β2 Integrin deficiency yields unconventional double-negative T cells distinct from mature classical natural killer T cells in mice
Summary
Expressed on leucocytes, β2 integrins (CD11/CD18) are specifically involved in leucocyte function. Using a CD18-deficient (CD18−/−) mouse model, we here report on their physiological role in lymphocyte differentiation and trafficking. CD18−/− mice present with a defect in the distribution of lymphocytes with highly reduced numbers of naïve B and T lymphocytes in inguinal and axillary lymph nodes. In contrast, cervical lymph nodes were fourfold enlarged harbouring unconventional T-cell receptor-αβ (TCR-αβ) and TCR-γδ CD3+ CD4− CD8− (double-negative; DN) T cells that expanded in situ. Using adoptive transfer experiments, we found that these cells did not home to peripheral lymph nodes of CD18wt recipients but, like antigen-experienced T or natural killer (NK) T cells, recirculated through non-lymphoid organs. Lacking regulatory functions in vitro, CD18−/− TCR-αβ DN T cells did not suppress the proliferation of polyclonally activated CD4+ or CD8+ (single-positive; SP) T cells. Most interestingly, CD18−/− TCR-αβ DN T cells showed intermediate TCR expression levels, an absent activation through allogeneic major histocompatibility complex and a strong proliferative dependence on interleukin-2, hence, closely resembling NKT cells. However, our data oppose former reports, clearly showing that, because of an absent reactivity with CD1d-αGalCer dimers, these cells are not mature classical NKT cells. Our data indicate that CD18−/− TCR-αβ DN T cells, like NKT and TCR-γδ T cells, share characteristics of both adaptive and innate immune cells, and may accumulate as a compensatory mechanism to the functional defect of adaptive immunity in CD18−/− mice.
Introduction
The dynamic recirculation of leucocytes surveying for pathogens and neoplastic transformation in organs is characteristic of the immune system. This requires coordinated leucocyte transmigration through endothelial barriers, and cell–cell contacts for leucocyte activation. Different membrane receptors are involved, including the β2 integrins (CD11/CD18).1 Their common β2 chain (CD18) combines with one of four α chains termed αLβ2 (lymphocyte function-associated antigen 1; LFA-1), αMβ2 macrophage-1 antigen (MAC-1), αXβ2 (p150/95) and αDβ2 (CD11d/CD18), which are diversely expressed on leucocytes.2 Major ligands for the β2 integrins are the immunoglobulin superfamily members of intercellular adhesion molecules.2
Ligation of β2 integrins on B and T lymphocytes influences a series of processes like transmigration, cytokine production, proliferation and cytotoxicity.3,4 Deficiency in LFA-1 crucially reduces cellular trafficking of naïve lymphocytes through high endothelial venules in peripheral lymph nodes (pLN).3,4 In humans, rare genetic defects can affect the function or expression of CD18 heterodimers leading to leucocyte adhesion deficiency-1.5,6 Analogously, a murine model lacking all CD11/CD18 heterodimers (CD18−/−) has been generated7 resembling the human disease characterized by a peripheral leucocytosis,7,8 impaired wound healing9 and recurrent bacterial infections.7,10 We previously showed that in CD18−/− mice, T cells were unable to extravasate from blood vessels into inflamed skin with the majority of them characterized by a distinct activation or memory phenotype.11
Along with the prevailing conventional T-cell receptor-αβ (TCR-αβ) expressing CD4+ and CD8+ T cells, which are selected in the thymus and contribute to adaptive immunity, a variety of unconventional T cells such as natural killer (NK) T cells, TCR-αβ (αβ) and TCR-γδ (γδ) exist.12,13 Unconventional T cells take an intermediate place between innate and adaptive immunity with the characteristic ability to complement or control the development of the adaptive immune response. The populations of unconventional T cells are heterogeneous with CD3+ CD4− CD8− double-negative (DN) T cells represented within each population. Under physiological circumstances the majority of unconventional T cells occur in thymus. Different subsets typically reside in immune and non-lymphoid organs14,15 and protect against malignancies, infection and autoimmunity16–20 and critically contributing to the maintenance of tissue integrity.14 Unconventional T cells often have autospecific oligoclonal TCR repertoires recognizing non-classical major histocompatibility complex (MHC)19,21 for binding of high-density ligands.22 Furthermore, the existence of phylogenetically conserved αβDN T cells with a canonical repertoire and a complementary role to classical (type-I) NKT cells22 indicates that unconventional T cells comprise diverse subsets. Although only rarely investigated, they are attributed pivotal functions in innate immunity,14,23 regulation of adaptive immunity and peripheral tolerance.20,24,25
The DN T cells can be generated by different mechanisms: (i) increased production, (ii) increased proliferation and/or (iii) reduced apoptosis. In lymphoproliferative lpr and gld mice, αβDN T cells originate from autoreactive single-positive (SP) T cells in the periphery, which down-regulate their CD4 and CD8 coreceptors.24,26 These cells become refractory and contribute to the maintenance of peripheral tolerance.25 Furthermore, autoreactive αβDN T cells with controversial functional properties are represented in the periphery of different TCR transgenic models.27,28αβDN T cells may display an activation/memory state, being functionally anergic and suppressing proliferation of CD4+29 and CD8+27 T cells. Conversely, they can be naïve, but interleukin-2 (IL-2) responsive and devoid of regulatory function.28
We demonstrate that lack of CD18 leads to an increase in unconventional αβDN and γδDN T cells. These cells are most prominent in secondary immune organs of CD18−/− mice, particularly in pLN. They are activated, proliferate strongly, respond to IL-2, and recirculate to non-lymphoid organs, hence resembling antigen-experienced cells. Although sharing typical features with NKT and γδ T cells, we show that DN T cells from CD18−/− mice, lacking CD1d-αGalCer binding capacity, are neither mature classical (type-I) NKT cells, nor can they be primed via classical MHC molecules. But they are activated to proliferate without rigid requirements for costimulation, similar to antigen-experienced SP or γδ T cells. Besides, unlike classical (type-I) NKT cells that are generally found with highest incidences among hepatic leucocytes,12,15,30 DN T cells are not increased in livers of CD18−/− mice but accumulate within their pLN. Collectively, these unconventional T cells from CD18−/− mice show a TCR-αβint CD4− CD8− NK1.1− CD1d-αGalCer− CD62L− CD44hi phenotype, and are mainly and massively generated within their lymph nodes upon an antigenic, homeostatic and/or cytokine-mediated stimulus that currently remains speculative.
Materials and methods
Mice
CD18−/− 129X1/SvJ × C57BL/6 (H-2b) mice were generated as described previously.7 CD18+/+ litters from heterozygote crosses served as wild-type (CD18wt) control mice. All experiments were performed with age-matched and sex-matched male or female mice, which were maintained under specific pathogen-free conditions. Depending on the experimental setting, the appropriate age of mice was chosen, as indicated in the figure legends. All experiments were performed in compliance with the German Law for Welfare of Laboratory Animals, as approved by the Regierungspräsidium Tübingen, Germany.
Antibodies
Monoclonal antibodies (mAb) to CD3 (145-2C11), CD4 (RM4-5), CD8α (53-6.7), CD16/CD32 (2.4G2), CD19 (1D3), CD28 (37.51), CD44 (IM7), CD62L (MEL-14), B220 (RA3-6B2), NK1.1 (PK136), CD49b (DX5), TCR-γδ (GL3), TCR-αβ (H57-597), Gr-1 (RB6-8C5), rat immunoglobulin G2a (IgG2a; R35-95), rat IgG2b (A95-1) and rat IgM (R4-22) were purchased from BD Pharmingen. The mAb to CD8α (5H10), F4/80 (CI:A3-1) were purchased from Caltag; mAb to Foxp3 (FJK-16s) from eBioscience; polyclonal rabbit transforming growth factor-β1 (TGF-β1) from MLB International Corporation (Woburn, MA). All antibodies were purified, or conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin-chlorophyll-protein complex (PerCP), PerCP-cyanin5·5 or allophycocyanin. The CD1d α-galactosylceramide (αGalCer)–PE dimer was kindly provided by Dr Jörg Reimann (Department of Medicine, Microbiology Section, Ulm University, Ulm).
Lymphocyte isolation from secondary immune organs
Lymph nodes or spleens were homogenized through 40-μm cell strainers (BD Falcon, Bedford, MA). Red blood cells from spleens were lysed osmotically. Cells were washed with phosphate-buffered saline (PBS), counted and stained further with mAb.
Lymphocyte isolation from non-lymphoid organs
Lungs and livers were digested in RPMI-1640 with 5% fetal calf serum containing 150 U/ml collagenase type CLS-II (Biochrom AG, Berlin, Germany) for 1 hr at 37°. Livers were homogenized and hepatocytes were removed after sedimentation for 4 min at 50 g. Cells from one liver were resuspended in 7·5 ml Percoll 35% (Biochrom AG), and stratified over 2 ml Percoll 75%. Cells were separated after centrifugation for 20 min at 780 g. Lymphocytes from lung suspensions, obtained by enzyme digestion, were separated using FicoLiteM (Linaris) gradient separation for 20 min at 1030 g. In both cases, lymphocytes from the interphase were analysed by fluorescence-activated cell sorting (FACS).
FACS staining
Lymphocytes from pLN, spleens, livers and lungs (1 × 106 cells per 50 μl PBS) were blocked with purified CD16/CD32 FcγII/III mAb to prevent non-specific binding. Subsequently, cells were stained with ≤ 1 μg mAb and analysed using a FACSCalibur (BD, Franklin Lakes, NJ).
Bromodeoxyuridine in vivo incorporation
Lymphocytes were prepared from the pLN of CD18wt and CD18−/− mice (3 months) that had received 0·8 mg/ml bromodeoxyuridine (BrdU) (Sigma, St Louis, MO) in drinking water for 2 days. Having stained TCR-αβ and TCR-γδ with mAb, intracellular staining for BrdU incorporation using an FITC BrdU Flow Kit (BD Pharmingen) was performed according to the manufacturer’s instructions.
Adoptive transfers experiments
Cell transfers were performed as described previously.3 Lymphocytes were prepared from pLN from 4-week-old CD18wt and CD18−/− mice. Cells (107/ml) were stained with different cell-tracker dyes, 1 μm 5-chloromethylfluorescein diacetate (CMFDA) or 5 μm of a rhodol-based fluorophore (CMRA) (Molecular Probes, Carlsbad, CA), for 30 min at 37° then washed and mixed at a ratio of 1 : 1. Before the transfers, percentages and absolute counts of CD18wt and CD18−/− T-cell and B-cell subsets contained in the 1 : 1 cell suspensions were determined to allow an exact quantitative assessment of the respective homing properties for each cell subset afterwards. Subsequently, 30 × 106 total cells (15 × 106 CD18wt : 15 × 106 CD18−/−) were injected intravenously into the tails of CD18wt recipients. For assessment of short-term migration, blood, pLN, spleens, livers and lungs from the recipient mice were harvested 18 hr after adoptive cell transfer and analysed for transferred lymphocytes using a FACSCalibur. This particular time frame was chosen from a time–course analysis for cell recirculation after 6, 18 and 24 hr. Time-points of 1–24 hr have previously been used for similar lymphocyte recirculation experiments by others.3,31 In initial experiments, we detected no quantitative differences in cell redistribution after 18 or 24 hr for either CD18−/− or CD18wt donor lymphocytes (data not shown). Note that recipient mice were not irradiated before adoptive transfer for the short-term migration studies here described, according to previously published protocols.3,31
For an exact quantitative assessment of the respective homing properties for each cell subset the numbers of the different cell subsets detected in the recipient organs were corrected by recalculation according to the count/percentage of the respective cell subset contained in the starting cell suspension before the transfer.
CFSE proliferation assays
Cells from pLN from CD18wt and CD18−/− mice (107/ml) were labelled with 5 μm carboxyfluorescein succinimidyl ester (CFSE) dye (Molecular Probes) in PBS and then used for magnetic-activated cell sorting (MACS) of total T cells. CD18wt and CD18−/− total T cells comprising CD4+, CD8+, αβDN and γδDN T cells were used for in vitro proliferation assays. For these, 2 × 105 cells/well were cultured with immobilized anti-CD3 (5 μg/ml) alone, immobilized anti-CD3 (5 μg/ml) and soluble anti-CD28 (2·5 μg/ml) or 100 U/ml IL-2 for 4 days in 96-well U-bottom plates. Finally, cellular proliferation of CD4+, CD8+, αβ and γδ T cells was analysed by FACS.
Enrichment of total T cells by MACS
Lymphocytes from pLN were CFSE-stained. T cells were purified using a self-made cocktail of mAb because of the absence of CD11b and CD11c on CD18−/− leucocytes. Using CD19, CD49b, NK1.1, F4/80, MHC II and Gr-1 PE-conjugated mAb, macrophages, B cells, NK cells and residual granulocytes were depleted. The MACS (Miltenyi Biotech, Bergisch Gladbach, Germany) was performed according to the manufacturer’s instructions and the depleted fraction (total T cells) had a purity > 90%.
Coculture suppression assays
Cells from pLN of CD18−/− mice were cultured in complete RPMI medium (RPMI-1640/GlutaMAX-I (Gibco, Carlsbad, CA), 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 1% sodium pyruvate, 1% non-essential amino acids, 2·5% HEPES buffer, 0·05 mmβ-mercaptoethanol) supplemented with 100 U/ml IL-2 (PeproTech EC, London, UK) for 7 days. Medium with IL-2 (50 U/ml) was changed every second day for enrichment of αβDN T cells prior to MACS purification. Macrophages, B, CD4+, CD8+, γδDN T and NK cells were depleted as described above. The αβDN T cells obtained (with a purity > 80–90%) were used as effector cells for in vitro suppression assays. The CFSE-labelled, MACS-sorted CD18wt CD4+ or CD18wt CD8+ responder lymphocytes (105/well) were activated with 5 μg/ml immobilized anti-CD3 and 2·5 μg/ml soluble anti-CD28. Responder cells were cultured alone or with the indicated numbers of CD18−/−αβDN T cells in 96-well plates for 3 days. The proliferation of responder cells was assessed by CFSE dilution.
Mixed lymphocyte reaction with allogeneic bone marrow-derived dendritic cells
Femurs from BALB/c mice were flushed out and the cells (106/ml) were cultured in complete RPMI medium with 100 U/ml IL-4 (PeproTech EC) and 50 ng/ml granulocyte–monocyte colony-stimulating factor (Promokine, Heidelberg, Germany). Culture medium containing cytokines was changed on day 3. On day 5, bone marrow-derived dendritic cells (BM-DC) were purified by 15·5% Histodenz (Sigma) (20 min at 600 g). Then, 104 BM-DC were cultured with 105 CFSE-labelled, MACS-sorted CD18wt and CD18−/− total T cells or CD18−/−αβDN T cells in 96-well plates. After 6 days, T-cell subset proliferation was assessed.
Results
Peripheral LN from CD18−/− mice display a severe lymphadenopathy
Isolated deficiency of LFA-1 in mice significantly decreases the numbers of naïve B and CD4+, CD8+ T cells in pLN.3 In contrast, reactive lymphadenopathy of cervical lymph nodes (cLN) was observed in a murine model of β2 integrin deficiency.7 To investigate the reasons underlying this apparent discrepancy, we first performed comparative analyses of cervical, axillary and inguinal LN cells from CD18−/− and CD18wt mice. Lymphocytes were decreased up to sixfold in absolute cell counts per LN (inguinal or axillary) in CD18−/− mice (Fig. 1a). By contrast, cLN revealed a fourfold increase in cellularity. Notably, the hypocellularity of inguinal and axillary LN from CD18−/− mice was the result of a decrease in SP T and B cells (Fig. 1b). Significantly enlarged, the cLN from CD18−/− mice showed identical absolute counts of CD4+ and CD8+ T cells per LN but an eightfold increase in CD19+ cells (mostly B cells) when compared with CD18wt pLN (Fig. 1c). Represented as percentages of total lymphocytes, CD19+ cells were 2·5-fold increased in cLN of CD18−/− mice (Fig. 1d), while CD18−/− CD4+ and CD18−/− CD8+ T cells were decreased three and eightfold, respectively, compared with CD18wt CD4+ and CD18wt CD8+ T cells (Fig. 1f). This discrepancy between absolute counts and percentages of lymphocyte subsets in cLN from CD18−/− mice unexpectedly revealed a substantial amount of γδ (Fig. 1c,e) as well as αβ T cells lacking both CD4 and CD8 (CD3+ CD4− CD8−) (Fig. 1c,f), which classified the DN T cells as unconventional. These unconventional T cells also existed in CD18wt mice, but in CD18−/− mice αβDN T cells were up to 12-fold increased with regard to percentages (Fig. 1f) and even 35-fold increased in absolute numbers in CD18−/− cLN (Fig. 1c). These data show that β2 integrin deficiency severely affected the distribution and numbers of lymphocyte subsets. In analogy to LFA-1−/− mice, a decrease in B and SP T cells in axillary and inguinal LN was observed, but deviant from this, a marked increase in unconventional αβDN and γδDN T cells occurred in cLN.
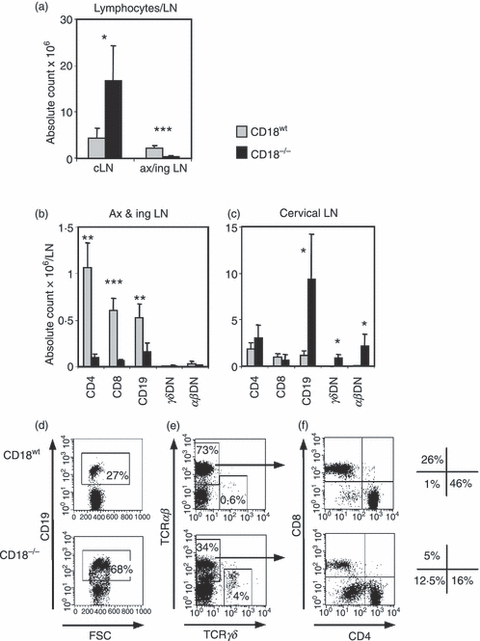
Cervical lymph nodes (cLN) from CD18−/− mice reveal an increase in absolute numbers of unconventional αβ or γδ double-negative (DN) T cells and B cells. (a) Cell suspensions from cervical and pooled axillary and inguinal LN were prepared from 2-month-old wild-type mice (CD18wt; grey bars, n = 5) and CD18−/− mice (black bars, n = 5). Absolute numbers of CD4+, CD8+, CD19+, αβ+ and γδ+ subpopulations were calculated using fluorescence-acitvated cell sorting analysis of pooled inguinal and axillary (ax&ing) (b) and cervical (c) LN, subsequently expressed as counts per one LN. Statistics were performed using alternate t-test: *P < 0·05, **P < 0·01 and ***P < 0·001. Representative dot plots of cLN from CD18wt and CD18−/− mice depict CD19+ cells (mostly B cells) gated on forward scatter (FSC; x-axis) versus CD19+ (y-axis) (d), γδ and αβ T cells gated on T-cell receptor (TCR) γδ (x-axis) versus TCR-αβ (y-axis) (e). As an example, total TCR-αβ+ T cells were further gated (as indicated by arrows) for CD4 (x-axis) versus CD8 (y-axis) expression (f). The resulting dot plots (f) allowed differentiation of total TCR-αβ+ T cells from CD18wt and CD18−/− mice into CD4+ (lower right quadrant), CD8+ (upper left quadrant) and αβDN T cells (lower left quadrant) (f). The corresponding percentages of T-cell subsets are shown aside. Data depict one representative out of three experiments. Data are expressed as mean ± SD.
CD18−/− CD4+, CD18−/− CD8+ and B lymphocytes do not home to pLN but efficiently recirculate through non-lymphoid organs
Under physiological conditions, lymphocyte composition in pLN (cervical, axillary, brachial and inguinal LN) reflect their homeostatic turnover.32 The reduced cellularity of inguinal and axillary LN from CD18−/− mice indicates an impaired lymphocyte homing, whereas the increase in cellularity and DN T-cell numbers in the enlarged cLN suggests that naïve CD18−/− cells preferentially migrate to and/or accumulate in cLN. To uncover the causes for these substantial changes in recirculation of SP T and B cells from CD18−/− mice, we performed adoptive co-transfer experiments with naïve (CD62Lhi CD44lo; data not shown) CD18wt and CD18−/− lymphocytes into CD18wt recipients. This allowed a comparison of the homing mechanisms dependent only on CD18 deficiency of transferred lymphocytes without a bias introduced by the disturbed lymphoid architecture in CD18−/− mice. The short-term recirculation of CD18wt and CD18−/− donor cells in the organs of recipient mice was visualized by loading with CMRA and CMFDA dye trackers and detected after 18 hr. Figure 2(a) exemplifies the distribution of transferred CD18wt cells (CMRA+, upper left gate) and CD18−/− cells (CMFDA+, lower right gate) in recipient spleens. Further analysis revealed the principal lymphocyte subsets of CD18wt (CMRA) or CD18−/− (CMFDA) donors according to their expression of B220 (Fig. 2b), CD4 (Fig. 2c) or CD8 (Fig. 2d) as found in spleens of recipient mice. Other immune and non-immune organs from recipients were investigated in the same way for the presence of donor cells. Contrasting the distribution of the corresponding CD18wt subsets, CD18−/− SP T and B220+ cells showed an impaired homing to pLN of the recipient CD18wt mice 18 hr after transfers (Fig. 2a). These data show that cellular transmigration through high endothelial venules was largely mediated by β2 integrins.
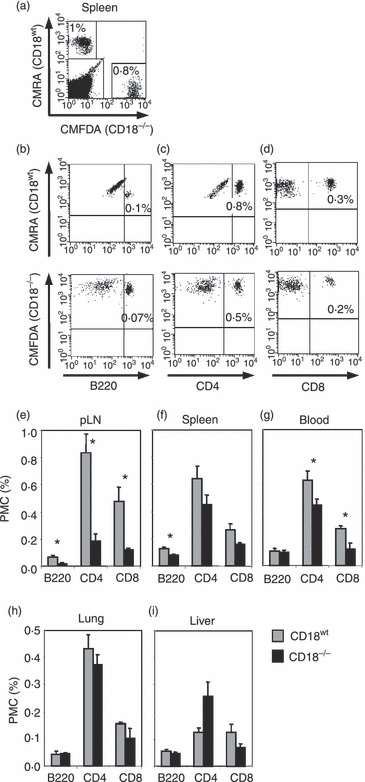
CD18−/− B220+ lymphocytes and CD4+, CD8+ T lymphocytes reveal an impaired homing to peripheral lymph nodes (pLN) and normal recirculation to non-lymphoid organs. CD18wt (5 μm CMRA-labelled) and CD18−/− (1 μm CMFDA-labelled) lymphocytes from pLN of 4-week-old donors were cotransferred intravenously at a ratio of 1 : 1 (30 × 106 cells/mouse) into 2-month-old CD18wt recipients. Donors were chosen at a young age (i.e. 4 weeks) to obtain a maximum of naïve (CD62L+) versus antigen-experienced (CD62L−) lymphocytes to be able to study lymph node homing. After 18 hr, secondary immune and non-immune organs of recipient mice (n = 3) were analysed for the presence of CD18wt (grey bars) and CD18−/− (black bars) donor cells. The cellular subtypes of transferred cells were defined after staining with CD4, CD8 and B220 monoclonal antibodies and fluorescence-activated cell sorting analysis. A representative dot plot from a spleen of a recipient mouse (a) demonstrating the percentages of CD18wt and CD18−/− transferred cells labelled with CMRA and CMFDA dye trackers, respectively. In addition, CMRA-labelled CD18wt and CMFDA-labelled CD18−/− donor cells detected in spleens of recipient mice were further differentiated into B220+ cells (b), CD4+ (c) and CD8+ (d) T cells. The upper right quadrants give the percentages of CD18wt (upper dot plots) or CD18−/− (lower dot plots) donor cells as detected in 100% of total (donor + recipient) lymphocytes. Data are representative of at least three different experiments. For quantitative assessment, results are presented in bar diagrams as percentage of total lymphocytes in LN (e), spleen (f), blood (g), lungs (h) and liver (i) of recipient mice. Significances were calculated by alternate t-test *P < 0·05.
Besides, CD18−/− SP T and B cells did not accumulate in the spleen (Fig. 2f), blood (Fig. 2g) and lungs (Fig. 2h) of CD18wt recipients. Remarkably, only in the case of livers from recipient mice, more CD4+ T cells were found from CD18−/− than from CD18wt donor mice (Fig. 2i). Interestingly, the retention of activated CD4+ T cells in liver has been described previously.33,34 In line with the latter reports, we earlier demonstrated that a higher percentage of CD4+ T cells from CD18−/− than from 18wt mice show an activated phenotype.11 This increase in CD4+ T cells with an activated phenotype could possibly explain the preferential accumulation of CD18−/− CD4+ T cells in the recipient livers that was observed in this study.
CD18−/−αβDN and γδDN T cells recirculate through liver and lungs
Elevated numbers of αβDN and γδDN T cells in cLN of CD18−/− mice indicate that these cells either selectively recirculate into, or alternatively, are generated by in situ-expansion in cLN. To address these hypotheses, adoptive transfer experiments were performed. As a result, CD18−/−αβDN and γδDN T cells did not home to pLN of CD18wt recipient mice (Fig. 3a). Both DN T-cell subsets were slightly increased in spleen (Fig. 3b) and blood (Fig. 3c), whereas they accumulated significantly more in non-lymphoid organs such as liver (Fig. 3d) and lungs (Fig. 3e). In contrast, SP T cells derived from CD18−/− mice showed an equal distribution in lymphoid and non-lymphoid organs. Representative dot plots indicating percentages of the CMRA-loaded CD18−/− donor lymphocyte subsets are also provided (Fig. 3f–j).
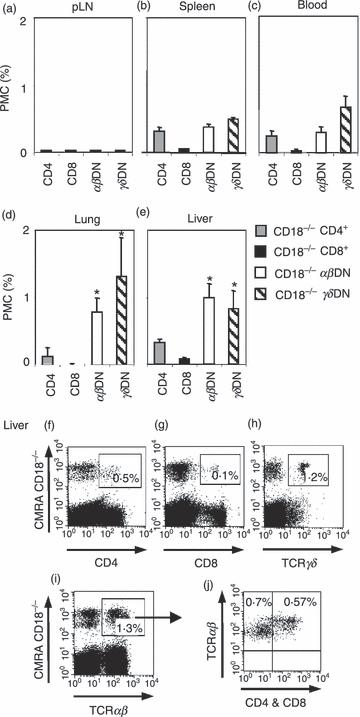
αβ and γδ double-negative (DN) T cells from CD18−/− mice selectively accumulate in non-lymphoid organs. CD18−/− CD4+, CD8+ and unconventional αβDN and γδDN T-cell subpopulations from 8- to 10-month-old mice were compared in their ability to recirculate through secondary lymphoid and non-lymphoid organs of CD18wt recipients. In this experiment, CD18−/− mice at an age of 8–10 months were used as donors because these were richer sources of unconventional DN T cells in comparison to CD18−/− young mice. Donor lymphocytes from cervical lymph nodes (cLN) of CD18−/− mice were labelled with 5 μm cell tracker dye CMRA and intravenously injected (15 × 106 cells/mouse) into CD18wt recipients (n = 4). After 18 hr, peripheral LN (pLN) (a), spleen (b), blood (c) and the non-lymphoid organs lungs (d) and liver (e) of recipient mice were analysed for the presence of transferred CD18−/− T-cell subpopulations CD4+ (grey bars), CD8+ (black bars), αβ (white bars) and γδDN (striped bars) T cells. The detected donor cells are presented as percentages of total (donor + recipient) PMC. Significances were calculated by alternate t-test *P < 0·05. Besides, representative dot plots show CMRA-labelled CD4+ (f), CD8+ (g), γδDN (h) and total αβDN (i) T cells from pLN of CD18−/− donors as detected in livers of CD18wt recipient mice. The percentages of the respective CD18−/− CMRA-labelled donor cell subset are given in the upper right gate. Dot plot (i) depicts further gating of total αβ T cells derived from CD18−/− donors (CMRA+) and subsequent (indicated by an arrow) assessment of their expression of CD4 and CD8 in dot plot (j). The latter plot subdivides these CMRA+αβ T cells (y-axis) from CD18−/− donors into conventional (SP) T cells that express CD4 or CD8 (x-axis), both stained in the same fluorescence channel (upper right quadrant), and unconventional αβ DN T cells, staining neither for CD4 nor for CD8 (upper left quadrant). Data are representative of three different experiments.
These data collectively suggest that migration of CD18−/−αβDN and γδDN T cells into liver and lungs was independent of β2 integrins. Moreover, our data do not support a selective homing of CD18−/−αβDN and γδDN T cells into cLN. However, we cannot currently exclude that impaired egress of these cells from cLN, as observed for CD18−/− plasma cells,35 contributes to the increase of DN T cells in cLN.
CD18−/−αβDN and γδDN T cells display an antigen-experienced phenotype
To next address the question whether DN T cells may be activated to expand in situ in cLN of CD18−/− mice, we compared CD18wt and CD18−/− lymphocytes for activation markers, with a CD62Llow CD44high expression identifying antigen-experienced T lymphocytes. A twofold decrease in CD62L-expressing cells was observed in CD18−/−αβDN (Fig. 4a) and γδDN (Fig. 4b) T cells compared with CD18wt cells, as also shown by representative dot plots. The majority of all αβDN T cells revealed a CD44hi expression (Fig. 4a), while significantly more CD18−/−γδDN T cells were CD44hi compared with CD18wt cells (Fig. 4b). These data indicate that lack of β2 integrins may generate an environment promoting selective expansion of antigen-experienced αβDN and γδDN T cells. To confirm the pre-activated status of CD18−/−αβDN and γδDN T cells, we performed in vitro culture with 100 U/ml exogenous IL-2.36 Interestingly, after 4 days, CFSE dilution revealed a moderate proliferation of CD18wt and a vigorous expansion of CD18−/−αβDN T cells (Fig. 4c). Strong proliferation of more than two cellular divisions was also observed for all γδDN T cells (Fig. 4d) suggesting that β2 integrins do not modulate their responsiveness to IL-2. In contrast, only 2–3% of CD18wt and 10% of CD18−/− SP T cells (Fig. 4e) performed one cellular division. This result demonstrated that based on IL-2 responsiveness, unconventional DN T cells are distinct from conventional T cells.
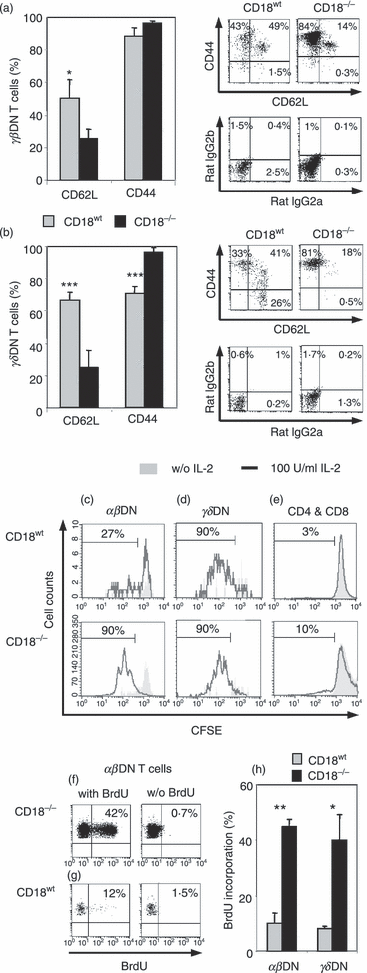
αβ and γδ double-negative (DN) T cells from CD18−/− mice show an antigen-experienced phenotype and vigorous proliferation with interleukin-2 (IL-2). Lymphocytes from peripheral lymph nodes (pLN) from 3- to 4-month-old CD18wt (n = 4) and CD18−/− (n = 5) mice were stained for CD4, CD8, T-cell receptor (TCR) αβ, TCR-γδ, CD44, rat immunoglobulin G2b (IgG2b), CD62L and rat IgG2a monoclonal antibodies and analysed by fluorescence-activated cell sorting (FACS). For quantitative assessment, bar graphs (left column) display percentages of αβ (a) and γδDN (b) T cells from CD18wt (grey bars) and CD18−/− (black bars) expressing CD62L or CD44. Besides, dot plots (right column) give FACS stainings of αβ (a) and γδDN (b) T cells from CD18wt (left plots) and CD18−/− (right plots) for the activation markers CD62L (x-axis) and CD44 (y-axis) (upper row) compared with dot plots of isotype control staining of rat IgG2a (x-axis) and rat IgG2b (y-axis) (lower row). Significances were calculated by alternate t-test *P < 0·05, ***P < 0·001. Magnetic antibody cell sorting (MACS) sorted CD18wt (n = 4, purity > 95%) and CD18−/− (n = 5, purity > 90%) total T lymphocytes from 3- to 4-month-old mice were labelled with CFSE (5 μm) and cultured with 100 U/ml IL-2 for 4 days. Cultures with IL-2 (black line) and controls without IL-2 (filled grey) are shown as analysed by FACS. Proliferating CD18wt (upper row) and CD18−/− (lower row) αβDN (c), γδDN (d), CD4+ and CD8+ (e) T-cell subsets are also presented as percentage of total cells of the indicated subsets. Histograms are representative of three experiments. Dot plots show percentages of bromodeoxyuridine (BrdU) incorporation in αβDN T cells obtained from cervical LN of 3- to 4-month-old CD18−/− (f, left plot) and CD18wt mice (g, left plot). Controls (right plots) of CD18wt and CD18−/− mice received water without BrdU. (h) Percentage of BrdU incorporation detected in αβ and γδDN T cells from pLN of CD18wt (grey bars) and CD18−/− mice (black bars) (n = 3). Significances were calculated by alternate t-test *P < 0·05, **P < 0·01.
To investigate whether αβDN T cells actively expand also in vivo, BrdU was added to the drinking water of CD18wt and CD18−/− mice. Already after 2 days, the αβDN T cells from cLN of CD18−/− mice had highly incorporated BrdU (Fig. 4f,h). In contrast, threefold less CD18wtαβDN T cells (Fig. 4g,h) had incorporated BrdU, each controlled by cohorts that received BrdU-free drinking water. Similarly, CD18−/−γδDN T cells proliferated significantly more in comparison to their CD18wt correspondents (Fig. 4h). Interestingly, BrdU incorporation was not found to be significantly increased in lymphocytes from lungs and livers of CD18−/− mice (data not shown), demonstrating that DN T cells were able to migrate selectively into these organs (Fig. 3d,e), but were not primarily generated there.
Our findings collectively demonstrate that antigen-experienced unconventional DN T cells from CD18−/− mice accumulate by in situ proliferation in vivo, with only IL-2 being a key mitogenic stimulus for a sustained in vitro expansion.
CD18−/−αβDN T cells do not reveal suppressive or classical regulatory functions
B220+αβDN T cells have been found to prevent the development of autoimmunity by suppressing the proliferation of autoreactive SP T cells in lpr and gld mice.25,26 Identically, CD18−/− mice also harbour αβDN T cells, but these are largely B220− (data not shown). We have addressed the question whether, similarly, αβDN T cells from CD18−/− mice can exert a regulatory function on polyclonally activated (iCD3 and anti-CD28) CD18wt SP T cells.
Therefore, CD18−/−αβDN T cells were cocultured with MACS-purified, polyclonally activated CD18wt CD4+ or CD18wt CD8+ responder T cells at different ratios for 4 days. In parallel, CD4+ CD25+ Treg cells from spleens of CD18wt mice served as controls, demonstrating a dose-dependent suppression of responder cell proliferation (data not shown). But remarkably, CD18−/−αβDN T cells did not have any significant suppressive effect on the proliferation of CD18wt responder cells (Fig. 5a). Furthermore, we investigated whether, similar to regulatory T cells, CD18−/−αβDN T cells produced TGF-β or IL-10. According to the lack of suppressive function, CD18−/− DN T cells produced no TGF-β (Fig. 5b) or IL-10 (data not shown). As positive controls, CD18wt CD4+ T cells stimulated with immobilized anti-CD3 and anti-CD28 mAb revealed a 22% increase in TGF-β expression (Fig. 5c). Next, we determined intracellular Foxp3 expression as marker mostly for natural Treg cells. The αβDN and γδDN T cells from cLN of CD18wt and CD18−/− mice were analysed for Foxp3 expression using natural Foxp3+ Treg cells, which are found among CD4+ T cells, as a positive control (Fig. 5d). Around 15% of both CD18wt CD4+ and CD18−/− CD4+ T cells stained positive for intracellular Foxp3 (Fig. 5d,e). Remarkably, among CD18wtαβDN T cells also about 6% proved to be positive for Foxp3. Interestingly, suppressive Foxp3+αβDN Treg cells have also recently been found in human patients with graft-versus-host disease.37 However, among CD18−/−αβDN T cells, only 1% or less were Foxp3+ (Fig. 5d,f), among γδDN T cells Foxp3 expression was even lower both from CD18wt and CD18−/− mice (Fig. 5d,g). In summary, these data clearly demonstrate that CD18−/−αβDN T cells do not possess distinct features of regulatory/suppressor T cells.
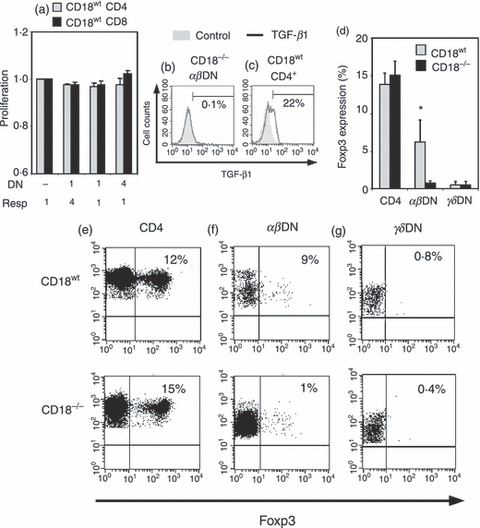
CD18−/−αβ double-negative (DN) T cells do not have suppressive or regulatory functions in vitro. (a) Suppressive function of in vitro expanded and sorted CD18−/−αβDN T cells (n = 3) from 4-month-old mice was assessed in cocultures with sorted, activated (5 μg/ml immobilized anti-CD3 and 2·5 μg/ml anti-CD28) CD18wt CD4+ (grey bars) or CD8+ (black bars) T cells from 2-month-old mice. All sorted subsets had a purity > 90%. CFSE (5 μm) labelled responder cells were mixed at ratios 4 : 1, 1 : 1 and 1 : 4 with CD18−/−αβDN T cells (x-axis) to obtain a total of 2 × 105 cells per well in 96-well U-bottom plates. The function of effector cells (y-axis) is presented as ratio from proliferation of responder cells from cocultures versus corresponding cell number of responder cells alone. The data are representative of two experiments. (b) CD18−/−αβDN T cells (n = 3) were investigated for transofrming growth factor-β (TGF-β) production after 4 days coculture with responder cells. Fluorescence-activated cell sorting stainings for TGF-β (black line) and isotype control (filled grey) are shown. TGF-β-positive cells are given as percentage of the indicated cell subset. (c) CD18wt CD4+ cells were stimulated in vitro by iCD3 and anti-CD28 for 3 days to obtain positive controls for TGF-β staining. (d) Intracellular Foxp3 expression was measured as per cent of Foxp3-expressing CD18wt (grey bars) and CD18−/− (black bars) CD4+ SP, αβ and γδDN T cells. Four CD18wt and CD18−/− mice at an age of 2–4 months were analysed. Significances were calculated by alternate t test *P < 0·05. Representative dot plots showing intracellular Foxp3 staining (x-axis) versus CD4+ (e), TCR-αβ (f) or TCR-γδ (g) staining (y-axis). The upper right quadrant indicates percentages of Foxp3 expression in CD4+, αβDN and γδDN T cells. The upper row represents lymphocytes from CD18wt and the lower row from CD18−/− mice.
CD18−/−αβDN T cells are activated by mechanisms deviant from naïve conventional T cells
Antigen-specific activation of naïve SP T cells involves CD4 or CD8 molecules, which along with the TCR make contact with MHC I or MHC II. The CD18−/−αβDN T cells lack CD4 and CD8, suggesting a different activation mechanism. Using BM-DC, we investigated whether enriched CD18−/−αβDN T cells could be activated by allo-MHC in vitro. The CD18−/−αβDN T cells did not proliferate (Fig. 6a), which revealed that absence of CD4 or CD8, combined with CD18 deficiency, leads to an inability to recognize allo-MHC. Analogously, CD18−/− CD8+ T cells proliferated very weakly (Fig. 6b), whereas CD18−/− CD4+ T cells revealed a higher proliferation of up to 12% (Fig. 6c). Although absence of β2 integrins substantially increases T-cell activation thresholds,38,39 CD18−/− T cells could partially overcome their defect in activation performing weak proliferation. In controls, CD18wt CD8+ and CD4+ T cells showed a strong proliferation of 70% and 40%, respectively (Fig. 6d,e).
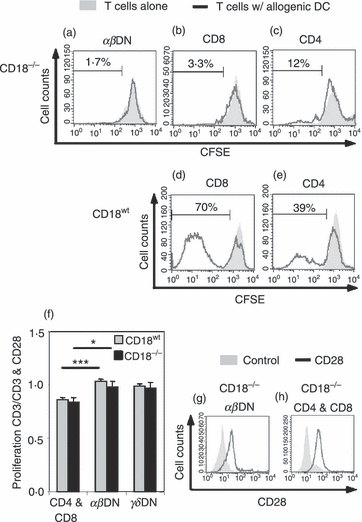
Unconventional CD18−/−αβ double-negative (DN) T cells are activated through mechanisms that deviate from conventional T cells. To investigate properties with regard to activation of CD18−/−αβDN T cells, we performed stimulation/proliferation assays using typical T-cell mitogens/stimuli. (a) Allogeneic bone marrow-derived dendritic cells (BM-DC) do not activate CD18−/−αβDN T cells. CD18−/−αβDN T cells were magnetic antibody cell-sorted, labelled with 5 μm CFSE and cultured for 6 days with previously prepared allogeneic BM-DC from BALB/c. Sorted CD18−/−αβDN T cells from 4-month-old mice were cultured alone (filled grey) or with DC at a ratio of 10 : 1 (black line) (n = 3). Control stimulations of CD18−/− and CD18wt CD8+ (b and d) and CD4+ (c and e) T cells were performed with allogeneic DC under the same conditions. (f) Full activation of αβDN T cells is independent from CD28-mediated costimulation. Total T cells from 4-month-old CD18wt (n = 4, grey bars) and CD18−/− (n = 4, black bars) peripheral lymph nodes were MACS-sorted, CFSE-labelled (5 μm) and cultured in vitro with either immobilized anti-CD3 (5 μg/ml) and anti-CD28 (2·5 μg/ml), or with immobilized anti-CD3 alone. The effect of costimulation of anti-CD28 monoclonal antibody (mAb) on proliferation of the indicated lymphocyte subsets is presented as the ratio of proliferation upon stimulation by anti-CD3 alone versus proliferation upon a combined stimulation by anti-CD3 and anti-CD28 mAb, as measured by fluorescence-activated cell sorting (FACS). A ratio of 1 indicated an equal proliferation and ratios < 1 occurred in the case that stimulation by anti-CD28 mAb further increased the anti-CD3-induced T-cell proliferation. Alternate t-test *P < 0·05, ***P < 0·001. FACS histograms show CD28 (black line) and isotype (filled grey) mAb staining of CD18−/−αβDN (g), CD4+ and CD8+ SP T cells (h). Representative data from three experiments are shown.
Full T-cell activation requires a combined signalling through TCR and costimulatory molecules. To analyse the role of CD28-mediated costimulation in CD18−/−αβDN T-cell activation, CFSE-labelled CD18wt and CD18−/−αβDN T cells were stimulated with immobilized anti-CD3 alone or with a combination of immobilized anti-CD3 and anti-CD28 mAb. Subsequently, the ratio of immobilized anti-CD3 versus immobilized anti-CD3 and anti-CD28 induced proliferation was determined. The SP T cells from CD18wt and CD18−/− mice employed as controls revealed a ratio below 1 (Fig. 6f), indicating a higher proliferation when additional CD28-mediated costimulation was provided. Interestingly, all αβDN and γδDN T cells proliferated similarly when stimulated with either immobilized anti-CD3 alone or both immobilized anti-CD3 and anti-CD28. Hence, proliferation could not be further increased by CD28 ligation as shown by ratios close to 1. To assess whether CD28 was at all expressed by the investigated DN T cells, FACS analyses were carried out but when compared with CD18−/− SP T cells, CD28 was expressed also by αβDN (Fig. 6g) and γδDN (data not shown) T cells (Fig. 6h) suggesting that the lack of costimulatory efficacy via CD28 was not caused by the absence of CD28 from cell surfaces of DN T cells. This may indicate that DN T cells from CD18−/− mice stand in line with cytotoxic cells like CD8+, TCR-γδ and NK cells using an alternative costimulation e.g. via NKGd2 to induce effector functions or proliferation.
CD18−/−αβDN T cells reveal features of NKT cells but are not mature classical NKT cells
According to our characterization, the phenotype of the unconventional T cells accumulating in pLN of CD18−/− mice that we describe is crucially defined by the absence of both CD4 and CD8 (i.e. they are DN). This particular feature, under physiological conditions, matches with either intrathymically maturing DN thymocytes or with peripheral unconventional T cells belonging to the phenotypically pleiotropic NKT cell population, only. Beyond being DN, the unconventional T cells from CD18−/− mice investigated here are not markedly increased intrathymically (data to be published elsewhere), are antigen-experienced (CD44hi CD62Llo), and recirculate to non-lymphoid organs. They resemble DN NKT cells more closely than intrathymic DN thymocytes but unlike classical (type-I) NKT cells that are normally found with highest incidences among hepatic leucocytes,12,15,30 DN T cells are not significantly increased in the livers of CD18−/− mice but accumulate within their pLN.
Therefore, we next analysed CD18−/−αβDN T cells for NKT markers such as reduced/intermediate TCR expression levels and NK1.1 defining distinct NKT subsets.30 As in CD18wt mice, CD18−/− DN T cells showed intermediate TCR expression when compared with SP T cells (Fig. 7a). However, analyses of pLN demonstrated that a significantly higher percentage of CD18−/−αβDN T cells compared to CD18wt cells expressed NK1.1 (Fig. 7b,c). The maximum value of this percentage was around 10%, showing that the majority of CD18−/− DN T cells did not belong to the NK1.1+ NKT subset. Because NK1.1+ TCR-γδ non-classical NKT cells have also been described,40γδ DN T cells from CD18−/− mice were investigated for NK1.1 expression. In contrast to the low percentages of NK1.1+ CD18wtγδ T cells, significantly more CD18−/−γδ T cells expressed NK1.1 (Fig. 7b,d) suggesting sixfold enlarged pools of non-classical NKT cells among CD18−/−γδ NKT cells.
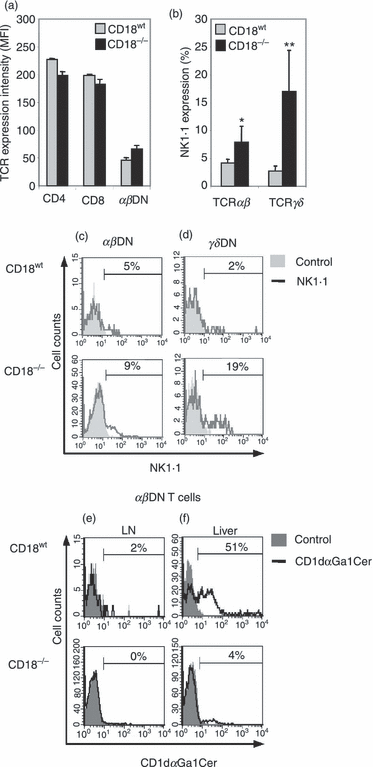
CD18−/−αβ double-negative (DN) T cells share common features with, but are different from natural killer (NK) T cells. (a) Lymphocytes from peripheral lymph node (pLN) from 2-month-old CD18wt (grey bars, n = 4) and CD18−/− mice (black bars, n = 4) were labelled with CD4, CD8, T-cell receptor (TCR) αβ monoclonal antibodies (mAbs). The intensity of TCR-αβ expression on indicated cellular subtypes was measured by fluorescence-activated cell sorting (FACS) and is given as mean fluorescent intensity (MFI) units. Besides, exclusively DN T cells were further investigated for the expression of distinct NKT cell markers by FACS-gating for TCR expression and absence of CD4 and CD8 (b–f). (b) Percentages of NK1.1-expressing cells from 2- to 4-month-old CD18wt and CD18−/−αβDN (n = 5) and γδDN T (n = 6) subsets are depicted. Significances were calculated by alternate t-test *P < 0·05, **P < 0·01. Representative histograms for NK1.1 expression by αβDN (c) and γδDN T cells (d) from CD18wt (upper row) and CD18−/− mice (lower row) are shown. Percentages represent NK1.1-expressing cells (black line), which are overlaid on control labelling with rat IgG2a (filled grey). αβDN T cells from pLN (e) of 2- to 4-month-old CD18wt (upper row, n = 4) and CD18−/− (lower row, n = 5) mice were investigated for invariant NKT-specific T-cell receptor reactivity using CD1d loaded with αGalCer dimers (black line) as compared with control staining (filled grey). αβDN T cells from livers (f) of the same mice, particularly of CD18wt mice, served as positive controls for CD1d-αGalCer dimer loading, and reflect the population of invariant NKT cells among the αβDN T cells. Percentages of dimer-reactive αβDN T cells are shown. Dot plots are representative of two experiments.
As NKT cell subsets devoid of NK1.1 expression have also been described,15,41,42 we next analysed whether CD18−/−αβDN T cells were NK1.1− type-I NKT cells that recognize αGalCer presented by CD1d. In fact, αβDN T cells from pLN (including cervical, axillary and inguinal LN) of CD18wt mice contained low percentages of CD1d-αGalCer+ classical (type-I) NKT cells, while corresponding CD18−/− cells totally lacked CD1d-αGalCer-reactive type-I NKT cells (Fig. 7e). Here it should be noted that in CD18−/− mice cervical LN were fourfold bigger and inguinal/axillary LN were sixfold smaller compared with those of WT mice. As a result, in preparations from pLN of CD18−/− mice, lymphocytes from cLN overwhelmingly outnumbered the ones from inguinal and axillary LN. Livers, which abundantly contain the whole variety of NKT subsets, were used as positive control for the stainings, revealing a significant difference in CD1d-αGalCer reactivity of 30–50% and 1·5–4% between CD18wt and CD18−/−αβDN T cells, respectively (Fig. 7f). Hence, these results even show an overall reduction in numbers of type-I NKT cells in CD18−/− mice. Interestingly, this is in line with previous findings on reduced numbers of classical (type-I) NKT cells in the livers of LFA-1-deficient (CD11a−/−) mice,31,43,44 although here only the DN subset of classical (type-I) NKT cells was investigated in CD18−/− mice.
In summary, the majority of αβDN T cells in cLN from CD18−/− mice display an NK1.1− CD1d-αGalCer− phenotype constituting a novel and unique group of unconventional T cells, which are not classical (type-I) NKT lymphocytes, even though they share typical features, such as intermediate TCR expression, antigen-experienced phenotype and recirculation to non-lymphoid organs.
Discussion
We have shown that CD18 is essential for proper lymphocyte homing to peripheral lymphoid tissues and, if absent, results in aberrant T-cell differentiation and trafficking of SP and αβDN T cells. Inversely, CD18 is not essential for the recirculation through non-lymphoid organs like liver and lungs, as CD18wt and CD18−/− naïve lymphocytes presented identical numbers. Impaired recirculation of naïve B and T cells to pLN resulted in an anticipated decrease in size and cellularity occurring in axillary and inguinal LN. Unexpectedly, cLN were fourfold enlarged in cellularity as the result of an increase in B cells, NK cells and αβDN and γδDN T cells. The increase in B cells was not surprising because IL-6, which is responsible for the expansion of B cells, is elevated,10 and in addition B cells/plasma cells are known to be retained in cLN of CD18−/− mice.35 However, we excluded the possibility that CD3+ CD4− CD8− cells are plasmacytoid cells or plasmacytoid DC because they stained CD19−B220− CD138Ig− (data not shown), and were TCR-αβ+ or TCR-γδ+. Also, CD18−/− DN T cells were not neoplastically expanding cells because both subsets always occurred in parallel. In large numbers of mice studied up to now, TCR-αβ+ and TCR-γδ+ DN T cells never outgrew one another. Despite the considerable lymphadenopathy of cLN,7,35 histopathological analyses of cLN from CD18−/− mice neither showed an infiltrating malignant growth of lymphoid cells (n > 10),7,10 nor did CD18−/− mice succumb to leukaemia or lymphoma.7,10
Our data strongly indicate that the increase in unconventional DN T cells is owed to a reactive expansion in cLN of CD18−/− mice. First, BrdU experiments indicated extensive in situ expansion of unconventional DN T cells in cLN. Second, CD18−/− DN T cells were highly sensitive to IL-2-induced proliferation, showing features of antigen-experienced lymphocytes. Third, our data revealed no alterations or delay in apoptosis of CD18−/− DN T cells (data not shown). Fourth, adoptive lymphocyte transfer experiments did not support a potential accumulation because of an increased homing of CD18−/− DN T cells to cLN, as for instance could have been mediated by alternative adhesion molecules.3,4,45,46 However, in contrast, we currently cannot exclude that CD18 deficiency may contribute to the retention and, thereby, accumulation of unconventional DN T cells in cLN. Interestingly, in collaboration with Pabst et al.,35 we were able to demonstrate that a marked accumulation of plasma cells occurred in cLN of CD18−/− mice. Intercellular adhesion molecule-1, the main ligand of the β2 integrins, was found abundantly expressed in the medullary cords of the cLN and for this reason the absence of β2 integrins on plasma cells is likely to be the principal mechanism for the accumulation of these cells within the cLN of CD18−/− mice.
Adoptive transfers demonstrated that CD18−/− DN T cells were unable to (re-)enter into cLN on the one hand, and BrdU labelling revealed that the massive increase of DN T cells within the cLN of CD18−/− most likely occurred as the result of primary expansion within the cLN, so it seems likely that one major cause for the marked increase of CD18−/− DN T cells in cLN was the oral ulcers often observed in CD18−/− mice.7,35 These could enhance antigenic stimulation and inflammation in the draining cLN, generating a cytokine environment which may then perpetuate expansion of unconventional DN T cells. Nevertheless, it remains elusive how far this process may depend on the presence of cognate (or ‘invariant’) antigen.
In absence of a specific antigen, homeostatic lymphocyte proliferation has been found to give rise to ‘antigen experienced-like’ lymphocytes and other ‘unconventional’ subpopulations, among them DN T cells.47–49 As shown herein, CD18−/− mice present a phenotype similar to antigen-experienced cells. Remarkably, initial data revealed a high susceptibility of CD18−/− DN T cells to be maintained long-term with and/or proliferate upon key homeostatic cytokines such as IL-7 and IL-15 (data to be published elsewhere). Hence, homeostatic expansion may be another mechanism contributing to the observed increase of CD18−/− DN T cells, induced by the markedly reduced numbers of naïve lymphocytes in the periphery of CD18−/− mice.
The occurrence of αβDN T cells was earlier described in several murine models of disease.50 The lpr and gld mouse models, which carry mutations affecting apoptosis signalling pathways, are characterized by the accumulation of refractory regulatory B220+αβDN T cells.24,26,50 Typically, lymphocytes in anergy can maintain peripheral tolerance by the suppression of proliferation of other lymphocytes in vivo.51 Their irresponsiveness may be reversed by exogenous IL-2,52 as also here observed for CD18−/−αβDN T cells. In contrast to the anergic B220+αβDN T cells from lpr and gld mice, the αβDN T cells from CD18−/− mice herein investigated were B220− and not anergic. This was demonstrated by their vigorous response upon polyclonal activation by immobilized anti-CD3. Although not anergic, we further investigated whether CD18−/−αβDN T cells nevertheless had a suppressive effect on proliferating CD18wt SP responder T cells in vitro. But CD18−/−αβDN T cells did not exert any suppressive function. Moreover, the absence of Foxp3 and TGF-β expression supported that CD18−/−αβDN T cells were not untypical regulatory T cells.
To characterize the properties of unconventional T cells in immune responses, we used classical stimulation assays employing (i) allogeneic BM-DC, and (ii) polyclonal stimulation with immobilized anti-CD3 and anti-CD28 mAb primarily acting independently of cellular adhesion. Similar to CD18−/− SP T cells, no proliferation of purified CD18−/−αβDN T cells was induced when cultured with allogeneic BM-DC. These data showed that CD18 was required for SP T cells to lower the activation thresholds. As a result of the paucity of DN T cells in CD18wt mice along with the relatively high amount of these cells required for mixed lymphocyte reactions,7 we could not fully clarify the contribution of CD18 for αβDN T-cell activation.
The discrepancy of activated CD18−/− DN T cells being unable to proliferate upon allogeneic BM-DC prompted us to investigate their activation mechanisms as compared with conventional SP T cells.21,53 As only antigen-experienced T cells can proliferate upon CD3 stimulation alone,36,54 we investigated the dependence of these cells on CD28-mediated costimulation. Consistently, we detected a strong TCR-mediated response with more than 80% proliferating CD18wt and CD18−/− DN T cells after stimulation with immobilized anti-CD3 mAb. This response was unaffected by the signalling through CD28, although expressed by CD18−/−αβDN T cells. We demonstrated that CD18wt and CD18−/− DN T cells can be expanded independently of CD28, hence sharing analogous activation mechanisms with γδDN T cells.55 The ability to recognize unprocessed soluble antigen without CD4 or CD8 coreceptors53 and without CD2855 but still requiring cell–cell interaction demonstrates that the activation threshold of CD18−/−γδDN T cells is not modulated by the same receptors as in SP T cells. In summary, CD18−/−αβDN and γδDN T cells demonstrated an antigen-experienced behaviour showing activation mechanisms mainly controlled by TCR signalling and differing distinctly from naïve CD4+ and CD8+ T cells.
Analysis of cytokines secreted from CD18−/− DN T cells after immobilized CD3 and CD28 stimuli in vitro did not reveal a clear-cut role of these cells in the immune response in the strict understanding from conventional T cells. Apart from their ability to produce the central pro-inflammatory cytokine tumour necrosis factor-α, CD18−/−αβDN T cells showed a profound deficiency in secreting T help type 1 (Th1; interferon-γ, IL-2) and Th2 (IL-4, IL-5) cytokines (data not shown). In this regard, the impaired IL-2 secretion from CD18−/−αβDN T cells was analogous to that of CD18−/− CD4+ and CD8+ T cells11,56 while absence of interferon-γ and IL-4 production distinguished them from other unconventional T cells such as classical NKT or γδDN T cells. Nevertheless, CD18-deficient αβDN and γδDN T cells are able to produce IL-17, outlining an important role in granulocyte homeostasis57 and potentially also in the pathogenesis of CD18-dependent chronic inflammation and autoimmunity.56,58
Acquisition of CD44 and shedding of CD62L identifies distinct states of T-cell activation. During transition from the naïve to the antigen-experienced state, T cells replace the rolling-mediating CD62L by the rolling receptor CD44 and the recirculation from pLN changes to places of inflammation and non-lymphoid organs.45 We found that CD18−/−αβDN and γδDN T cells were mostly antigen-experienced showing a CD44hi CD62Llo expression pattern. By contrast, CD18−/− antigen-experienced DN T cells showed a marked accumulation in non-lymphoid organs (liver, lungs), not observed for CD18−/− SP T-cell subsets. Interestingly, liver is known to be most abundantly populated by all NKT subsets,12,13 whereas NKT cells rarely reside in pLN.59 However, although CD18−/− DN T cells preferentially recirculated to the liver and lungs of CD18wt recipient mice after adoptive transfers, BrdU incorporation was not found to be significantly increased in DN T lymphocytes from lungs and livers of CD18−/− mice (data not shown), demonstrating that DN T cells were able to migrate selectively into these organs, but were not primarily generated there. But BrdU staining was highly increased in DN T cells detected in the cLN of these mice implying that these cells are generated, or at least expanded, in the cLN and not in the liver. This is one feature that makes these cells, although ‘NKT-like’ in phenotype, distinctly different from classical NKT cells.
In their detailed work, the group of Emoto et al.43,44,60 investigated the role of CD11a/CD18 (LFA-1) for distinct NKT cell subsets using CD11a−/− mice. They found that an absence of LFA-1 resulted in a reduction of NKT cell subsets in livers of CD11a−/− mice.43,44 Similar findings may be expected also for livers of CD18−/− mice. In the absence of CD18, necessarily leading to a lack also of LFA-1, it is possible that NKT cells are reduced in the livers of CD18−/− mice. However, invariant NKT cells and other previously defined NKT cell subsets have so far not been investigated in detail in CD18−/− mice.
Apart from a preferential recirculation to non-lymphoid organs, our findings of a pronounced IL-2 responsiveness along with a TCRintCD44hi CD62Llo phenotype revealed that CD18−/−αβDN T cells share other similarities with NKT cells.41 In accordance with our results, Stark et al.57 also reported on the presence of the herein characterized NK1.1−αβDN T cells in CD18−/− C57BL/6 mice, but claimed that these cells were NKT cells. Indeed, because NK1.1 is expressed only by some NKT cells and NK1.1− NKT cells also exist,61 NK1.1−αβDN T cells found in CD18−/− mice do not per se disqualify as NKT cells.41,62 However, we have demonstrated for the first time that αβDN T cells from CD18−/− mice do not bind CD1d-αGalCer+ dimers clearly proving that these cells are not classical (type-I) NKT cells. In this regard, our data are novel and distinctly contrast with former studies.57 However, we cannot currently exclude the possibility that CD18−/−αβDN T cells are CD1d-restricted but αGalCer non-reactive non-classical (type-II) NKT cells,16,17,63 but further experiments are underway. Most interestingly, only very recently, Coquet et al. identified an immature NKT cell subset with an NK1.1− CD4− (CD8−) phenotype, a phenotype identical to the DN T cells reported here, which were shown to be precursors of the CD4− NK1.1+ NKT lineage.64 Additionally, most of these cells presented with a CD44hi phenotype and were able to produce IL-17 at high levels,64 findings made also for the here underlying DN T cells in CD18−/− mice (Fig. 4).57 Therefore, it remains to be seen whether the unconventional DN T cells accumulating in CD18−/− mice can also be assigned to this specific NKT cell lineage in the future.
We suggest that CD18−/−αβDN T cells represent either (i) an expanded population of unconventional NKT phenotypic T cells physiologically existing also in wild-type mice at dramatically lower numbers, but which are not mature classical (type-I) NKT cells; or (ii) a unique population of T cells occurring as the result of CD18 deficiency. Importantly, the existence of highly conserved canonical αβDN T cells in humans and mice was reported. These cells show a memory phenotype in humans.22 In either case, CD18−/−αβDN T cells most likely stand in line with γδ+ T23 and NKT cells.65 Showing a restricted repertoire selected by autoantigens and able to recognize evolutionarily conserved ligands, unconventional T cells are physiologically endowed with the unique role of linking innate and adaptive immunity.16,20,23,65
Interestingly, unconventional T cells, which have restricted TCR repertoires and are evolutionarily more ancient, recognize phylogenetically conserved high-density antigens22 even in the absence of accessory molecules.66 However, recognition of protein antigen involves coreceptors and costimulatory and adhesion molecules, which, in combination, modulate TCR-mediated signals in the control of the immune response. Similarly, antigen-experienced CD18−/−αβDN T cells, probably constituting an expanded unconventional T-cell subset, might recognize high-density ligands independently of coreceptors and CD18 adhesion molecules.
We show that the absence of CD18 prevents naïve T cells from locating to pLN where T cells encounter antigen-presenting cells; and effective antigen-presenting cell–T-cell interaction is required for the development of immune responses. Relative unresponsiveness of the adaptive immunity in the absence of CD187,38,67 is paralleled by a concomitant increase of αβDN T cells. This increase is the result of a sustained in situ expansion of these cells in vivo rather than of a selective recirculation or decrease in apoptosis. Interestingly, these cells largely share phenotypic and functional similarities not only with effector/memory CD4+ and CD8+ T cells, but also with NKT and γδ T cells. Although they share key features particularly with NKT cells, they are not classical NKT cells. But combining traits of innate and adaptive immunity, CD18−/−αβDN T cells may regulate the immune response like NKT and γδ T cells do. We propose that, alternatively, CD18−/−αβDN T cells may be antigen-experienced lymphocytes with less stringent requirements for cell activation replacing the impaired classical adaptive immunity in CD18−/− mice. Spontaneously enriched in the CD18−/− mouse model, αβDN T cells are most suitable to investigate the relationship between CD18 expression and their development and function. Collectively, β2 integrins serve to maintain physiological levels of lymphocyte subsets in the periphery. An impaired recirculation of CD18−/− naïve B-cell and T-cell subsets to pLN may hence contribute to the increased susceptibility for infection as the result of immunodeficiency observed in CD18−/− mice7,10,68 as well as leucocyte adhesion deficiency-1 patients.5,67
Acknowledgements
This work was supported by the Theodor Nasemann Scholarship (T.P.); and by grants from SFB497-C7 ‘Signals and Signal Processing during Cellular Differentiation’, Ulm University, from the Fritz Thyssen Stiftung (AZ. 10.02.2.134), and the DFG grants 411/12-1 (K.S.-K.). The authors thank Dr Jörg Reimann (Department of Medicine, Microbiology Section, Ulm University, Ulm, Germany) for his helpful advice, and for critically reading this manuscript as well as for providing CD1d-αGalCer–PE dimers.




