodo-like flagellate numbers increased with advancing gill tissue changes but prostomateans correlated negatively with gill lesions. The phase of the disease correlated with micronodule, mucous cell, chloride cell, amoeba, Ichthyobodo-like flagellate and prostomatean abundance.
| Parameter | ANOVA | Tukey–Kramer method | ||
|---|---|---|---|---|
| F | P | Season | P | |
| Phase | 49.1 | 0.000 | Winter–summer | 0.000 |
| Winter–autumn | 0.000 | |||
| Spring–summer | 0.000 | |||
| Spring–autumn | 0.000 | |||
| Summer–autumn | 0.000 | |||
| Micronodules | 9.8 | 0.000 | Winter–autumn | 0.009 |
| Spring–autumn | 0.000 | |||
| Summer–autumn | 0.000 | |||
| Mucous cells | 25.2 | 0.000 | Winter–autumn | 0.001 |
| Spring–autumn | 0.000 | |||
| Summer–autumn | 0.000 | |||
| Chloride cells | 18.9 | 0.000 | Winter–summer | 0.000 |
| Winter–autumn | 0.000 | |||
| Spring–summer | 0.000 | |||
| Spring–autumn | 0.000 | |||
| Scuticociliates | 4.6 | 0.004 | Winter–spring | 0.040 |
| Spring–summer | 0.049 | |||
| Spring–autumn | 0.007 | |||
- Numerator d.f. = 4, denominator d.f. = 138, F ≥ 2.55; P ≤ 0.05 test accepted.
| Parameter | ANOVA | |
|---|---|---|
| F | P | |
| Phase | 3.9 | 0.049 |
| Micronodules | 11.8 | 0.001 |
| Chloride cells | 16.4 | 0.000 |
| Amoebae | 8.1 | 0.005 |
| Ichthyobodo-like flagellates | 11.8 | 0.001 |
| Prostomateans | 5.0 | 0.028 |
- Numerator d.f. = 1, denominator d.f. = 141, F ≥ 3.92; P ≤ 0.05 test accepted.
| Parameter | Regression(s) | |||||||
|---|---|---|---|---|---|---|---|---|
| Simple | Multiple | |||||||
| R 2 (%) | T | F | P | Predictors | T | P | R 2 (%) | |
| Phase | ||||||||
| Micronodules | 29 | 7.5 | 55.7 | 0.000 | Micronodules | 4.4 | 0.000 | 83 |
| Mucous cells | 57 | 13.7 | 186.6 | 0.000 | Mucous cells | 14.1 | 0.000 | |
| Chloride cells | 45 | −10.6 | 112.3 | 0.000 | Chloride cells | −11.4 | 0.000 | |
| Amoebae | 11 | 4.2 | 17.5 | 0.000 | Ich-flagellates | 3.1 | 0.003 | |
| Ich-flagellates | 3 | 2.1 | 4.3 | 0.040 | ||||
| Prostomateans | 4 | −2.2 | 5.0 | 0.026 | ||||
| Micronodules | ||||||||
| Mucous cells | 19 | 5.5 | 33.0 | 0.000 | Mucous cells | 4.5 | 0.000 | 34 |
| Chloride cells | 8 | −3.6 | 12.5 | 0.000 | chloride cells | −2.2 | 0.027 | |
| Amoebae | 6 | 3.0 | 9.3 | 0.003 | Amoebae | 4.3 | 0.000 | |
| Scuticociliates | 3 | −2.1 | 4.4 | 0.036 | Ich-flagellates | −4.5 | 0.000 | |
| Mucous cells | ||||||||
| Chloride cells | 8 | −3.4 | 11.3 | 0.001 | Prostomateans | −3.2 | 0.002 | 12 |
| Trichodinid ciliates | 5 | 2.8 | 7.6 | 0.006 | Trichodinid ciliates | 3.4 | 0.001 | |
| Prostomateans | 5 | −2.6 | 6.6 | 0.001 | ||||
| Chloride cells | ||||||||
| Amoebae | 8 | −3.6 | 12.8 | 0.000 | Amoebae | −3.2 | 0.002 | 13 |
| Ich-flagellates | 4 | −2.5 | 6.4 | 0.013 | Prostomateans | 1.9 | 0.054 | |
| Trichodinid ciliates | 4 | −2.3 | 5.2 | 0.024 | Trichodinid ciliates | −2.4 | 0.019 | |
| Neoparamoeba sp. | ||||||||
| Amoebae | 7 | 3.3 | 10.8 | 0.001 | Amoebae | 3.3 | 0.001 | 7 |
| Ich-flagellates | 5 | 2.6 | 6.7 | 0.011 | ||||
| Amoebae | ||||||||
| Micronodules | 6 | 3.0 | 9.3 | 0.003 | Micronodules | 5.3 | 0.000 | 46 |
| Chloride cells | 8 | −3.6 | 12.8 | 0.000 | Neoparamoeba sp. | 2.4 | 0.020 | |
| Ich-flagellates | 33 | 8.3 | 68.0 | 0.000 | Ich-flagellates | 9.1 | 0.006 | |
| Neoparamoeba sp. | 7 | 3.3 | 10.8 | 0.001 | ||||
| Scuticociliates | ||||||||
| Micronodules | 3 | −2.1 | 4.5 | 0.036 | Micronodules | −2.1 | 0.036 | 3 |
| Ich-flagellates | ||||||||
| Phase | 3 | 2.1 | 4.3 | 0.040 | Amoebae | 8.3 | 0.000 | 33 |
| Chloride cells | 4 | −2.5 | 6.4 | 0.013 | ||||
| Neoparamoeba sp. | 5 | 2.6 | 6.7 | 0.011 | ||||
| Amoebae | 33 | 8.3 | 68.0 | 0.000 | ||||
| Trichodinid ciliates | ||||||||
| Mucous cells | 5 | 2.8 | 7.6 | 0.006 | Mucous cells | 3.0 | 0.003 | 13 |
| Chloride cells | 4 | −2.3 | 5.2 | 0.006 | Chloride cells | −2.0 | 0.052 | |
| Prostomateans | 3 | 2.0 | 4.2 | 0.043 | Prostomateans | 2.8 | 0.006 | |
| Prostomateans | ||||||||
| Phase | 4 | −2.2 | 5.0 | 0.026 | Mucous cells | −3.2 | 0.006 | 10 |
| Mucous cells | 5 | −2.6 | 6.6 | 0.001 | Trichodinid ciliates | 2.8 | 0.002 | |
| Trichodinid ciliates | 3 | 2.0 | 4.2 | 0.043 | ||||
- Numerator d.f. = 1–4, denominator d.f. = 141–138, F ≥ 3.92; T ≥ ±1.98; P ≤ 0.05 test accepted.
- Ich-flagellates, Ichthyobodo-like flagellates.
Micronodules
Micronodules (Fig. 2c), consisting of two or more fused lamellae, were observed throughout the study period. The highest micronodule densities were observed in autumn and the lowest in spring (Table 2). In addition, higher micronodule densities were observed in 2002 than 2001 (Table 3). Amoeba densities increased with increasing micronodule densities in the gill but scuticociliates correlated negatively with the number of micronodules (Table 4). Numbers of micronodules in the gill associated with the phase of the disease, mucous cells, chloride cells, amoebae, scuticociliates and Ichthyobodo-like flagellate numbers (Table 4).
Mucous cells
Mucous cells were either absent, or observed in low numbers, along unaffected gill filaments. However, high densities were present along hyperplastic gill epithelium and on lamellae adjacent to the hyperplastic regions (Fig. 2a–d). The highest mucous cell densities were observed in summer–autumn and the lowest in spring (Table 2). Mucous cell densities did not vary between the two study years. Trichodinid ciliate densities increased and prostomatean densities decreased with increasing mucous cell numbers (Table 4). Mucous cell densities correlated with the phase of the disease, micronodules, chloride cells, trichodinid and prostomatean densities (Table 4).
Chloride cells
Chloride cells were abundant along healthy gill lamellae, but were rare along hyperplastic epithelium. The highest chloride cell densities were observed in spring and the lowest in summer–autumn (Table 2). Furthermore, higher chloride cell densities were observed in 2001 (Table 3). Amoeba, Ichthyobodo-like flagellate and trichodinid abundance increased with decreasing chloride cell densities (Table 4). Chloride cell densities associated with the phase of the disease, micronodule, mucous cell, amoeba, Ichthyobodo-like flagellate, trichodinid ciliate and prostomatean densities (Table 4).
Gill amoebae
Neoparamoeba sp. (Fig. 3a–d) were observed in all phases of the disease, however they were not generally associated with lesions. Neoparamoeba sp. densities did not vary over time or between sample years. Neoparamoeba sp. densities correlated positively with amoeba and Ichthyobodo-like flagellate densities (Table 4).
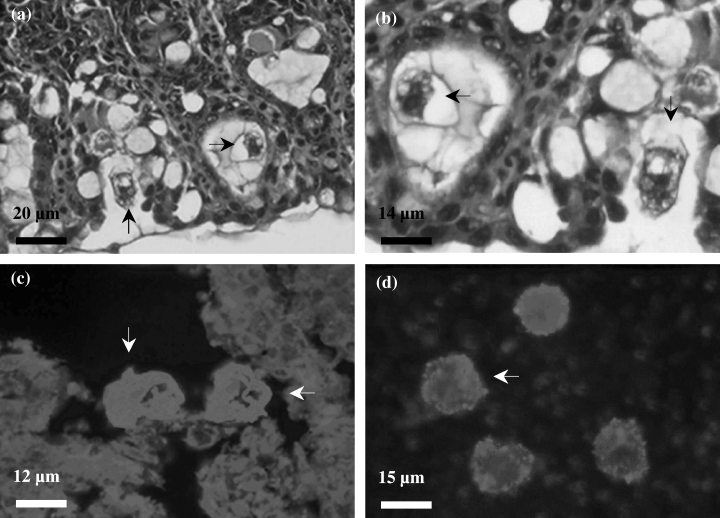
(a–d) Neoparamoeba sp. (arrow) visualised by histology (H&E stained, a–b), by the immunofluorescent staining technique (c), and by the immunofluorescent antibody technique (IFAT, d) (arrow indicates fluorescing Neoparamoeba sp.)
Amoebae other than Neoparamoeba sp. (Fig. 4a–c) were observed in all phases of the disease. Amoeba densities did not vary over the seasons. However, higher amoeba densities were observed in 2001 (Table 3). Amoebae were rare in healthy gills and densities increased with augmenting gill lesions (Table 4). Amoeba densities correlated positively with the progression of gill tissue changes, Neoparamoeba sp. and Ichthyobodo-like flagellate densities (Table 4).
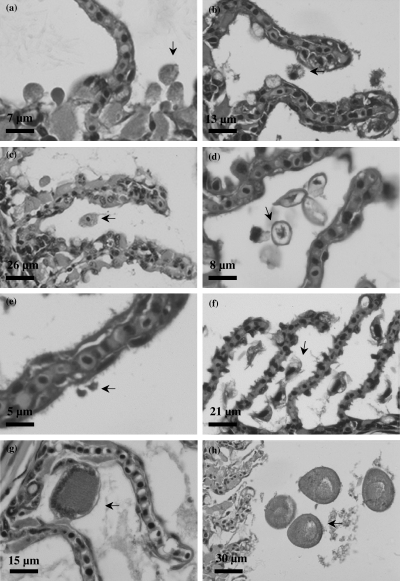
(a–h) Microfauna of the gills. a–c. Amoebae (arrow) colonising the gills. (d) Scuticociliates (arrow) in the gills. (e) Ichthyobodo-like flagellates (arrow) attached to the lamellar epithelium. (f) Trichodinid ciliate (arrow) colonising the gill. (g) Prostomatean trophont (arrow) attached to primary gill lamellae. (h) Prostomatean theront (arrow) in interlamellar space.
Scuticociliates
High densities of scuticociliates (Fig. 4d) were observed colonizing the gill. The highest scuticociliate densities occurred in spring and the lowest in autumn (Table 2). Scuticociliate densities did not vary between years. Scuticociliate densities decreased with advancing gill lesions (Table 4).
Ichthyobodo-like flagellates
Ichthyobodo-like flagellate (Fig. 4e) densities increased with augmenting gill tissue changes (Table 4). Ichthyobodo-like flagellate densities did not vary over time but higher numbers were observed in 2001 (Table 3). Ichthyobodo-like flagellate densities correlated positively with the progression of gill lesions, Neoparamoeba sp. and amoeba densities (Table 4).
Trichodinid ciliates
High densities of trichodinid ciliates (Fig. 4f) were observed in the gills with advanced gill lesions. Trichodinid ciliate densities did not vary significantly over time or between years. Trichodinid ciliates increased with advancing gill tissue changes (Table 4). They also correlated positively with the progression of gill lesions and prostomatean densities (Table 4).
Prostomatean ciliates
Prostomateans (Fig. 4g–h) were observed in the gills throughout the study period. Their densities did not vary significantly over time but greater prostomatean densities were observed in 2001 (Table 3). Prostomatean densities decreased with advancing gill lesions but correlated negatively with the phase of the disease, mucous cell numbers and positively with trichodinid numbers (Table 4).
Discussion
The progression of gill tissue changes described in this study was similar to that reported previously, where AGD was characterized by prominent but variable gill epithelial hyperplasia, with amoebae adhering to, or in close proximity to hyperplastic regions (Munday, Zilberg & Findlay 2001). However, the lesions observed in this study were not as extreme as in AGD cases reported in the literature (Kent, Sawyer & Hedrick 1988; Munday et al. 1990) or personally observed in Tasmania. Gill tissue changes progressed from healthy gill (phase 1) with an increase in mucous cell numbers, coinciding with a reduction in chloride cell densities (phase 2). This was followed by thickening of the individual lamellae (phase 3), by their fusion (phase 4–6), culminating in structural reorganization of gill tissue (phase 7). Neoparamoeba sp., other amoebae or other gill parasites were not always present when gill lesions were observed.
In this study, in accordance with earlier reports, micronodules were observed in the gills throughout the year, however, they progressively increased in abundance over the summer months. Clark & Nowak (1999) also found the greatest tissue changes were observed in summer and autumn, and the least in spring. The presence of micronodules in the gills of Atlantic salmon has been described previously (Clark & Nowak 1999; Bermingham & Mulcahy 2004), and associated with early AGD lesions (Nowak & Munday 1994). Micronodules and plaques (clubbing and fusion of many secondary lamellae, Nowak & Munday 1994), in the absence of amoebic colonization, have been reported in the gills of Atlantic salmon smolts post-transfer to sea water (Nowak & Munday 1994; Clark & Nowak 1999).
Mucous cell densities increased with advancing gill lesions. The presence of excess mucus on the fish gills and an increase in the number of mucous cells have been associated with AGD (Roberts & Powell 2003; Bermingham & Mulcahy 2004). An increase in the abundance of mucous cells is also a common finding in other gill diseases (Ferguson, Morrison, Ostland, Lumsden & Byrne 1992; Tumbol, Powell & Nowak 2001).
Chloride cell densities decreased with augmenting gill tissue changes. A reduction in chloride cell densities along the surface of hyperplastic epithelium has been previously reported in AGD-affected gills (Zilberg & Munday 2000; Bermingham & Mulcahy 2004). Apoptosis and necrosis of chloride cells was reported in post-smolt Atlantic salmon experimentally infected with the sea louse Lepeophtheirus salmonis (Krøyer) (Nolan, Reilly & Wendelaar Bonga 1999).
A variety of organisms was observed colonizing the gills including Neoparamoeba sp., other amoebae, scuticociliates, Ichthyobodo-like flagellates, trichodinids and prostomateans. Certain protozoans have been reported to cause serious gill disease in fish, for example, Neoparamoeba sp., Thecamoeba sp., Amyloodinium sp., Cryptobia sp., Ichthyobodo (formerly Costia) necator, Cryptocaryon irritans, Trichodina sp. and Myxobolus exiguus (Ferguson 1989).
Neoparamoeba sp. were observed in the gills throughout the year, however, their presence and abundance failed to correlate with gill lesions. This supports the findings of an earlier study in Ireland, where Neoparamoeba sp. abundance actually correlated negatively with gill tissue changes (Bermingham & Mulcahy 2004). In the present study Neoparamoeba sp. densities correlated positively with the other amoebae, however, amoebic colonization of the gill preceded that of Neoparamoeba sp. Competitive interspecific interactions were also noted in the earlier study, though Neoparamoeba sp. appeared to be out-competed by the other amoebae colonizing the gills during the warmer months of mid-summer to early autumn (Bermingham & Mulcahy 2004).
Amoebae other than Neoparamoeba sp. were rare in healthy gills, but their densities increased with augmenting gill lesions. A significant positive association was also observed between amoebae and Ichthyobodo-like flagellate densities, however, amoebae were the earlier colonizers of the gill. The question of the involvement of amoebae other than Neoparamoeba sp. in gill disease been raised elsewhere, with amoeba genera other than Neoparamoeba isolated from gills of Atlantic salmon in Tasmania and turbot in Spain, the most common being species belonging to the genera Platyamoeba and Vannella (Dykováet al. 2000; Howard 2001). In accord with earlier findings, this study suggests that amoebae other than Neoparamoeba sp. are involved in the disease in Ireland, though earlier work carried out elsewhere identified Neoparamoeba as the agent associated with the pathological lesions observed in AGD (Zilberg, Nowak, Carson & Wagner 1999).
Scuticociliates were observed in the gills and their densities decreased with augmenting gill lesions. Scuticociliates have been isolated along with Neoparamoeba sp. from the gills of clinically diseased Atlantic salmon, turbot, sea bream and sea bass (Dyková & Novoa 2001; Bermingham & Mulcahy 2004). However, it may be that the observed scuticociliates in this study were merely commensals, feeding on the microbial flora of the gills, as marine scuticociliates are known to occur abundantly in coastal areas, particularly in eutrophic mariculture waters (Song 2000).
Trichodinid ciliate densities increased with augmenting gill tissue changes. Trichodina sp. is the most frequently encountered marine ectoparasitic protozoan (Lom & Dyková 1992). During the 1995 AGD outbreak in Ireland Trichodina sp. was recovered with Neoparamoeba sp. in the gills of cultured Atlantic salmon (Rodger & McArdle 1996).
Prostomatean densities peaked in spring and autumn, but decreased as augmenting gill lesions increased. The prostomatean, C. irritans has been reported to cause white punctate lesions in the integument and gills of marine fish (Lom 1970). The negative association between prostomateans and gill lesions may be indicative of species succession in the gill, as prostomateans appeared as the primary colonizers, but over time were replaced by amoebae and trichodinid ciliates.
Ichthyobodo-like flagellate densities increased with progressive gill lesions. A number of Ichthyobodo-like flagellates have been associated with gill damage including Ichthyobodo necator, Cryptobia branchialis (Chen) and Piscinoodinium sp. These mastigophoran parasites are known to effect fatal epizootics, resulting from gill obstruction and mucus hypersecretion (Lom & Lawler 1973).
The observations of the progression of gill tissue changes in this study have, as in earlier findings, indicated that colonization of the gills occurs following micronodule formation (Bermingham & Mulcahy 2004). Kent et al. (1988) also suggested that amoebic infection results only in gills with pre-existing lesions; and it was observed that gill lesions resulting from jellyfish contact and ‘clubbing and necrosis syndrome’ were rapidly colonized by Neoparamoeba pemaquidensis (Handlinger 1991). Nevertheless, Zilberg et al. (1999) did observe the attachment of N. pemaquidensis to healthy gill epithelium. The amoebae in that situation may have been extremely virulent, as infection was established by cohabitation with AGD infected fish, and there is some evidence that Neoparamoeba sp. becomes more virulent with sequential passage through naïve hosts (Munday et al. 2001).
A sequence of colonization within the gill parasite community as described in this study was also evident in an earlier field study, where Neoparamoeba sp. was out-competed by accompanying amoebae in gills of Atlantic salmon at certain times of the year (Bermingham & Mulcahy 2004). Natural parasite communities are non-saturated with species, the different parasite species rarely co-occur at high densities, and interspecific competition does not play a major structuring role. However, when the same community is subjected to environmental perturbation, strong interaction occurs between the species, leading to a reduction in species richness while favouring increased virulence, culminating in disease/host death (Leibold, Holyoak, Mouquet, Amarasekare, Chase, Hoopes, Holt, Shurin, Law, Tilman, Loreau & Gonzalez 2004).
In conclusion, the microfaunal association with gill lesions in this study was an opportunistic one, involving pathogenic colonization of pre-existing micronodules by a diverse polyphyletic protozoan array including Ichthyobodo-like flagellates, trichodinid ciliates, and amoebae other than Neoparamoeba sp. The sequence of colonial succession observed within the parasite community is indicative of a disruption in the natural gill infracommunity equilibrium. The absence of association between Neoparamoeba sp. and gill lesions has suggested that the use of this genus as a diagnostic marker in the Irish situation may not be justified, indicating that diagnosis of AGD should now be based on gill lesions in association with amoebae, but not necessarily Neoparamoeba sp.
Acknowledgements
The authors are grateful for study funding from Marine Harvest Ireland, Fanad, Co. Donegal; help and advice from Jan Feenstra, Catherine McManus, Nigel Teape, Noreen McConigley and the other staff of Marine Harvest; and for an invitation to their laboratory and for the supply of anti-Neoparamoeba sp. antibodies from the late Dr Barry Munday, Dr Mark Powell, Dr Dina Zilberg, and Dr Marianne Douglas-Helders of the University of Tasmania.




