Human immunodeficiency virus (HIV) infection during pregnancy induces CD4 T-cell differentiation and modulates responses to Bacille Calmette-Guérin (BCG) vaccine in HIV-uninfected infants
Re-use of this article is permitted in accordance with the terms and conditions set out at http://www3.interscience.wiley.com/authorresoures/onlineopen.html
Summary
Human immunodeficiency virus (HIV)-negative infants born to HIV-positive mothers frequently exhibit a range of immunological abnormalities. We tested the hypothesis that HIV during pregnancy affects the ability of CD4 T cells of HIV-negative infants to respond to vaccine challenge by recruiting HIV-negative infants born to HIV-negative and HIV-positive mothers and measuring their responses to Bacille Calmette-Guérin (BCG) vaccine given at birth. At 2 weeks, maternal HIV status did not influence CD4 T-cell counts or differentiation, but by 10 weeks CD4 counts of infants born to HIV-positive mothers fell to a level characteristic of HIV-positive infants. Among the CD4 T-cell populations, markers of differentiation (CCR7− CD45RA− CD27−) and senescence (CD57, PD-1) were more common among infants born to HIV-positive mothers than among infants born to HIV-negative mothers. At 2 weeks of age, we assessed the effector response to heat-killed BCG and tuberculin purified protein derivative (PPD) by overnight interferon (IFN)-γ enzyme-linked immunosorbent spot-forming cell assay (ELISpot), but found no measurable effect of maternal HIV status. At 10 weeks, we assessed CD4 T-cell memory by measuring proliferation in response to the same antigens. We observed a bimodal response that allowed infants to be classified as high or low responders and found that fewer infants born to HIV-positive mothers were able to mount a robust proliferative response, suggesting that their reduced CD4 counts and increased differentiation indicated a deficiency in their ability to develop immunological memory.
Introduction
Over 3·5 million women of childbearing age are infected annually with human immunodeficiency virus (HIV) in Sub-Saharan Africa,1 and large numbers of children are born to HIV-positive women. Infants infected in utero or perinatally have a poor prognosis, with as many as 25% developing acquired immune deficiency syndrome (AIDS) within the first year.2 However, even in the absence of interventions such as antiretroviral therapy (ART) or caesarean section, most infants born to HIV-positive women are not infected. The rapidly expanding availability of ART to prevent perinatal transmission throughout Africa makes it likely that the overwhelming majority of infants born to HIV-positive mothers will remain HIV-negative. These ‘seroreverters’3 are born with maternally derived antibodies to HIV that they later lose. They frequently have lower birth weights than infants born to HIV-negative mothers,4 and a low maternal CD4 count was found to be a strong risk factor for infant mortality and hospital admission in this infant population.5
The implication that seroreverters are physiologically and immunologically disadvantaged is supported by their lower CD4 counts and higher proportions of differentiated T cells3,6,7 than infants born to HIV-negative mothers. As low CD4 counts and large differentiated T-cell subpopulations are associated with reduced immune responses in the context of HIV infection8–10 and aging,11,12 it is consistent that seroreverters also show relatively poor interleukin (IL)-26 and IL-1213 production in response to polyclonal stimuli.
Impairment of seroreverters’ immune systems is of particular concern in Sub-Saharan Africa, which has both a high HIV prevalence and a high burden of infectious disease.14 Immune impairment suggests a mechanism for the frequent poor health of seroreverters5,15 but no conclusion can be drawn without establishing whether the impairment affects antigen-specific responses and the development of immune memory to natural infectious challenge or vaccination.
One of the most widely used vaccines world-wide is the Bacille Calmette-Guérin (BCG) strain of Mycobacterium bovis, which protects against miliary tuberculosis and tuberculosis meningitis in childhood.16 BCG is given at birth in most African countries and induces a strong cellular response, especially in CD4 T cells.17,18 Thus BCG represents a robust model for assessing antigen-specific CD4 T-cell responses in infants.
We therefore tested the hypothesis that HIV during pregnancy reduces CD4 T-cell responses in healthy infants by comparing the development of immunity to BCG between seroreverters and a control group born to HIV-negative mothers. We administered the BCG vaccine at birth and assessed the T-cell effector response at 2 weeks and the T-cell memory response at 10 weeks, while quantifying CD4 T-cell subpopulations at both time-points. At 2 weeks of age, we found no discernable differences in CD4 T-cell subpopulations or the effector response to BCG. However, by 10 weeks, seroreverters had lower CD4 counts, higher levels of CD4 T-cell differentiation and reduced proliferative capacity in response to mycobacterial antigens. Together these data indicate an impaired ability to both induce CD4 T-cell differentiation and stimulate a pool of memory T cells through vaccination.
Methods
Recruitment and immunization
Women with known HIV status were asked to provide informed consent and were recruited when they gave birth at Queen Elizabeth Central Hospital (QECH), Blantyre. The exclusion criteria were any serious complication during pregnancy or delivery, and infants that had birth weights of below 2 kg or required emergency postnatal care. All HIV-positive mothers were given 200 mg nevirapine during labour and their infants were given 2 mg/kg nevirapine within 72 hr post-partum according to national guidelines.19
Neonates were weighed and measured at birth and APGAR scores (a standard measure of neonatal health with a range from one to ten, was recorded at 1 min and 5 min after delivery) were recorded. Basic socioeconomic information was collected from their mothers.
The protocol was approved by the University of Malawi’s College of Medicine Research and Ethics Committee and the Liverpool School of Tropical Medicine’s Research Ethics Committee.
Vaccine preparations
Infants were vaccinated at birth with oral trivalent poliomyelitis vaccine (OPV) (Sanofi-Pasteur) and BCG. The BCG was Danish strain 1331 at 1–4 × 105 colony-forming units (cfu) per dose (Statens Serum Institute, Copenhagen, Denmark).
For the assays, heat-killed BCG was prepared by heat-inactivating the contents of one vial (2–8 × 106 cfu) at 80° for 20 min20 and confirming inactivation by establishing no growth after 10 weeks on Löwenstein–Jensen medium (E&O Laboratories, Bonnybridge, UK). As each vial also contained 3·75 mg of sodium glutamate, a negative control solution containing an equivalent concentration was also prepared.
Sampling schedule and HIV diagnosis
At birth, a sample of umbilical cord blood serum was collected and stored. When the infant reached 2 weeks of age, a venous blood sample was collected into heparin. For the enzyme-linked immunosorbent spot-forming cell assay (ELISpot), peripheral blood mononuclear cells (PBMCs) were isolated using lymphoprep (Axis-Shield, Dundee, UK). At 10 weeks, a second sample was taken and PBMCs were collected for the proliferation assay if sufficient numbers were isolated. If the mother was HIV-positive, 0·5 ml of the sample was collected into ethylenediaminetetraacetic acid (EDTA) and used to diagnose the infant’s HIV status using the Amplicor PCR kit (Roche, Basel, Switzerland). Infected infants were referred for further clinical management at QECH.
CD4 T-cell subpopulations
Lymphocytes were enumerated with an HmX haematology analyser (Beckman-Coulter, Fullerton, CA). The CD4 T cells and their subpopulations were identified by flow cytometry, for which all reagents and equipment were from Becton Dickinson (Franklin Lakes, NJ) unless otherwise specified. The CD4 T cells were identified by staining with PerCP-labelled CD4 and subpopulations were further characterized using a panel of fluorescein isothiocyanate (FITC)-labelled anti-PD-1 (eBioscience, San Diego, CA) and anti-CD57, phycoerythrin (PE)-labelled anti-CD28 and anti-CD45RA, allophycocyanin (APC)-labelled anti-CD27 and Alexa Fluor 647-labelled anti-CCR7 antibodies. Erythrocytes were lysed using FACSlyse.
The samples were acquired on a FACSCalibur using cellquest pro software (Becton Dickinson) and data were analysed with flowjo 7.2.2 for Windows (Treestar, Ashland, OR). Concentrations of subpopulations in peripheral blood were calculated by multiplying the percentage of lymphocytes within each subpopulation by the lymphocyte count.
ELISpot
The assay was carried out on 96-well plates with polyvinylidene difluoride (PVDF)-based wells (Millipore, Billerica, MA). The PBMCs were resuspended in R10F [90% v/v RPMI-1640 containing 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mm l-glutamine, and 10% v/v fetal bovine serum (FBS)] and 2 × 105 PBMCs were placed in each well of the plate. All wells were costimulated with antibodies to CD28 and CD49d (Becton Dickinson), and duplicate wells were stimulated with 10 μg/ml tuberculin purified protein derivative RT50 (PPD) (Statens Serum Institute), heat-killed BCG at a final dilution of 1 : 1500, negative control solution or 20 μg/ml phytohaemagglutinin (PHA).
After 18 hr at 37° in 5% CO2, interferon (IFN)-γ-producing cells were quantified using monoclonal antibodies (Mabtech, Nacka Strand, Sweden) and an alkaline phosphatase detection system (Bio-Rad, Hercules, CA). Spots were counted using an ELRO4 ELISpot reader with elispot reader version 4.0 software (AID, Straßberg, Germany). Results are expressed as specific spot-forming units (SFU), calculated by subtracting the mean negative control count from the count for each treatment and expressed per 106 cells.
Proliferation assay
Cells were labelled with 250 nm carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA) as previously described21 and resuspended at 2 × 106 PBMCs/ml in R10AB [90% v/v RPMI-1640 (Sigma, St Louis, MO) containing 100 U/ml penicillin (Sigma), 100 μg/ml streptomycin (Sigma) and 2 mm l-glutamine (Sigma), and 10% v/v human serum (Sigma)].
Cells were costimulated with antibodies to CD28 and CD49d (Becton Dickinson), and treated with either 25 μg/ml PPD, a 1 : 100 dilution of heat-inactivated BCG, negative control solution with a final concentration of 375 μg/ml sodium glutamate or 2 μg/ml PHA.
Cells were incubated for 8 days at 37° with a 5% CO2 atmosphere before being harvested and stained with PerCP-conjugated anti-CD3, PE-conjugated anti-CD8 and APC-conjugated anti-CD4 antibodies (Becton Dickinson) and analysed as before by flow cytometry.
Assessment of antibody responses
Antibody levels in umbilical cord blood serum and heparinized plasma collected at 10 weeks of age were assessed by enzyme-linked immunosorbent assays (ELISAs) detecting immunoglobulin G (IgG) to Mycobacterium tuberculosis (Demeditec, Kiel-Wellsee, Germany) and polio (IBL-Hamburg, Hamburg, Germany).
Statistical analysis
All statistical analyses were restricted to infants who remained HIV-negative throughout the course of the study. Subpopulation sizes and ELISpot responses were compared by Mann–Whitney U-test. Proliferation data were assessed by dividing subjects into those with high and low levels of division and comparing by Yates-corrected chi-squared test. Differences in growth were assessed by analysis of covariance (ancova) in which the equivalent parameters at birth were used as a covariate. Levels of anti-M. tuberculosis and anti-polio IgG at 10 weeks were assessed by ancova with the equivalent levels of IgG in umbilical cord blood as a covariate. Differences were considered significant when P < 0·05. The analysis was carried out using stata 8.0 (Statacorp, College Station, TX) and minitab 15 (Minitab Inc., Coventry, UK).
Results
Cohort characteristics
All pregnancies were full term and both pregnancies and deliveries were uncomplicated. The 16 HIV-positive and 21 HIV-negative mothers were similar in terms of the socioeconomic parameters recorded. Ages were similar [median 23 years (interquartile range (IQR) 20·0–26·0 years) for HIV-negative mothers versus 24 years (IQR 22·5–27·5 years) for HIV-positive mothers], as were lengths of time at school [median 9·0 years (IQR 7·0–10·0 years) for HIV-negative mothers versus 7·5 years (IQR 2·8–10·8 years) for HIV-positive mothers]. All 21 HIV-negative mothers who gave information were married, while 10 of 13 HIV-positive mothers (77%) were married.
Three infants born to HIV-positive mothers were diagnosed with HIV infection and the remainder tested negative and were classified as seroreverters. The few data from the three HIV-positive infants showed high variance and revealed no clear trends, so they were excluded from all further analyses (data not shown).
At birth, seroreverters tended towards smaller sizes and lower weights, although the difference was only statistically significant for mid-upper arm circumference (MUAC) (Table 1). While infants born to HIV-positive mothers tended to have slightly lower APGAR scores at 1 min, all but two infants had maximal APGAR scores of 10 by 5 min (Table 1). There were no differences in growth during the 10 weeks of the study based on changes in weight, length, head circumference or MUAC (data not shown).
| Characteristic | Infants born to HIV-negative mothers | Infants born to HIV-positive mothers | P | ||||
|---|---|---|---|---|---|---|---|
| Median | IQR | n | Median | IQR | n | ||
| Birth weight (kg) | 3·2 | 2·7–3·4 | 21 | 3·0 | 2·9–3·5 | 13 | NS |
| Head circumference at birth (cm) | 35 | 33·5–37 | 21 | 35 | 33–35 | 11 | NS |
| Length at birth (cm) | 47 | 45–51 | 21 | 46 | 45–49 | 11 | NS |
| MUAC (cm) | 12·0 | 11·0–13·8 | 20 | 10·0 | 10·0–10·0 | 11 | 0·002 |
| APGAR score at 1 min | 8 | 8–9 | 21 | 8 | 7–9 | 13 | NS |
| APGAR score at 5 min | 10 | 10–10 | 21 | 10 | 10–10 | 13 | NS |
| CD4 count at 2 weeks (%) | 46·2 | 42·5–55·7 | 16 | 44·9 | 38·0–48·6 | 12 | NS |
| CD4 count at 10 weeks (%) | 42·0 | 31·4–46·3 | 10 | 30·7 | 26·0–35·3 | 11 | 0·024 |
| CD4 count at 2 weeks (106 cells/ml) | 3·23 | 2·28–3·88 | 16 | 2·66 | 1·86–3·01 | 12 | NS |
| CD4 count at 10 weeks (106 cells/ml) | 2·61 | 2·32–3·04 | 10 | 1·98 | 1·73–2·75 | 11 | 0·041 |
- Comparisons were carried out by Mann–Whitney U-test.
- IQR , interquartile range; MUAC, mid-upper arm circumference; NS, not significant.
Infants born to HIV-positive mothers had reduced CD4 counts and more differentiated CD4 T cells by 10 weeks of age
At 2 weeks of age, maternal HIV status had no discernable effect on the infants’ CD4 counts, but by 10 weeks of age, the CD4 counts of seroreverters had dropped below those of infants born to HIV-negative mothers in terms of both concentration in peripheral blood (P = 0·041) and percentage of total lymphocytes (P = 0·024) (Table 1).
Based on CD45RA and CCR7 expression, CD4 T cells may be classified as naïve (CCR7+ CD45RA+), central memory (CCR7+ CD45RA−), effector or effector memory (CCR7− CD45RA−) and effector memory RA (CCR7− CD45RA+) cells.22–24 Maternal HIV status did not influence concentrations or relative proportions of the four subpopulations at 2 weeks of age. However, by 10 weeks, the concentrations of naïve and central memory cells in seroreverters had fallen substantially, indicating that the loss of CD4 T cells occurred mainly among the less differentiated subsets (Fig. 1a,b).

Maternal human immunodeficiency virus (HIV) infection increases CD4 T-cell differentiation in 10-week-old HIV-negative infants. (a) CD45RA and CCR7 expression in representative 10-week-old HIV-negative infants with HIV-positive and HIV-negative mothers, gated on CD4 T cells. (b) Concentrations of CD4 T-cell subpopulations defined by CD45RA and CCR7 in 2- and 10-week-old infants with HIV-positive and HIV-negative mothers. (c) CD27 and CD28 expression in representative 10-week-old HIV-negative infants with HIV-positive and HIV-negative mothers, gated on CD4 T cells. (d) Concentrations of CD4 T-cell subpopulations defined by CD27 and CD28 in 2- and 10-week-old infants with HIV-positive and HIV-negative mothers. On composite plots, grey bars indicate medians. All statistical analysis was performed using the Mann–Whitney U-test.
As CD4 T cells differentiate, they lose expression of CD27 and then CD28, allowing classification into early (CD27+ CD28+), intermediate (CD27− CD28+) or late (CD27− CD28−) differentiated subpopulations.12,25 At 2 weeks, seroreverters had slightly fewer early differentiated and more intermediate CD4 T cells than infants born to HIV-negative mothers. This difference increased by 10 weeks (Fig. 1c,d). Fully differentiated cells were infrequent at both time-points. The same trends were evident whether the analysis was based on the cell concentration in peripheral blood or proportions of the total CD4 T-cell population (data not shown).
Surface expression of CD5726 or PD-127,28 has been associated with replicative senescence in CD4 T cells, and by 10 weeks of age, expression of both was more frequent on the CD4 T cells of seroreverters (Fig. 2).
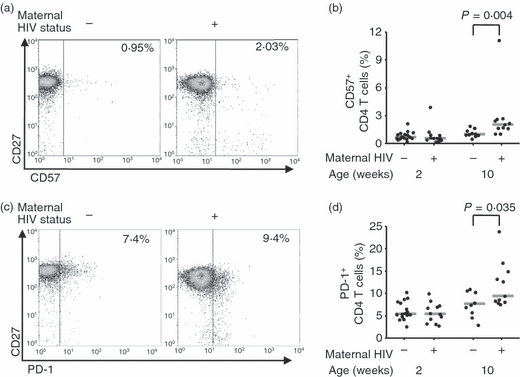
Maternal human immunodeficiency virus (HIV) infection leads to a higher proportion of CD4 T cells expressing markers of senescence in 10-week-old HIV-negative infants. (a) Representative CD57 and CD27 expression by CD4 T cells of 10-week-old HIV-negative infants with HIV-positive and HIV-negative mothers. Percentages of CD4 T cells expressing CD57 are given. (b) Percentage of CD4 T cells expressing CD57 at 2 and 10 weeks of age in HIV-negative infants born to HIV-negative and HIV-positive mothers. Bars indicate medians. (c) PD-1 and CD27 expression by the CD4 T-cells of HIV-negative infants with HIV-positive and HIV-negative mothers. Percentages of total CD4 T cells expressing PD-1 are given. (d) Percentage of CD4 T cells expressing PD-1 at 2 and 10 weeks of age in HIV-negative infants born to HIV-negative and HIV-positive mothers. Bars indicate medians. All statistical comparisons were by Mann–Whitney U-test.
Both markers tended to be more prevalent among differentiated cells of seroreverters. In particular, CD57 was considerably more prevalent among the effector memory (CCR7− CD45RA−) subpopulation (P = 0·017) of seroreverters than in that of infants with HIV-negative mothers, and PD-1 was more prevalent among the fully differentiated (CD27− CD28−) subpopulation (P = 0·026) of seroreverters (data not shown).
HIV infection during pregnancy did not influence infant effector responses to BCG at 2 weeks
BCG vaccination rapidly induces a large population of IFN-γ-producing T cells,17,18,29 so the effector response was evaluated by assessing the ex vivo IFN-γ response to PPD and heat-killed BCG at 2 weeks. Background responses, measured in negative control wells, were similar, with a median of 70 (IQR 38–180) SFU/106 PBMCs among infants born to HIV-negative mothers and a median of 73 (IQR 26–101) SFU/106 PBMCs among seroreverters.
More IFN-γ-producing cells were present following treatment with PPD or killed BCG than in negative control cultures, indicated by positive SFUs, but these responses were not influenced by maternal HIV status (Fig. 3).

Maternal human immunodeficiency virus (HIV) status has no discernable effect on interferon (IFN)-γ release in 2-week-old infants. The peripheral blood mononuclear cells (PBMCs) of 2-week-old infants were stimulated with (a) heat-killed Bacille Calmette-Guérin (BCG) or (b) purified protein derivative (PPD) and IFN-γ responses measured by enzyme-linked immunosorbent spot-forming cell assay (ELISpot) after 18 hr. Values are expressed as negative control subtracted from treatment, so cases where proliferation was greater in the negative control return negative values.
HIV infection during pregnancy led to reduced proliferative responses at 10 weeks
Functional T-cell memory was assessed at 10 weeks of age by measuring specific proliferation in response to PPD and heat-killed BCG. Percentages of divided cells in negative control cultures appeared unaffected by maternal HIV status, as the median numbers of divided CD4 T cells in the negative control treatments were 5·4% (IQR 3·2–8·7%) in infants with HIV-negative mothers and 5·4% (IQR 3·2–10·0%) in seroreverters, while median numbers of divided CD8 T cells in the negative control treatments were 5·0% (IQR 2·6–6·2%) in infants with HIV-negative mothers and 3·0% (IQR 1·9–15·3%) in seroreverters.
Antigen-specific division was calculated by subtracting the percentage of divided cells in negative controls from the percentage of divided cells in antigen-treated cultures. Neither heat-killed BCG nor PPD stimulated much division among CD8 T cells, as the percentage of divided cells was < 5% in all cases. In CD4 T cells, heat-killed BCG stimulated slightly more division but there was no discernable difference based on maternal HIV status, as the median percentage of divided cells was 5·3% (IQR 1·1–11·6%) in seroreverters and 4·2% (IQR 0·1–30·2%) in infants with HIV-negative mothers. However, PPD induced more division in the CD4 T cells of infants with HIV-negative mothers (median 7·6%; IQR 0·8–28·0%) than in those of seroreverters (median 5·6%; IQR 1·2–11·4%), representing a 26% reduction in the percentage of divided cells.
It was possible to carry out this assay on relatively few infants as some had withdrawn from the study, and the number of cells recoverable from 10-week-old infants was often insufficient to carry out the proliferation assay. Nonetheless, a bimodal distribution was discernable in proliferative responses to BCG and PPD, allowing infants to be classified as high responders in which at least an eighth of the cells remaining at the end of the culture period had divided, and low responders in which less than an eighth had divided (Fig. 4). Few infants were classed as high responders based on their CD8 T-cell responses, but when classified by CD4 T-cell responses to BCG, there were more high responders among infants whose mothers were HIV-uninfected (three of eight) than among seroreverters (one of eight). There was a similar disparity in responses to PPD between infants whose mothers were HIV-uninfected (five of 10) and seroreverters (one of 10).
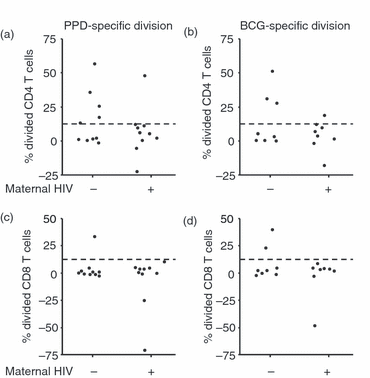
Maternal human immunodeficiency virus (HIV) infection leads to a reduced antigen-specific proliferation in 10-week-old HIV-negative infants. Plots indicate percentages of divided CD4 T cells, measured by carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution, following 8 days of culture after stimulation with (a) purified protein derivative (PPD) and (b) Bacille Calmette-Guérin (BCG), and percentages of divided CD8 T cells after stimulation with (c) PPD and (d) BCG. The dashed line indicates delineation of high-level responders. Values are expressed as negative control subtracted from treatment, so cases where proliferation was greater in the negative control return negative values.
Maternal HIV status did not affect maternal antibody transfer or infant antibody responses
To assess the influence of HIV in pregnancy on antibody levels, we assessed levels of both anti-M. tuberculosis and anti-polio IgG at 10 weeks, controlled for levels in umbilical cord blood. There were no significant differences either in the umbilical cord blood or infant blood at 10 weeks.
Median anti-M. tuberculosis IgG at 10 weeks was 19·64 (IQR 10·63–26·39) U/ml in infants with HIV-negative mothers and 15·80 (IQR 4·92–22·49) U/ml in seroreverters, and median levels of anti-polio IgG were 1·64 (IQR 1·07–1·97) IU/ml in infants with HIV-negative mothers and 1·43 (IQR 0·51–1·77) IU/ml in seroreverters.
Discussion
Childhood responses are influenced by a number of factors that affect pregnancy. Many, such as poor nutrition30–33 and malaria,34 are highly prevalent in Sub-Saharan Africa. HIV infection during pregnancy has also been shown to have a profound and long-lasting effect on childhood immune responses.3
We found that, by 10 weeks, the CD4 count of seroreverters had dropped to levels characteristic of HIV-positive African infants of equivalent age and well below those typical of HIV-negative infants.35 The phenotypic differences based on maternal HIV status indicate that the losses had come from early and undifferentiated cells, identified by expression of CCR7 and CD27,25,36 while higher proportions of CD4 T cells expressed CD57 and PD-1, which are associated with poor replication and function.26–28 While the same changes in the CD4 T-cell compartment are associated with progressive HIV infection,27,37,38 similar changes are associated with the aging of healthy adults,11,12,39 implying that they are indicative of general immune activation rather than a specific response to HIV.
The central memory T-cell population that proliferates in response to antigen is characterized by the CCR7+ CD45RA− phenotype;40 hence the smaller central memory subpopulation of seroreverters at 10 weeks was associated with a bimodal proliferative response to PPD with some high responders and some low responders. The differences in central memory subpopulation size appeared between 2 and 10 weeks, suggesting that the subpopulation structure at the time of first exposure to the antigen was less important to the magnitude of the recall response than the subpopulation structure at the time of re-exposure.
The greater importance of central memory subpopulation size at the time of re-exposure than at first exposure is supported by evidence from a previous study on proliferative responses to the measles vaccine.21 There, central memory subpopulation size differed between treatments at the time of vaccination but not by the time of re-exposure, and proliferative responses following re-exposure were also similar between the treatment groups. Loss of CCR7 is associated with a shortening of telomeres and loss of proliferative capacity,40 so it is likely that the central memory cells are the precursors for proliferation and the ability of seroreverters to mount a strong proliferative response is lost with them.
Our results beg the question of how HIV in pregnancy can affect the development of the immune system without actually infecting the infant. All infants were healthy at birth, which rules out the possible influence of preterm birth or intrauterine growth retardation. One possibility is that the infant responses are influenced by HIV antigens crossing the placenta, either in solution or during an infection that is later cleared. HIV-specific T-cell responses in seroreverters have been widely reported, as reviewed by Kuhn et al.,41 and, although there is considerable variation in the assay methods and frequency of responding infants, stimulation with peptide pools derived from the gag, env and nef HIV proteins induced detectable responses in 90–100% of tested infants.3,42 This suggests that prenatal or possibly perinatal exposure to HIV antigens is common enough to account for the almost ubiquitous effects that we observed. However, it remains to be established whether exposure to HIV antigens without infection can exert such a powerful influence.
Another possibility is that the influence on the infant immune system is not derived directly from HIV contact but from exposure to a maternal immune system under the influence of HIV, which induces considerable activation and differentiation among both CD4 and CD8 T cells.10,23,26-28,43,44 This reprogramming is likely to dramatically affect the milieu of cytokines and chemokines present in maternal blood and lymphoid systems. Although we are aware of no direct evidence for vertical transfer of cytokines and chemokines to the fetal circulation, maternal cells have been detected in fetal blood in around 40% of healthy pregnancies,45 demonstrating that transplacental traffic is commonplace. Transplacental transfer of activating cytokines would directly expose the immune system of the fetus to an immunological environment conducive to differentiation. This is the subject of current investigations.
Although polyclonally induced IFN-γ and IL-12 production has been shown to be reduced in the umbilical cord blood cells of seroreverters,13 we found that, at 2 weeks, there was little or no effect of HIV in pregnancy on differentiation or the effector phase of the immune response to BCG. Taken together, these findings suggest that seroreverters’ immune systems are impaired at birth, but that the extent of the impairment increases during the first weeks of life until the generalized effects that we observed at 10 weeks become apparent.
Unfortunately the sample volumes available were not sufficient to repeat the assay for overnight IFN-γ production at 10 weeks, but the CD4 T cells were more differentiated and appeared less able to respond to BCG in the seroreverters. The implication is that either the mechanism behind immune impairment is initiated late in pregnancy, or that the increased levels of regulation induced by HIV infection46 counter the stimulatory influences during pregnancy but wane faster after birth. In either case, we suggest that the reported effects on umbilical cord blood cytokine production are merely the first hints of a process that is far from complete by 2 weeks after birth.
The scope of the findings of this study is limited by several factors. Firstly, although we tried to restrict recruitment to infants with healthy birth weights in order to exclude confounding with the prenatal undernutrition often associated with HIV in pregnancy,4,5,47 the lower MUACs of seroreverters indicated slightly poorer nutrition during pregnancy. Secondly, the possible influence of nevirapine treatment could not be assessed as it was unethical to deny treatment to any HIV-positive mother–infant pairs or to administer it in the absence of HIV infection. Nevirapine has been associated with slight immune activation at birth, in the form of elevated levels of neopterin and sL-selectin in umbilical cord blood serum,48 but its longer term effects on the development of the immune system have not been assessed. Finally, the low volumes of blood available limited our ability to provide detailed insights into CD4 phenotype and CD8-mediated immune memory.
In conclusion, given the importance of CD4 T cells in protection against tuberculosis,49–51 it is of concern that the large number of infants born to HIV-positive mothers in Africa may have impaired CD4 T-cell responses and increased vulnerability to tuberculosis and possibly a range of other infections, especially in the light of the evidence that immune deficiencies in such infants may last for several years.3,13 It is of particular interest to know whether the levels of CD4 T-cell differentiation continue to rise, stabilize or recover after 10 weeks, and whether antigen-specific proliferative responses follow the trend set by the differentiation. A number of strategies to improve protection of seroreverters may be possible, such as boosting the vaccine later in infancy, or using novel delivery systems that may not be appropriate for general use, but may be considered for a high-risk group that is often identified by antenatal HIV testing.52
Acknowledgements
We would like to thank the Blantyre District Health Office for assistance in acquiring vaccines and recruiting study subjects, and the nurses of the Queen Elizabeth Central Hospital Labour and Postnatal wards, and of the Ndirande and Limbe Health Centre antenatal clinics. Dr Newton Kumwenda of Johns Hopkins University provided advice on several aspects of the study, and Dr Sarah White of the Malawi-Liverpool-Wellcome Trust Clinical Research Programme provided invaluable guidance on statistical analysis. Mr Felanji Simulkonda provided technical assistance.




