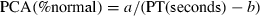Warfarin and miconazole oral gel interactions: analysis and therapy recommendations based on clinical data and a pharmacokinetic model
Summary
What is known and Objective: Miconazole is a strong inhibitor of CYP2C9, one of the main enzymes involved in the metabolism of warfarin. Concurrent use of the two drugs leads to potentially serious adverse effects. Although it is often assumed that use of the oral miconazole gel is acceptable with concomitant warfarin, because of the low bioavailability following buccal administration, drug–drug interactions have been reported following such use. We aimed to investigate case reports of such interactions and develop a pharmacokinetic model to model such interactions.
Methods: The Medline database from 1966 to October 2010 was used for literature search. Case reports of the potentiation of the anticoagulant effects of warfarin, such as the elevation of prothrombin time (INR), by concomitant administration of warfarin and miconazole oral gel were collected. We quantitatively estimated the extent of inhibition of warfarin metabolism by orally administered miconazole gel and compared our findings with case reports.
Results and Discussion: Metabolism of (S)-warfarin is inhibited potently following administration of a standard dose (200–400 mg/day in Japan) of miconazole gel. This may lead to in an increase in the blood concentration of warfarin and lead to serious adverse effects. The literature reports of clinical interactions with concomitant use of those drugs show that other factors may amplify the effects of any increase in blood concentration.
What is new and Conclusion: We summarize all reported, clinically significant, cases of drug interaction between miconazole oral gel and warfarin. Pharmacokinetic modelling shows that concomitant administration of warfarin and miconazole oral gel can lead to substantial increase in warfarin concentration. However, our PK/PD model fails to capture the dramatic increases seen in INR values, and hence bleeding complications, reported in the literature. Taken together, the evidence suggests that concomitant use of miconazole gel and warfarin should be avoided. Even over-the-counter products containing miconazole should be used with caution by patients receiving warfarin.
Introduction
Miconazole is an imidazole antifungal with strong antibacterial activity against Candida albicans. Various formulations of miconazole, such as oral gel (conventional dose: 200–400 mg/day in Japan), are now available throughout the world. The oral gel is available as an over-the-counter drug and is used for oral candidiasis and oesophageal candidiasis (conventional dose: 248 mg/day in the USA and Canada). Because the bioavailability of oral miconazole gel is as low as 27%, little attention has been paid to its systemic action and drug interactions (1). However, some case reports describing drug interactions between miconazole gel and warfarin have attracted considerable clinical attention (2–13).
Warfarin is one of the most widely prescribed oral anticoagulants but it may cause haemorrhage and bleeding of the gingiva or oral mucosa, as well as haematuria, particularly with overdoses. Warfarin is clinically used as a racaemic mixture of (R)-and (S)-enantiomers. As the latter has 3- to 5-fold the anticoagulation potency of the former, most of the anticoagulant action of warfarin is attributable to (S)-warfarin. Warfarin is eliminated from the liver and cytochrome P450(CYP)2C9 is responsible for more than 80% of the metabolism of the active (S)-warfarin (14).
Miconazole is a strong inhibitor of CYP2C9, with the in vitro Ki value (inhibition constant for 7-hydroxylation of (S)-warfarin) of 0·5 μm. Therefore, the inhibition of CYP2C9 is expected to result in an increase in the blood level of warfarin. Indeed, there are many case reports of miconazole gel (248–500 mg/day) potentiating the effect of warfarin and leading to elevation of prothrombin time (INR), intracutaneous haemorrhage, ecchymosis and epistaxis (2–13). Similar adverse reactions or prolongation of INR has been reported with the concomitant use of miconazole intravaginal cream, suppository or capsu1e (15–17).
Therefore, we aimed to critically appraise those case reports so as to allow a quantitative estimation of the extent of inhibition of warfarin metabolism following administration of oral miconazole gel. We develop a pharmacokinetic model, which we hope explains the case reports of interaction between the two drugs, following oral miconazole gel administration.
Methods
Case reports of interaction between miconazole and warfarin were searched in the Medline database (1966–October 2010) with the key words ‘miconazole gel’ and ‘warfarin’. The reports written in English or Japanese were chosen.
As most clinically significant drug–drug interactions are caused by the inhibition of metabolic enzymes, it is important, if possible, to quantitatively predict such interactions. Ito et al. (18) predicted the extent of increase in the blood level of tolbutamide in humans associated with the use of sulphaphenazole, a CYP2C9 inhibitor, from in vitro data by means of a model taking into consideration the concentration of metabolic inhibitor in the hepatic venous blood (18). Therefore, we aimed to predict quantitatively the extent of interaction with miconazole gel and warfarin by using the same model with minor modifications.
 (1)
(1)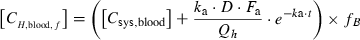 (2)
(2) (3)
(3)where τ represents the dosing interval.
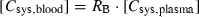 (4)
(4)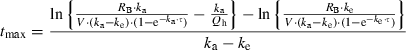 (5)
(5)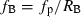 (6)
(6)Finally, the maximum unbound concentration of miconazole in the hepatic venous blood [CmaxH,blood,f] can be calculated by applying Eqs (3) and (6) to Eq. (2), and incorporating the pharmacokinetic parameters. Because warfarin is a racemic mixture, the extent of increase in AUC (ρ) was separately estimated for (R)- and (S)-warfarin. (S)-Warfarin is mainly hydroxylated to 7-hydroxywarfarin, whereas (R)-warfarin is mainly hydroxylated at the 6-position.
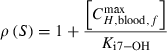 (7)
(7) (8)
(8)If warfarin is actively transported into the liver, then the assumption that [Cf] is equal to [CmaxH,blood,f] results in the underestimation of [Cf]. Unfortunately, it remains uncertain whether warfarin is actively transported into the liver or not. Therefore, as in a previous report, we assumed that [Cf] may range from [CmaxH,blood,f] to 10 × [CmaxH,blood,f] for calculation (18). Table 1 presents the parameters used for calculation.
| k a | = 0·829 (h-1)a |
| k e | = 0·821 (h−1)a |
| t lag | = 0·875 (h)a |
| F a | = 0·27 (1) |
| Dose | = 50, 100 mg (four times a day) |
| MW | = 416·13 |
| t max | = 1·17 (h)a |
| R B (CB/Cp) | = 0·63b |
| f p | = 0·093 (ultrafiltration) (1) |
| f B | = 0·148 (fp/RB) |
| τ | = 6 (h) |
| Q h | = 1460 (mL/min) (19) |
| K i7-OH | = 0·50 (μmol/L) (14) |
| K i6-OH | = 4·0 (μmol/L) (14) |
- aPharmacokinetic parameters (ka, ke, tlag) were calculated by applying to the one compartment open model. See text for detail.
- b R B was calculated based on the blood-to-plasma ratio of radioactivity after single oral administration of 3H-labelled miconazole in healthy subjects (20).
- k a, first-order absorption rate constant; ke, elimination rate constant; tlag, lag time; fa, fraction absorbed from the gastrointestinal tract into the portal vein; MW, molecular weight; tmax, time to reach the maximum concentration; RB, the blood-to-plasma concentration ratio; Qh, hepatic blood flow rate; fp, unbound fraction in the plasma; fB, unbound fraction in the blood, τ, dosing interval; Ki7-OH, inhibitory constant of miconazole for (S)-warfarin metabolism at the 7-position; Ki6-OH, inhibitory constant of miconazole for (R)-warfarin metabolism at the 6-position.
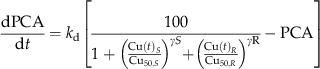 (9)
(9)Results
Table 2 summarizes the case reports of increase in the prothrombin time (INR) after concomitant use of warfarin and miconazole gel, vaginal tablet or vaginal cream. In most cases, the prothrombin time (INR) rose markedly within one or two weeks, accompanied with adverse events such as subcutaneous bleeding, ecchymosis or haematuria. In many cases, recovery of the prothrombin time (INR) took about a week after the cessation of coadministration. Then, we evaluated quantitatively the risk of interaction between miconazole gel and warfarin. [Cmaxsys,blood] was estimated to be 0·10 μm after the administration of miconazole gel at a dose of 200 mg/day and 0·20 μm after 400 mg/day. Furthermore, the maximum blood concentration of miconazole extracted from the gastrointestinal tract into the liver was estimated to be 0·12 μm after the administration of miconazole gel at a dose of 200 mg/day administration and 0·23 μm for 400 mg/day. The sum of the above concentrations gives the maximum unbound concentration in the hepatic venous blood [CmaxH,blood,f] as follows: [CmaxH,blood,f] = (0·10 + 0·12) × 0·148 = 0·033 μm for 200 mg/day of miconazole gel and [CmaxH,blood,f] = (0·20 + 0·23) × 0·148 = 0·064 μm for 400 mg/day of miconazole gel.
| Patient Age/sex | Miconazol daily dose (mg/day) | Warfarin daily dose (mg/day) | Duration of concomitance (day) | Baseline INR | Peak INR (complication) | Concomitant medicines | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 1 | 71/M | 500 | 1 | 10 | 2·5 | 17·9 | Furosemide, Amiloride | (2) |
| 2 | 43/F | 15 g | 7–8 | 11 | 2·1 | 13·1 (haematuria) | Alprenolol, Hydralazine, Bendrofluazide, Metformin, Glipizide | (3) |
| 3 | 57/M | 100 | 10 | 7 | 2·0–3·0 | 10 (retroperitoneal heamorrhage) | Not stated | (4) |
| 4 | 82/F | Not stateda (D) | 5 | 15 | 1·7 | 9·7 (bruise thigh) | Thyroxine, Digoxin, Calcium carbonate | (4) |
| 5 | 69/F | Not stateda(D) | 3·5 | 13 | 2·2 | 18·0 (elbow heamarthrosis) | Nuelin SR, Prednisone, Nifedipine, Calcium carbonate | (4) |
| 6 | 47/F | Not stateda(D) | 4 | 7 | Stable | 12 (epistaxis) | Furosemide, Spironolactone, Ranitidine | (4) |
| 7 | 70/F | Not stateda(D) | 1 | 14 | Stable | 7·5 | Medroxyprogesterone | (4) |
| 8 | 69/F | 2% gel 5 times/day a(D) | Not stated | 14 | Stable | 20 (bruises,intramuscular haematoma, blood-stained urine) | Not stated | (5) |
| 9 | 63/F | 30g a (D) | Not stated | 7 | Stable | 11·4 (haematuria,bruising) | Not stated | (5) |
| 10 | 66/F | Not stated 3-4 times/day | 2–4 | 7 | 2·9 | 15 | Azathioprine, Prednisolone, Betamethasone mouthwash | (6) |
| 11 | 73/M | 125 4 times /day | 1–3 | 14 | 1·5 | 10 | Metoprolol, Diltiazem, Aspirin isosorbide, Omeprazole | (7) |
| 12 | 73/F | Not stated | Not stated | 2 | Not stated | 24 (retroperitoneal haematoma, intramural haematoma in the small bowel.) | Not stated | (8) |
| 13 | 54/F | 500 | 6 | 10 | 3·4 | >17 (bruise, melaena) | Bumetanide, Ranitidine, Amiloride, dextromoramide, Amitriptyline, Lorazepam, Salbutamol, Beclomethasone dipropionate, Ipratropium bromide inhalers | (9) |
| 14 | 65/M | 500 | Not stated | 14 | Stable | Significantly elevated (petechial haemorrhage) | (9) | |
| 15 | 75/F | 500 | Not stated | 15 | 2·6 | 5·78 (petechiae, ecchymosis) | Furosemide, Digoxin | (10) |
| 16 | 65/M | 500 | Not stated | 10 | 2·48 | 10 (no evidence) | Transdermal nitrates, Ramipril, Digoxin, Furosemide | (10) |
| 17 | 57/F | 375 | Not stated | 7 | 2·19 | 8·69 (haematuria) | LH-RH agonists, megestrol | (10) |
| 18 | 52/M | 300–400 | 6 | 15 | 2 | >10 (bleeding from buccal) | Digoxin, Dipyridamole, Triamteren, Furosemide, Verapamil, Betamethasone | (11) |
| 19 | 69 | 200 | 3 | 9 | 2·5 | >9 | Not stated | (12) |
| 20 | 62/M | Not stated | (1) 5–5·5 reinstitute treatment by miconazole oral gel(2) 4·5 | 30 Not stated | 3·5–4·53·1 | 11·9 (haematuria)5.8 | Not stated | (13) |
- aA measuring spoon (5 mL) is provided with the gel. One spoonful contains approximately 124 mg of miconazole.
- Half a measuring spoon of gel four times daily. (D) represents coadministration with Daktarin.
- F, female; M, male; INR, international normalized ratio; Ref, reference.
Taking into consideration the hypothetical concentrative uptake of miconazole into the liver, [CmaxH,blood,f] is assumed to range from 0·03 to 0·33 μm and from 0·064 to 0·643 μm at doses of 200 and 400 mg/day, respectively. Therefore, the extents of increase in AUC (ρ) were estimated as follows
ρ (S) = 1·03–1·64, ρ (R) = 1·01–1·08 at a dose of 200 mg/day,
ρ (S) = 1·13–2·29, ρ (R) = 1·02–1·16 at a dose of 400 mg/day.
Figure 1 shows a simulation of the effect of miconazole (400 mg/day) on the unbound plasma concentration-time profile of (S)-warfarin based on the assumption of 10-fold concentrative uptake of miconazole into the liver.
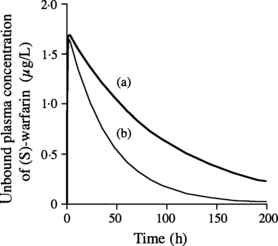
Estimated unbound plasma concentration-time profile of (S)-warfarin after oral administration of warfarin at a dose of 6 mg/day, (a) with miconazole gel at dose of 400 mg/day or (b) warfarin alone.
Moreover, based on the above estimation that the blood concentration of warfarin was increased 2·29-fold (S-enantiomer) and 1·16-fold (R-enantiomer) by concomitant use of miconazole gel, we calculated the time course of prothrombin time after oral administration of a clinical dose of warfarin (6 mg) with miconazole gel, using the previously reported indirect model (22, 23). The prothrombin time was predicted to increase slightly (Fig. 2), i.e. the maximum prothrombin time was predicted to be increased from 13·5 to 13·9 s by the concomitant administration of miconazole gel at a dose of 400 mg/day. Thus, the remarkable augmentation of prothrombin time described in several clinical cases was not predicted by our model.
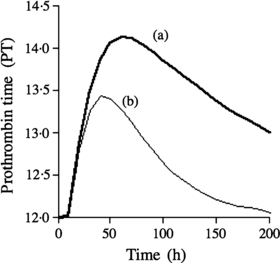
Estimated time course of prothrombin time of (S)-warfarin after oral administration of warfarin at a dose of 6 mg/day, (a) with miconazole gel at dose of 400 mg/day or (b) warfarin alone by using the pharmacodynamic model previously reported (22, 23).
Discussion
Several case reports describe the potentiation of the effects of warfarin, such as a remarkable increase in INR, subcutaneous bleeding, ecchymosis and nosebleed, with concomitant administration of warfarin and miconazole gel. These cases suggest a clinically significant drug-interaction. O’Reilly et al. (14) have shown in normal subjects that an oral miconazole tablet (125 mg/day) increased the elimination half life of (S)-warfarin from 35 to 135 h, decreased the oral clearance of (S)-warfarin by 80% and prolonged by almost 5-fold the AUC of prothrombin time at 24 h after the coadministration. Trough concentration of miconazole at 24 h after the administration was reported to be 3–26 ng/mL (7·21–62·48 nm) in the above study. This report implies that quite low concentrations of miconazole in the blood may lead to interaction with warfarin. The extent of increase in AUC (ρ) was estimated to be as follows: ρ(S) = 1·03–1·64, ρ (R) = 1·01–1·08 at a dose of 200 mg/day, ρ (S) = 1·13–2·29, ρ (R) = 1·02–1·16 at a dose of 400 mg/day.
Our present analysis also demonstrated that the metabolism of (S)-warfarin, which shows potent anticoagulant activity, can be potently inhibited by concomitant administration of miconazole gel at a therapeutic dosage. As the anticoagulant efficacy of (R)-warfarin is weaker than that of (S)-warfarin and the inhibition of metabolism by miconazole gel is less potent, (R)-warfarin may not significantly contribute to the adverse reactions to warfarin upon concomitant administration of miconazole gel.
One case report in Table 2 describes an increase in the blood concentration of warfarin from 1·3 to 2·6 mg/mL following the coadministration of 15 g/day of Daktarin™ (300 mg/day of miconazole) (2). This extent of increase is in accordance with our prediction. Our prediction also explains why the metabolism of warfarin is strongly impaired in vivo via inhibition of the 7-hydroxylation of (S)-warfarin by miconazole (Ki value 0·5 μm), although the calculated concentration of miconazole in the hepatic venous blood, as well as that in the systemic blood, is rather low.
Next, we considered the prolongation of the prothrombin time associated with the increase in the blood level of warfarin by applying the pharmacodynamic indirect model reported previously by Chan et al. (22). However, this model failed to predict the remarkable increase in the prothrombin time which has been reported in several clinical cases. A possible explanation for this failure is that there might be a large degree of interpatient variability in the pharmacokinetics, pharmacodynamics and/or extent of drug interaction, while we used the mean parameters obtained from independent experiments instead of individual cases. The pharmacokinetic parameters of warfarin have been reported to be affected by several factors, including age, and genetic polymorphism in CYP2C9 (24). Indeed, patients carrying either CYP2C9*2 or CYP2C9*3 allele have an increased risk of adverse effects of warfarin (25).
With respect to the pharmacodynamics, there might be also interpatient variation.
For example, the risk of major bleeding has been reported to be increased in elderly patients. Although this may be partly explained by impaired elimination of warfarin, which would lead to the elevation of blood warfarin level, pharmacodynamic response to warfarin may also be increased in the elderly population. It is possible that the patients in the case reports exhibited extreme anticoagulant reactions that cannot be described by our pharmacodynamic model using mean parameter values.
The clinical cases analysed in our study may be outlying cases in relation to PK/PD properties and therefore were not adequately modelled by population mean PK/PD parameters. With more precise data on interindividual variations in PK/PD parameters for warfarin, it may become feasible to further evaluate the appropriateness of the present model for predictive purposes. Another possibility is that, because warfarin does not inhibit the coagulation factor in the blood, but inhibits the protein synthesis of vitamin K-dependent clotting factors in the liver to exhibit its anticoagulant effect, the blood warfarin level may not be appropriate variable for estimating individual response to warfarin. Moreover, the extent of interaction is likely to be affected by several factors. It is quite feasible that interpatient variation in the pharmacokinetics of the metabolic inhibitor, miconazole, may strongly influence the extent of drug interaction.
To assess the influence of more extreme interindividual variation in warfarin pharmacokinetics on the predictions, we used a greater increase in the AUC of (S)-warfarin than the 8·2-fold increase predicted. However, event then, the prothrombin time was estimated to be prolonged from 13·5 to only 14·5, suggesting that other factors must have contributed to the remarkable increase in prothrombin time noted in some case reports. It is also possible that the indirect model employed in this study may not be applicable to clinical cases of adverse reactions, because the model was based on data from healthy volunteers, given a quite high dose of warfarin (1·5 mg/kg). In this study, we made various assumptions (e.g. bioavailability can be used in place of Fa, the entire miconazole dose was swallowed, etc.). These assumptions would affect the predicted increase in the blood level of warfarin. However, their impacts are likely to be smaller than the aforementioned interpatient variations. A significant increase in the blood concentration of warfarin associated with the use of miconazole gel was clearly predicted by the pharmacokinetic model, although the present analysis failed to quantitatively estimate the prolongation of prothrombin time described in the case reports. The bioavailability of miconazole oral gel is so low that the risk of drug interaction with other medications is often judged to be small.
What is New and Conclusion
We summarize all reported, clinically significant, cases of drug interaction between miconazole oral gel and warfarin. Pharmacokinetic modelling shows that concomitant administration of warfarin and miconazole oral gel can lead to substantial increase in warfarin concentration. The literature reports of clinical interactions with concomitant use of those drugs show that other factors may amplify the effects of any increase in blood concentration. However, our PK/PD model fails to capture the dramatic increases seen in INR values, and hence bleeding complications, reported in the literature. Taken together, the evidence suggests that concomitant use of miconazole gel and warfarin should be avoided. Even over-the-counter products containing miconazole should be used with caution by patients receiving warfarin, and such use should be carefully monitored by both physicians or pharmacists.