Regulation of triggering receptor expressed on myeloid cells 1 expression on mouse inflammatory monocytes
Summary
Triggering receptor expressed on myeloid cells 1 (TREM-1) is an activating receptor involved in inflammatory diseases and septic shock. The TREM-1 ligand(s) (TREM-1L) have not yet been identified. In this study, we performed a detailed analysis of the expression of mouse TREM-1 and its ligand(s). Our results demonstrate that TREM-1 is expressed on bone-marrow-derived dendritic cells (BMDC). On bone-marrow-derived macrophages (BMM) its expression is induced in vitro after stimulation by granulocyte–macrophage colony-stimulating factor, interleukin-3 or by myeloid differentiation primary response gene 88 (MyD88)-dependent Toll-like receptor (TLR) ligands. Under steady-state conditions mouse TREM-1 is detectable on a Gr-1− F4/80+ monocyte subpopulation bearing markers of resident monocytes, but not on Gr-1+ F4/80+ inflammatory monocytes. During lipopolysaccharide (LPS)-induced endotoxaemia TREM-1 was also up-regulated on inflammatory Gr-1+ F4/80+ cells in vivo. In tumour-bearing mice, TREM-1 was up-regulated on Gr-1+ F4/80+ monocytes, which phenotypically and functionally resembled mononuclear myeloid-derived suppressor cells. Using a soluble TREM-1 fusion protein, we demonstrate that after intravenous injection of LPS TREM-1L was induced on Gr-1+ granulocytes and monocytes but not on other cell populations in peripheral blood. This up-regulation on granulocytes was directly mediated by TLR ligands and required the adapter protein MyD88. In contrast to human, mouse platelets expressed TREM-1L neither under steady-state conditions nor after LPS injection in vivo. Our study reveals differential regulation of TREM-1 expression on mouse monocyte subpopulations and improves our understanding of the biological role of TREM-1 during disease.
Introduction
The innate immune system serves as a first line of defence during infectious and malignant diseases. The activity of its effector cells is controlled by multiple receptors including members of the immunoglobulin superfamily. The activating ‘triggering receptor expressed on myeloid cells-1’ (TREM-1) is expressed by neutrophils and the ‘classical’ CD14high monocyte subset in human peripheral blood.1 Stimulation of these cells with bacterial or fungal products results in an up-regulation of TREM-1.2–4
In mice, TREM-1 cell surface expression and messenger RNA transcripts are detected in peripheral neutrophils and monocytes/macrophages originating from spleen, peritoneum and liver.2,5 Furthermore, TREM-1 expression is up-regulated on monocytes/macrophages and neutrophils in the peritoneum, in peripheral blood and on splenic macrophages during septic shock.2,5 Moreover, an increased TREM-1 expression was described on peritoneal macrophages in gout6 and on monocytes from blood or in bronchoalveolar lavage fluid in melioidosis.7 However, detailed knowledge of TREM-1 expression on mouse monocyte subpopulations and its regulation is missing.
In mouse sepsis models, peritoneal injection of a recombinant soluble TREM-1 fusion protein or LP17, a 17-mer peptide derived of a conserved region in the extracellular part of TREM-1, attenuates the inflammatory response and improves host survival.2,8,9 To date, the TREM-1 ligand(s) (TREM-1L) has not been identified. Nevertheless, foreign10 or endogenous11 molecules were suggested as ligand(s) for TREM-1. By staining with soluble TREM-1 fusion proteins, TREM-1L was detected on the cell surface of resting human platelets12 and on granulocytes in a mouse model of experimental peritonitis.13 Granulocytes, monocytes and macrophages are among the most prominent cell populations mediating inflammatory immune responses,14 so it is of major importance to gain a detailed knowledge of TREM-1 and TREM-1L expression and their regulation on these cell populations.
Two F4/80+ monocyte subpopulations were described in mouse blood that show similarities to the CD14high CD16− and CD14+ CD16+ subpopulations in humans.15,16 One population expresses CD62L (l-selectin), low levels of CX3CR1 (fractalkine receptor) and high levels of Gr-1. As a result of their rapid recruitment to areas of inflamed tissues, this mouse subpopulation is named ‘inflammatory’ monocytes (MoIF). The other subpopulation is characterized by high levels of CX3CR1, low levels of Gr-1 and no expression of CD62L. This CX3CR1high CD62L−Gr-1low subset persists longer in blood and normal tissues and is called ‘resident’ monocytes (MoRT). In tumour-bearing mice and during certain infectious diseases an accumulation of a population of immature myeloid cells expressing Gr-1 and CD11b was described.17 These cells potently inhibit T-cell proliferation so they are called myeloid-derived suppressor cells (MDSC). Distinct subpopulations of MDSC, the Gr-1+ CD11b+ F4/80+ mononuclear MDSC (Mo-MDSC) and the low-density Ly6G+ polymorphonuclear MDSC (PMN-MDSC) have been characterized.18
The aim of this study was to determine the distribution of TREM-1 expression on myeloid cells generated in vitro from bone marrow cells and different monocyte subsets in the peripheral blood of mice under steady-state conditions, during inflammation and cancer in vivo. In this study, we report the modulation of TREM-1 expression on bone-marrow-derived macrophages by granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3) and certain Toll-like receptor (TLR) ligands in vitro as well as on distinct monocyte subsets during inflammation and in tumour-bearing mice in vivo. In addition, our data demonstrate that in mice TREM-1L was detectable on granulocytes, but not on platelets or any other peripheral blood cell population during lipopolysaccharide (LPS)-induced endotoxaemia.
Materials and methods
Mice
C57BL/6 mice were purchased from Charles River Laboratories (Sulzfeld, Germany). Myeloid differentiation primary response gene 88 (MyD88) -deficient mice were kindly provided by Professor Akira, Osaka, Japan. Mice were housed under specific pathogen-free conditions and in concordance with all standards of animal care. Experiments were performed with age-matched female mice at the age of 6–15 weeks. All animal experiments were approved by the appropriate governmental authorities.
Cell lines, cell preparation and flow cytometry
Peripheral blood leucocytes were collected via the orbital vein into heparin-containing tubes. Erythrocytes were removed by cell lysis in BD FACS™ lysing solution (BD Biosciences, Heidelberg, Germany). To obtain purified Gr-1+ or Ly6G+ granulocytes and CD11b+ monocytes, a magnetic antibody cell sorting-based method was applied. Erythrocytes were lysed with heparin-containing ammonium chloride buffer and granulocytes were enriched using an anti-Ly6G isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). CD11b+ monocytes were purified from Ly6G-depleted blood cells using an anti-CD11b isolation kit (Miltenyi Biotec). Enriched granulocytes and enriched CD11b+ cells were obtained at a purity of > 98% and > 80%, respectively.
The promyelocytic mouse cell line MPRO was cultured in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) medium containing 10% fetal calf serum (FCS), 2 mm l-glutamine, 100 U/ml penicillin/streptomycin (Invitrogen, Karlsruhe, Germany) supplemented with 10% GM-CSF containing supernatant harvested from cell line X6310-GM-CSF (kindly provided by Professor Thomas Decker, Vienna, Austria).
Cultured cells and blood cells were stained for flow cytometry in fluorescence-activated cell sorting (FACS) buffer [1× phosphate-buffred saline (PBS), 1% bovine serum albumin, 0·02% sodium aside]. Fc receptors were blocked by incubation with 10% FCS for 10 min at room temperature.
Monoclonal antibodies
For the characterization of different cell types by flow cytometry the following anti-mouse antibodies were used (all purchased from BD Bioscience): allophycocyanin-conjugated anti-Gr-1 (clone RB6-8C5), allophycocyanin-conjugated anti-CD11b (clone M1/70), fluorescein isothiocyanate-conjugated anti-CD11c (clone HL3), phycoerythrin (PE)-conjugated anti-CD86 (clone GL1), PE-conjugated anti-CD124 (clone mIL4R-M1) and peridinin chlorophyll protein-Cy5.5-conjugated anti-CD45.2 (clone 104). The PE-conjugated anti-CD115 (clone AFS98), biotin-conjugated anti-CD62L (clone MEL14) and polyclonal anti-CX3CR1 were obtained from eBioscience (Frankfurt, Germany), Alexa488-conjugated anti-F4/80 (clone BM8) was from Caltag (Karlsruhe, Germany), fluorescein isothiocyanate-conjugated anti-CD41 (clone MWreg30) was from AbD Serotec (Oxford, UK) and PE-conjugated anti-TREM-1 (clone 174031) was from R&D Systems (Wiesbaden, Germany).
Generation of bone-marrow-derived macrophages (BMM) and bone-marrow-derived dendritic cells (BMDC)
The BM cells were isolated from femurs and tibiae of the hind legs and washed once with ice-cold DMEM. For the generation of BMM or BMDC, BM cells were cultured overnight in DMEM medium with 10% FCS, 2mm l-glutamine, 100 U/ml penicillin/streptomycin (Invitrogen) and 10% M-CSF containing supernatant harvested from the cell line L929-MCSF (kindly provided by Professor Rainer Zawatzky, Heidelberg, Germany) or 10% GM-CSF containing supernatant harvested from the cell line X6310-GM-CSF, respectively. Adherent cells were further differentiated in the appropriate media for 5 days.
LPS-induced endotoxaemia
C57BL/6 mice were injected intravenously (i.v.) with 100 μg LPS (Escherichia coli serotype 0111:B4; Sigma-Aldrich, Munich, Germany) or 100 μl PBS (Invitrogen) and peripheral blood cells were analysed by flow cytometry at the indicated time-points.
Tumour model
Major histocompatibility complex (MHC) class I deficient RMA-S lymphoma cells19 were cultured in complete RPMI-1640 medium, containing 10% FCS, 1%l-glutamine, 1% penicillin/streptomycin (Invitrogen). For tumour cell injection, cells were washed at least three times with PBS and 106 cells were injected in 100 μl PBS (Invitrogen) into the shaved left flank of C57BL/6 mice. Tumour volume was monitored with a calliper measuring along the perpendicular axes of the tumours and expressed as the product of the three diameters.
Cell stimulation and cytokine measurement
Cells were stimulated in vitro for the indicated time intervals either on plates pre-coated with anti-TREM-1 monoclonal antibody (mAb; 10 μg/ml) or isotype control, in combination with LPS (1 μg/ml), with recombinant mouse GM-CSF (10 ng/ml; PeproTech, London, UK) or recombinant mouse IL-3 (1 ng/ml; PeproTech), or with the following TLR agonists: LPS (1 μg/ml, E. coli serotype 0111:B4; Sigma-Aldrich), poly(I : C) (1 μg/ml; Invivogen, Toulouse, France), Pam3Cys (5 μg/ml; Invivogen), recombinant flagellin (100 ng/ml; Invivogen) and imiquimod (10 μg/ml; kindly provided by Professor Klaus Heeg and Professor Alexander Dalpke, Heidelberg, Germany). The GM-CSF was neutralized by the addition of anti-GM-CSF mAb (25 μg/ml; Thermo Fisher Scientific, Schwerte, Germany) to the supernatant of stimulated BMM.
Supernatants were harvested at 16 hr and tumour necrosis factor-α (TNF-α) levels were measured as triplicates by enzyme-linked immunsorbent assay (ELISA; R&D Systems) following the manufacturer’s instructions.
Proliferation assay
The FACS-sorted Gr-1+ F4/80+ and Gr-1+ F4/80− cell populations were cocultured with 1 × 105 splenocytes from naïve C57BL/6 mice with concanavalin A (2 μg/ml, Sigma, Germany). Cells were cultured for 72 hr and 1 μCi [3H]thymidine (GE Healthcare, Munich, Germany) was added for the final 18 hr of culture. Cells were harvested, and [3H]thymidine incorporation was measured with a MicroBeta TriLux Counter (Perkin Elmer, Rodgau-Juegesheim, Germany).
Production of fusion proteins and detection of TREM-1 ligand(s)
The fusion proteins consist of the ectodomains of TREM-1 (amino acids 21–198) or H60 (amino acids 29–223) and a human immunoglobulin G (IgG) Fc mutein. The human IgG Fc mutein harbours three point mutations (L234A, L235E and G237A) by which the binding to Fc-receptors is reduced.20 The mouse TREM-1-human IgG fusion protein (mTREM-1-Ig-FP) and mouse H60-human IgG fusion protein (H60-Ig-FP) were produced by transfection of 293T cells with pcDNA3.1 vectors encoding the fusion proteins coupled to a TR2 leader sequence for secretion of the proteins.
Proteins were precipitated by the addition of saturated ammonium sulphate to supernatant of transfected 293T cells. The protein pellet was resuspended in 1× Tris–HCl buffer (pH 7·0) + 10% glycerol and proteins were purified by affinity chromatography over HiTRAP ProteinG–sepharose columns (Amersham) following the manufacturer’s instructions.
For detection of TREM-1L with mTREM-1-Ig-FP, the Fc receptors of peripheral blood leucocytes depleted of erythrocytes or purified granulocytes were blocked with 10% FCS for 10 min at room temperature. Afterwards, cells were incubated with either 10 μg mTREM-1-Ig-FP or H60-Ig-FP as a control, followed by staining using PE-conjugated anti-hIgG-PE F(ab′)2 antibodies (Jackson ImmunoResearch, Suffolk, UK) and analysed by flow cytometry.
Statistical analysis
Statistical significance was calculated by unpaired Student’s t-test. Differences were considered significant when P < 0·05.
Results
TREM-1 is expressed on a subpopulation of mouse blood monocytes
To determine TREM-1 expression on monocyte subsets in mouse peripheral blood, blood from C57BL/6 mice was stained with mAbs directed against F4/80 and Gr-1 (Ly6G/C) and analysed by flow cytometry. In concordance with a previous report,2 Gr-1+ F4/80− granulocytes expressed high levels of TREM-1 (Fig. 1a). In addition, our data reveal that F4/80+ blood monocytes are subdivided into two subpopulations: Gr-1− monocytes with high cell surface expression of TREM-1 and Gr-1+ monocytes, which did not express TREM-1. For further characterization, blood monocytes were analysed for the expression of markers defining MoRT and MoIF.16 TREM-1+ F4/80+ cells expressed CD11b (Mac-1) and CX3CR1, but not CD62L, indicating that they belong to the MoRT subset (Fig. 1b). In contrast, TREM-1−F4/80+ expressed CD11b and CD62L, but not CX3CR1 that were described to be markers of the MoIF subset. Our data indicate that in mouse blood under steady-state conditions TREM-1 is expressed on F4/80+ Gr-1− MoRT and on Gr-1+ granulocytes.
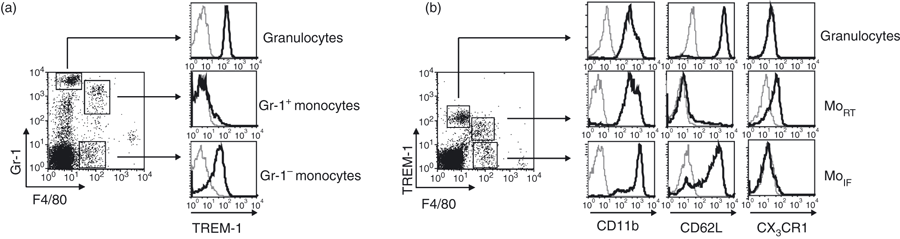
TREM-1 expression on mouse monocyte subsets. (a) Blood of naïve C57BL/6 mice was stained with monoclonal antibodies (mAbs) directed against F4/80, Gr-1 (Ly6C/6G) and TREM-1 and cells were analysed by flow cytometry. Histograms depict expression of TREM-1 (black lines) and staining with the isotype-matched controls (grey lines) gated on the indicated populations. (b) Granulocytes and monocytes were further characterized by staining with mAbs directed against F4/80 and TREM-1 in combination with mAbs directed against CD11b, CD62L and CX3CR1, respectively. Histograms depict expression of CD11b, CD62L and CX3CR1 (black lines) and staining with the isotype-matched controls (grey lines) gated on the indicated populations. Results are representative for five independent experiments.
TREM-1 expression is inducible by GM-CSF and IL-3 in vitro
Next, we investigated whether myeloid cells generated in vitro from bone marrow cells also expressed TREM-1. F4/80+ BMM did not express TREM-1. In contrast, on CD11c+ BMDC, expression of TREM-1 was detected (Fig. 2a). Therefore, we asked the question, whether the expression of TREM-1 on BMDC is induced by the GM-CSF that was used for the differentiation of bone marrow cells to BMDC. We stimulated BMM that were differentiated for 5 days in M-CSF-containing media with recombinant GM-CSF for 16 hr and analysed the cell surface expression of TREM-1 by flow cytometry. TREM-1 expression was induced by GM-CSF on BMM in a concentration-dependent manner (Fig. 2b, upper panel). Notably, the expression of CD11c, a cell surface marker for dendritic cells, was not detectable on these cells, indicating that these cells where not differentiated to dendritic cells (data not shown). Because the common beta chain of the GM-CSF receptor (CD131) also transmits signals of other cytokine receptors, such as IL-3 (reviewed in refs 21, 22), we tested, whether IL-3 up-regulated TREM-1 expression. Interleukin-3 also induced the expression of TREM-1 on BMM although to a lesser extent (Fig. 2b, lower panel). Other cytokines including IL-5, which also uses CD131 for signalling, or interferon-α (IFN-α), IFN-β, IFN-γ, and TNF-α did not up-regulate TREM-1 expression on BMM (data not shown).
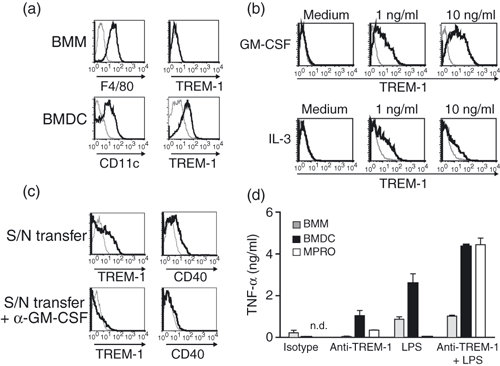
TREM-1 is up-regulated on bone-marrow-derived macrophages (BMM) after in vitro stimulation of the granulocyte–macrophage colony-stimulating factor (GM-CSF) receptor. (a) BMM (upper panel) were differentiated for 5 days in M-CSF containing media and stained with monoclonal antibodies (mAbs) directed against F4/80 (left panel) or TREM-1 (right panel). Bone-marrow-derived dendritic cells (BMDC; lower panel) were differentiated for 5 days in media containing GM-CSF and stained with mAbs directed against CD11c (left panel) or TREM-1 (right panel). (b) BMM were stimulated with different amounts of recombinant mouse GM-CSF (upper panel) or recombinant mouse interleukin-3 (IL-3; lower panel) for 16 hr and analysed for TREM-1 expression by flow cytometry. (c) Supernatant (S/N) of BMM stimulated with 10 ng/ml GM-CSF for 16 hr were transferred to naïve BMM in the absence (upper panel) or presence (lower panel) of neutralizing anti-GM-CSF mAb and TREM-1 (left panel) or CD40 (right panel) expression was analysed by flow cytometry. (d) BMM (grey bars) and BMDC (black bars) generated from C57BL/6 mice or MPRO cell line (white bars) were stimulated with plate-bound isotype-matched mAbs or anti-TREM-1 mAbs in the absence or presence of lipopolysaccharide for 16 hr. Supernatants were harvested and analysed for tumour necrosis factor-α (TNF-α) by enzyme-linked immunosorbent assay. (a–c) Histograms depict bone-marrow-derived dendritic cells (BMDC) (a) and bone-marrow-derived macrophages (BMM) (a–c) stained with specific mAbs (black lines) or corresponding isotype controls (grey lines). (d) Data show mean ± SEM of triplicate cultures. n.d. = not detectable. Results are representative of three independent experiments.
To test whether the up-regulation of TREM-1 was a direct effect after stimulation of the GM-CSFR or an indirect effect by the autocrine production of an additional factor, we transferred the supernatant of BMM after stimulation with GM-CSF for 16 hr to unstimulated BMM in the absence or presence of a neutralizing anti-GM-CSF mAb and analysed the cell surface expression of TREM-1. Upon supernatant transfer, up-regulated TREM-1 expression was only observed in the absence of the neutralizing mAb (Fig. 2c). These data suggest that TREM-1 is directly induced by GM-CSF in vitro.
Engagement of TREM-1 on BMDC, but not on blood granulocytes or monocytes, results in production of TNF-α
In a further step, we investigated whether triggering of myeloid cells via TREM-1 using an agonistic mAb resulted in the release of inflammatory cytokines. The BMDC, BMM and the promyelocytic mouse cell line MPRO that is cultured in GM-CSF and expresses TREM-1 (data not shown) were stimulated via TREM-1. Secretion of TNF-α was detectable after TREM-1 ligation of BMDC and MPRO cells (Fig. 2d). Furthermore, we observed highly increased levels of TNF-α released from BMDC and MPRO cells, when TREM-1 was engaged in the presence of LPS. As expected, TNF-α was not produced upon TREM-1 cross-linking by BMM that did not express TREM-1 (Fig. 2d). In addition, the combination of LPS and anti-TREM-1 mAb induced similar amounts of TNF-α compared with LPS alone (Fig. 2d). Notably, we neither detected TNF-α production by freshly isolated CD11b+ Ly6G− monocytes nor by Ly6G+ granulocytes after cross-linking of TREM-1 alone or in the presence of LPS (data not shown). Similar results were obtained when different time intervals (2–48 hr) of stimulation or the production of other pro-inflammatory mediators, including macrophage inflammatory protein (MIP-1α; CCL3) and MIP-2 (CXCL1), were analysed (data not shown). Our data suggest that TREM-1 triggering induces the release of pro-inflammatory cytokines in vitro by BMDC but not by TREM-1 expressing monocytes and granulocytes.
TREM-1 is up-regulated on BMM in vitro or MoIFin vivo after stimulation with MyD88-dependent TLR agonists
The induction of TREM-1 on primary monocytes and macrophages by microbial products was reported previously in mouse and human.2,3,23,24 We further investigated how TREM-1 expression is regulated on mouse bone marrow-derived macrophages by different TLR ligands. TREM-1 was up-regulated on BMM after stimulation with MyD88-dependent TLR agonists, such as LPS and R848 (Fig. 3a). In contrast, poly(I:C), a ligand of the MyD88-independent TLR3, did not induce TREM-1 up-regulation. The costimulatory molecule CD40 was up-regulated after stimulation with TLR agonists indicating that cells became activated and that all ligands used in the assay were functional. To test whether the adapter protein MyD88 was required for TREM-1 up-regulation, BMM from MyD88-knockout mice were stimulated with LPS and the expression of TREM-1 was analysed by flow cytometry. BMM from MyD88-knockout mice did not up-regulate TREM-1 after stimulation with LPS (Fig. 3b). In contrast, an induction of CD40 that does not require MyD88 was observed indicating that BMM from MyD88-knockout mice became activated. Our data indicate that induction of TREM-1 after TLR signalling is MyD88-dependent. In a next step, we investigated the modulation of TREM-1 cell surface expression on mouse blood monocytes in the context of acute inflammation in vivo. Systemic endotoxaemia was induced by administration of LPS i.v. and peripheral blood leucocytes were analysed by flow cytometry after 4 hr. After LPS injection, higher percentages of Gr-1+ MoIF among F4/80+ blood monocytes were detectable compared with the PBS control (Fig. 3c). Of note, Gr-1− MoRT almost disappeared from mouse blood. In addition, similar data were obtained by the analysis of total cell numbers (data not shown). When we analysed MoIF 1 hr after the LPS injection, we observed an up-regulation of TREM-1 surface expression (Fig. 3d, upper panel). Up-regulated TREM-1 expression persisted as long as 24 hr after LPS administration (data not shown). To exclude that these cells were MoRT that had up-regulated Gr-1, we stained F4/80+ Gr-1+ monocytes for CD62L, a marker reported to be expressed on MoIF (Fig. 1b).16 Indeed, F4/80+ Gr-1+ monocytes expressed high levels of CD62L indicating that MoIF up-regulated TREM-1 after LPS injection (Fig. 3d, lower panel). In conclusion, certain inflammatory conditions result in TREM-1 up-regulation on the inflammatory monocyte subpopulation accompanied by a change of ratio of blood monocyte subsets in favour of TREM-1+ F4/80+ Gr-1+ monocytes.
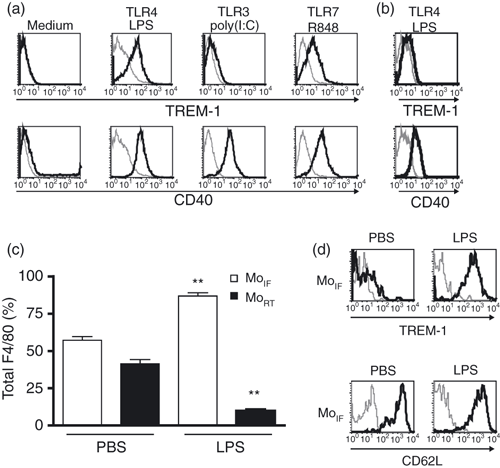
TREM-1 is up-regulated on bone-marrow-derived macrophages (BMM) in vitro or inflammatory monocytes (MoIF) in vivo after stimulation with myeloid differentiation primary response gene 88 (MyD88)-dependent Toll-like receptor (TLR) agonists. (a) Bone-marrow-derived macrophages (BMM) of C57BL/6 mice were stimulated for 16 hr with indicated TLR agonists and analysed for TREM-1 (upper panel) or CD40 (lower panel) expression by flow cytometry. (b) BMM of MyD88-deficient mice were stimulated with lipopolysaccharide (LPS; 1 μg/ml) for 16 hr and analysed for TREM-1 (upper panel) or CD40 (lower panel) expression by flow cytometry. (c) C57BL/6 mice were injected intravenously (i.v.) with 100 μg LPS or 100 μl phosphate-buffered saline (PBS) and the percentages of Gr-1+ MoIF (white bars) and Gr-1− resident monocytes (MoRT; black bars) among the F4/80+ population were determined after 4 hr. (d) Gr-1+ F4/80+ MoIF were analysed for TREM-1 (upper panel) and CD62L (lower panel) expression after i.v. injection of 100 μl PBS (left panel) or 100 μg LPS (right panel). (a+b+d) Histograms depict BMM (a+b) or Gr-1+ MoIF (d) stained with specific monoclonal antibodies (black lines) or corresponding isotype controls (grey lines). Representative data out of three independent experiments are shown. (c) Data show mean ± SEM (n = 6). Results of two independent experiments were pooled. Statistical differences compared to PBS control were determined by unpaired Student’s t-test (**P <0·01).
TREM-1L is up-regulated on granulocytes after TLR stimulation
We detected up-regulated TREM-1 expression after stimulation of certain TLR, so we investigated further the expression of the putative TREM-1L monitored by mTREM-1-Ig-FP. To investigate whether TREM-1L is expressed upon in vitro stimulation of TLR, mouse peripheral blood leucocytes were stimulated in the presence of different TLR agonists. No TREM-1L expression was detectable on peripheral blood cells before stimulation (Fig. 4a). All tested TLR ligands were potent inducers of TREM-1L surface expression on Gr-1+ granulocytes. No specific staining was detectable with the irrelevant control H60-Ig-FP. The binding of mTREM-1-Ig-FP to TREM-1L was blocked by anti-TREM-1 mAb confirming binding specificity of the reagent (data not shown). To test, whether the TLR adaptor protein MyD88 had an effect on TREM-1L modulation, we analysed the expression of TREM-1L on Gr-1+ blood cells from MyD88-knockout mice after stimulation with LPS for 3 hr. In these experiments, we did not detect the ligand(s) of TREM-1 on Gr-1+ blood cells of MyD88-knockout mice, indicating that the expression of TREM-1L is regulated by signalling downstream of the adapter protein MyD88 (Fig. 4b). In addition, TREM-1L surface expression was induced by LPS on highly purified granulocytes (purity > 98%) from wild-type C57BL/6 mice suggesting that a direct effect of LPS on granulocytes was responsible for the observed TREM-1L up-regulation (Fig. 4c).
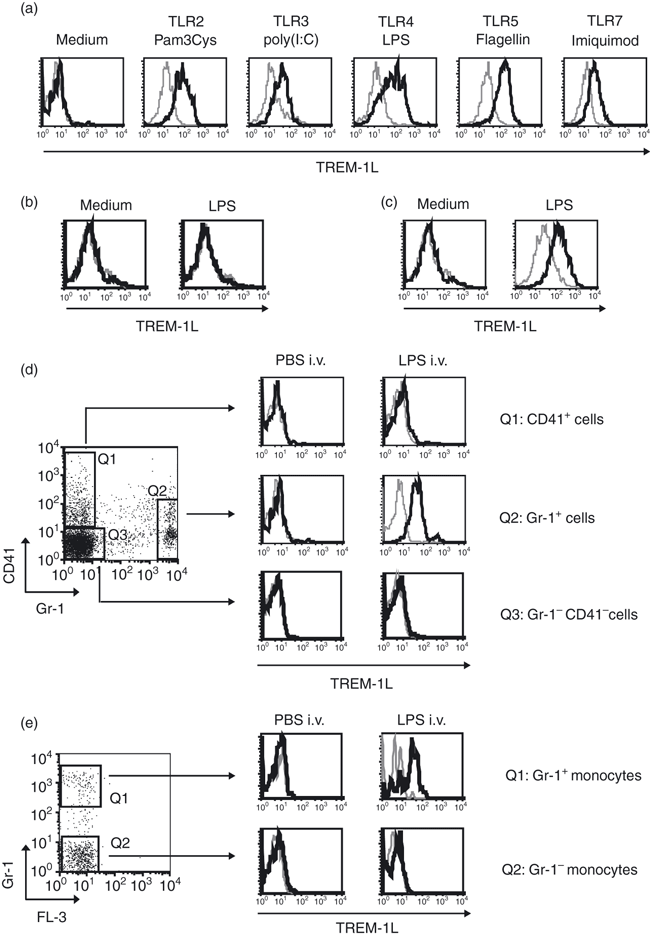
TREM-1 ligand(s) is up-regulated on granulocytes but not on platelets after lipopolysaccharide (LPS) stimulation. (a) Whole blood cells isolated from C57BL/6 mice were stimulated in vitro for 2 hr with the indicated Toll-like receptor (TLR) agonists or left untreated in medium and Gr-1+ cells were analysed for TREM-1L expression by flow cytometry. (b) Whole blood cells isolated from myeloid differentiation primary response gene 88 knockout (MyD88-KO) mice were stimulated in vitro for 3 hr with LPS (1 μg/ml)or left untreated in medium and Gr-1+ cells were analysed for TREM-1L expression by flow cytometry. (c) Magnetic antibody cell-sorted granulocytes (> 98% purity) were incubated in medium (left panel) or stimulated with 1 μg/ml LPS for 2 hr (right panel) and analysed for TREM-1L expression. (d) C57BL/6 mice were injected intravenously (i.v.) with 100 μl phosphate-buffered saline (PBS; left panel) or 100 μg LPS (right panel). Two hours after injection, whole blood cells were stained with mAbs directed against CD41 for the identification of platelets or against Gr-1 for granulocytes and analysed for the expression of TREM-1L. (e) C57BL/6 mice were injected i.v. with 100 μl PBS (left panel) or 100 μg LPS (right panel). 2 hr after injection, monocytes gated on according to their forward and side scatter profile, were analysed for the expression of Gr-1. TREM-1L expression was analysed on the Gr-1+ (upper histograms) and Gr-1− (lower histograms) monocyte subpopulation. (a–e) Cells were stained with mTREM-1-Ig-FP (black lines) or H60-Ig-FP (grey lines). Results are representative of three independent experiments.
To analyse the surface expression of TREM-1L after LPS-induced endotoxaemia, C57BL/6 mice were injected i.v. with 100 μg LPS. Before and 2 hr after LPS injection, Gr-1+ granulocytes, CD41+ platelets and the remaining Gr-1− CD41− cell population from blood were analysed for expression of TREM-1L. Under steady-state conditions, no TREM-1L expression was observed on any cell population in the peripheral blood of mice (Fig. 4d, left panel). Importantly, i.v. administration of LPS efficiently induced TREM-1L expression on Gr-1+ cells, but no TREM-1L was detectable on CD41+ platelets or on Gr-1− CD41− cells (Fig. 4d, right panel). In addition, we observed that TREM-1L+ Gr-1+ cells included the Gr-1+ monocyte subpopulation (Fig. 4e).
In conclusion, our data demonstrate that in mice TREM-1L was induced on Gr-1+ granulocytes and monocytes after stimulation by TLR ligands, but not on other cells in peripheral blood.
TREM-1 is up-regulated on Gr-1+ F4/80+ mononuclear myeloid-derived suppressor cells in tumour-bearing mice
Monocytes and macrophages are the major leucocyte population present in tumours and involved in both the promotion of tumour growth25 and anti-tumour immune responses.26 Therefore, we investigated TREM-1 expression patterns on monocytes in tumour-bearing mice. C57BL/6 mice were injected subcutaneously (s.c.) with the MHC class I-deficient mouse T-cell lymphoma RMA-S. After injection of 105 RMA-S cells, tumours were rejected by natural killer cells because of the lack of MHC class I expression,19 whereas higher cell numbers (106) led to progressive tumour growth. In tumour-bearing mice, the ratio between F4/80+ Gr-1− and F4/80+ Gr-1+ monocytes in the blood did not change (data not shown). Importantly, both monocyte subpopulations in the blood of tumour-bearing mice expressed TREM-1 on their cell surface (Fig. 5a). Enhanced TREM-1 up-regulation on F4/80+ Gr-1+ cells correlated with increasing volume of the established tumours (Fig. 5b, left panel). Furthermore, the up-regulation of TREM-1 on F4/80+ Gr-1+ blood monocytes was observed in other tumour models including s.c. B16-BL6 melanoma and s.c. MHC class I expressing RMA lymphoma (data not shown).
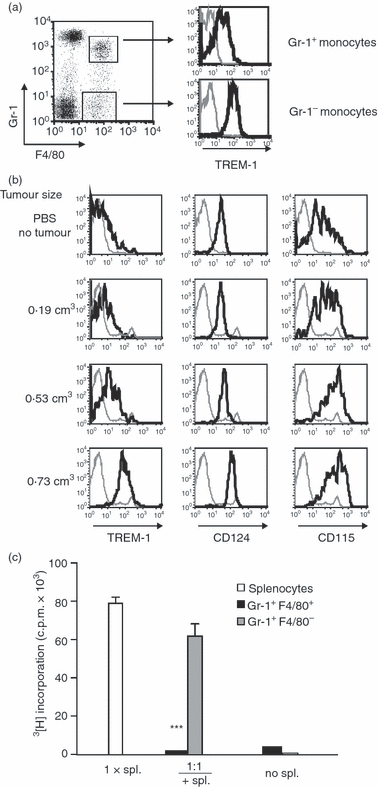
TREM-1 is up-regulated on mononuclear myeloid-derived suppressor cells. (a) C57BL/6 mice were injected subcutaneously (s.c.) with 106 RMA-S cells. Twelve days after tumour cell inoculation, TREM-1 expression was analysed on Gr-1+ F4/80+ (upper histogram) and Gr-1− F4/80+ (lower histogram) monocytes in the peripheral blood by flow cytometry. (b) F4/80+ Gr-1+ blood monocytes of mice with s.c. RMA-S tumours of different sizes (as indicated on the left) and control mice injected with phosphate-buffered saline (PBS) were analysed for the surface expression of TREM-1 (left panel), CD124 (middle panel), and CD115 (right panel) on day 12 after tumour cell inoculation. (c) Sorted Gr-1+ F4/80+ and Gr-1+ F4/80− cells isolated from tumour-bearing mice on day 21 after tumour cell inoculation were cocultured with splenocytes from naïve mice for 72 hr with concanavalin A at a 1 : 1 ratio. Proliferation was assessed by a [3H]thymidine incorporation assay. Data show mean ± SEM of triplicate cultures. Statistical differences compared to 1× splenocytes were determined by unpaired Student’s t-test (***P < 0·0001). Spl. = splenocytes. (a+b) Staining with specific mAbs is depicted as black lines and corresponding isotype controls as grey lines. Results are representative of five (a, b) or three (c) independent experiments.
Recently, in tumour-bearing mice an accumulation of immature myeloid cells was reported.17 These cells inhibit T-cell proliferation and cytokine production and therefore are called MDSC. These MDSC express Gr-1 and CD11b and up-regulate M-CSF-R (CD115) and IL-4Rα (CD124).18,27 Distinct subsets of mouse MDSC including the F4/80+ Gr-1+ Mo-MDSC and the low-density Ly6G+ PMN-MDSC have been described.18 To investigate, whether TREM-1+ F4/80+ Gr-1+ monocytes from the blood of tumour-bearing mice phenotypically resembled Mo-MDSC, surface expression of CD115 and CD124 was analysed by flow cytometry. Indeed, concomitantly with an increased TREM-1 surface expression, an up-regulation of CD115 and CD124 on F4/80+ Gr-1+ monocytes was observed (Fig. 5b, middle and right panels). Again, this up-regulation was dependent on the size of the resulting tumours. In addition, these TREM-1+ F4/80+ Gr-1+ monocytes efficiently suppressed concanavalin A-induced splenic T-cell proliferation in vitro, confirming that they functionally resembled MDSC (Fig. 5c). In concordance with a previous study,28 Gr-1+ F4/80− cells suppressed T-cell proliferation to a lesser extent. We also analysed the expression of TREM-1 on tumour-infiltrating cells. Tumour-infiltrating Gr-1+ F4/80+ macrophages and Gr-1+ granulocytes expressed TREM-1 on their cell surface 12 and 21 days after s.c. tumour cell injection (data not shown). TREM-1L expression was detectable in neither blood cells nor tumour-infiltrating cells. In summary, in tumour bearing mice, Mo-MDSC and F4/80+ Gr-1+ macrophages in the tumours expressed TREM-1.
Discussion
Several recent studies emphasized that TREM-1 plays a pivotal role in the amplification of inflammatory responses.1,29 The goal of our study was to provide a detailed analysis of TREM-1 and TREM-1L expression in mice and its regulation during disease.
In blood of naïve mice, two monocyte subpopulations were described as existing at approximately similar numbers.15,16 These MoIF and MoRT are characterized by their expression of distinct cell surface markers including CD62L, CX3CR1 and Gr-1 and their migratory capacity. MoIF and MoRT are considered to be the mouse counterparts of the human ‘classical’ and ‘non-classical’ monocyte subpopulations, respectively. In humans, TREM-1 is expressed on a ‘classical’ CD14high monocyte subset in human peripheral blood.1 However, our data demonstrate that in naïve mice TREM-1 is expressed on the cell surface of MoRT, which correspond to the CD14+ CD16+ subpopulation in humans,15,16 whereas MoIF expressed TREM-1 only after injection of LPS (1, 3).
We have evidence that neither TREM-1-expressing mouse monocytes nor granulocytes isolated from mouse blood produced TNF-α (Fig. 2d) or pro-inflammatory chemokines including MIP-1α (CCL3) and MIP-2 (CXCL1) (data not shown) upon stimulation via TREM-1. The anti-TREM-1 mAb used in the study was agonistic, because it efficiently stimulated TNF-α production by the cell line MPRO and by BMDC (Fig. 2d). The addition of GM-CSF used for cell culture of BMDC and MPRO cells or additional factors produced during inflammatory conditions might be required for efficient activation of mouse cells via TREM-1.
In contrast to our observations in mice, human neutrophils and monocytes were reported to produce cytokines including IL-8 and TNF-α after cross-linking of TREM-1. The levels of these pro-inflammatory cytokines were further increased in the presence of TLR agonists.3,23 The reason for this discrepancy is currently unknown. It is possible that activation via TREM-1 might require additional coreceptors or signalling components that are absent on mouse but present on human primary blood monocytes and granulocytes. Of note, mice used in our experiments are held under pathogen-free conditions and are exposed to a different pathogen repertoire as compared with humans that might also impact the activation status of innate immune cell populations.
In accordance with Hara et al.,30 TREM-1 was expressed on the cell surface of in vitro-generated BMDC (Fig. 2a). To investigate whether TREM-1 was also expressed on primary mouse dendritic cells, we analysed the expression of TREM-1 on peripheral blood CD11c+ cells. TREM-1 expression was detectable on a subset of CD11c+ cells (data not shown). This subset also expressed CD11b, F4/80 and CX3CR1, but was negative for Gr-1, B220 and CD8a. In humans, TREM-1 is neither expressed on in vitro-generated monocyte-derived DC nor on DC from peripheral blood.3 It is possible that the expression of TREM-1 on in vitro-generated BMDC is induced by the exposure to GM-CSF similar to BMM (Fig. 2b).
Up-regulation of TREM-1 surface expression was detected on Gr-1+ F480+ cells in tumour-bearing mice (Fig. 5). Our further phenotypical and functional analyses revealed that this TREM-1+ Gr-1+ F480+ cell population isolated from the blood of tumour-bearing mice resembled Mo-MDSC. In humans, MDSC accumulation correlates with high plasma levels of certain factors including GM-CSF.31 We observed that GM-CSF and IL-3 that share the common beta-chain receptor induced cell surface expression of TREM-1 on BMM (Fig. 2b). The GM-CSF also up-regulated TREM-1 on human primary monocytes.23 Substantial levels of GM-CSF were detectable in RMA-S tumour lysates (data not shown). Whether GM-CSF that is present in tumour-bearing mice induces the expression of TREM-1 on Mo-MDSC is currently unknown. In addition, Gr-1+ F480+ cells isolated from RMA-S tumour tissue also expressed TREM-1 (data not shown). Accordingly, tumour-associated macrophages isolated from patients suffering from non-small cell lung cancers expressed TREM-1.32 Using the mTREM-1-Ig-FP, we did not detect TREM-1L expression on any cell population in the peripheral blood of tumour-bearing mice. Whether the expression of TREM-1 is of importance for MDSC function is currently unknown.
So far, the nature of TREM-1L has not been described. In humans, a putative ligand(s) was expressed on platelets12 and present in the sera of septic patients.33 In mice, a putative ligand of TREM-1 was detected on granulocytes after induction of experimental peritonitis.13 Our data demonstrate that upon i.v. injection of LPS, TREM-1L expression was only detectable on granulocytes, but not on other cells (Fig. 4). Although s.c. applied tumours induced up-regulation of TREM-1 expression similar to LPS-induced endotoxaemia, TREM-1L was not detected in the blood of tumour-bearing mice or on tumour-infiltrating cells by staining using the mTREM-1-Ig-FP. In addition, TREM-1L was not detectable on the cell surface of RMA-S tumour cells (data not shown). It is possible that soluble forms of TREM-1L might exist that were not detectable in our experimental setting. We also cannot exclude that the TREM-1L might be expressed in tissues that we did not analyse. In addition, soluble TREM-134,35 might affect the detection of TREM-1L in our experimental set-up. In our experiments, however, no soluble TREM-1 was detectable in the sera of mice analysed 2–24 hr after LPS-induced endotoxaemia by ELISA (data not shown).
TREM-1L surface expression was induced by TLR agonists on purified granulocytes in vitro suggesting a direct effect of TLR signalling on TREM-1L expression. Our data indicate that the adapter MyD88 was required for the TREM-1L up-regulation via LPS. The poly(I : C) that stimulates TLR3 does not require MyD88 up-regulated TREM-1L, so we assume that additional pathways for TREM-1L up-regulation exist. TREM-1L up-regulation is an early event occurring already 2 hr after LPS injection. Addition of the RNA synthesis inhibitor actinomycin D or the vesicle transport inhibitor brefeldin A during LPS stimulation of granulocytes partially inhibited TREM-1L up-regulation, suggesting that its surface expression is to some extent the result of de novo protein synthesis and transport (data not shown). It is also possible that some preformed TREM-1L might exist that becomes accessible to the mTREM-1-Ig-FP by oligomerization, by induction of a conformational change, or the release from intracellular vesicles after TLR-stimulation.
Our data indicate that after LPS application Gr-1+ granulocytes and monocytes express both TREM-1 and TREM-1L (1, 4). The consequences of the expression of both, TREM-1 and its putative ligand(s), on the cell surface of Gr-1+ are currently unknown. On these cells TREM-1 and TREM-1L might interact not only in trans but also in cis. Cis interactions of TREM-1 and its ligand(s) can serve to increase or to decrease the threshold for functional activation. TREM-1-expressing cells might become stimulated upon the expression of TREM-1L in cis and ‘armed’ for high activation in an inflammatory environment. In contrast, the coexpression of TREM-1 and TREM-1L in cis might reduce the interaction of TREM-1 with other putative ligand(s) in trans counteracting activation. In addition, not only signals delivered via TREM-1, but also via TREM-1L, might determine activation pathways in TREM-1 and TREM-1L-expressing cells. Of note, also TREM-2, another member of the TREM family, and its ligand(s) are expressed on the surface of monocytes (reviewed in ref. 1). In this context it was reported that TREM-2 binds to plexin-A1 in cis and to additional ligands in trans36 resulting in a multimeric complex. Accordingly, the binding of TREM-1 to its ligand(s) in cis and to additional ligand(s) in trans may also be a part of a multimeric complex.
Monocytes and neutrophils serve as major players to propagate inflammatory responses. After microbial infection, TREM-1 is up-regulated on inflammatory monocytes and it has been shown that this subpopulation is selectively recruited to sites of inflammation.16 Of note, Soehnlein et al.37 showed that PMN preceded a second wave of recruitment of Gr-1+ inflammatory monocytes to the site of inflammation in a peritonitis model. This selective recruitment of MoIF was dependent on PMN-secreted products and CCR2. We observed that TREM-1 was up-regulated on MoIF upon LPS challenge and that microbial products induced the expression of TREM-1L on granulocytes. Subsequently, at the site of microbial infection this interaction of TREM-1+ MoIF with TREM-1L+ granulocytes might result in the production of high levels of pro-inflammatory cytokines and chemokines.
The exact role of TREM-1 in the context of inflammation and tumour growth remains to be elucidated. In our study, we describe the finding that TREM-1 expression is differentially regulated on monocyte subpopulations during inflammation and cancer. The identification of TREM-1L will be crucial for our understanding of the role of TREM-1 in these diseases.
Acknowledgements
We thank Carmen Mader and Joachim Neuert for excellent technical assistance; Professor Klaus Heeg and Professor Alexander Dalpke for providing TLR ligands; Professor Rainer Zawatzky for providing cells and for helpful discussions; Professor Akira for kindly providing MyD88-deficient mice and Dr Annelies Verbrugge, Dr Anja Tessarz, Dr Ioanna Galani and Martina Kegel for critically reading the manuscript. This work was supported by the Marie Curie Excellence Grant MEXT-CT-2003-2739 to PD Dr A. Cerwenka.
Conflict of interest
The authors declare that they have no conflicting financial interests.




