Anti-ribosomal phosphoprotein autoantibody triggers interleukin-10 overproduction via phosphatidylinositol 3-kinase-dependent signalling pathways in lipopolysaccharide-activated macrophages
Summary
Anti-ribosomal phosphoprotein autoantibodies have been shown to be significantly associated with multiple manifestations of systemic lupus erythematosus (SLE). High levels of interleukin-10 (IL-10) have been demonstrated to contribute to lupus susceptibility and severity. In this study, we investigated the molecular mechanisms of anti-ribosomal phosphoprotein monoclonal antibody (anti-P mAb)-induced autoimmune responses. Anti-P mAb promoted IL-10 overproduction in a dose- and time-dependent manner in both lipopolysaccharide (LPS)-activated RAW 264.7 cells and primary human macrophages. Anti-P mAb enhanced phosphorylation of Akt (PKB; protein kinase B), extracellular signal regulated kinase 1/2 (ERK1/2) and c-Jun NH2-terminal kinase 1/2 (JNK1/2), while phosphorylation of p38 remained unaltered. Furthermore, anti-P mAb decreased glycogen synthase kinase 3 (GSK3) activity and reduced the phosphorylation of IκBα in LPS-activated macrophages. The Syk, phosphatidylinositol 3-kinase (PI3K), protein kinase C (PKC), JNK and ERK signalling pathways involved in anti-P mAb-triggered IL-10 secretion were also confirmed using various pharmacological inhibitors. In addition, nuclear factor (NF)-κB had negative regulatory effects on anti-P mAb-triggered IL-10 secretion. Using reporter plasmids containing the nuclear factor binding sites of NF-κB, cAMP-enhanced activation protein 1 (AP-1), serum response element (SRE) or cyclic AMP response element (CRE), treatment of anti-P mAb led to activation of the corresponding factors that bind to the AP-1 site, SRE and CRE in the LPS-activated macrophages. Furthermore, by transfection with reporter plasmids bearing various lengths of the IL-10 promoter, the AP-1 binding site, SRE and CRE were shown to be required for anti-P mAb-induced effects. Collectively, our results provide a molecular model for anti-P mAb-induced IL-10 overproduction in LPS-activated macrophages, which may play a role in the pathogenesis of SLE.
Introduction
Anti-ribosomal phosphoprotein antibodies (anti-P Abs) are detectable in 12–40% of patients with systemic lupus erythematosus (SLE)1 and are strongly associated with SLE-related psychiatric,2 nephritic3 and hepatic manifestations.4 In eukaryotic cells, acidic ribosomal phosphoproteins (P0, P1 and P2) and other components form the 60S ribosomal subunit, while ribosome-free P proteins are located in the cytoplasm.5 However, the P0 protein has also been found on the cell surface of multiple cell types, including fibroblasts6 and human hepatoma, neuroblastoma and endothelial cells.7 In our previous study, we demonstrate that anti-P monoclonal antibody (anti-P mAb) can bind to and penetrate various cell types and induce apoptosis of human T cells.8 These results suggest that anti-P Abs can play a role in the pathogenesis of lymphopenia or lymphocyte dysfunction in SLE.9
Macrophages are the major mediators of inflammation in innate immunity, acquired immunity and tissue homeostasis. In response to infection, macrophages secrete a variety of inflammatory cytokines, lipid mediators, toxic radicals of oxygen, and nitride. These macrophages-derived substances can be triggered by various microbial components such as lipopolysaccharide (LPS) from Gram-negative bacteria.10 As a result, a key effector function of activated macrophages is the production of inflammatory cytokines and chemokines. SLE patients have an increased overall risk of pathogen infection and are susceptible to the development of urinary infections, pneumonia and bacteraemia. Activated macrophages are considered one of the key mediators responsible for induction of inflammation in SLE.11,12 Recent studies indicate that inflammatory cytokines are important for pathogenesis of SLE, especially the involvement of T helper type 1 (Th1)/Th2 cytokines.13,14
Interleukin (IL)-10, a cytokine synthesis inhibiting factor (CSIF) produced mainly by Th2 cells and macrophages,15 is a stimulator of B lymphocytes but an inhibitor of antigen-presenting cells and T lymphocytes.16 Previous studies have shown that high levels of IL-10 are present in the sera of SLE patients and in murine models of lupus.17,18 Thus, levels of serum IL-10 may serve as a useful biomarker for SLE disease progression.19,20 In addition, increased production of IL-10 causes immune dysregulation, which can lead to overt SLE.21 In our previous studies, we showed that anti-P mAb inhibits the production of IL-12 and tumour necrosis factor (TNF)-α in macrophages, but regulates IL-10 expression in a different manner.22
It has been shown that LPS induces IL-10 production through binding to the Toll-like receptors (TLRs) that activate nuclear factor (NF)-κB, mitogen-activated protein kinases (MAPKs), phosphatidylinositol 3-kinase (PI3K), glycogen synthase kinase 3 (GSK3) and tyrosine kinase Syk.23–27 Given that anti-P mAb is significantly associated with SLE pathogenesis and pathogen infections are common in the context of SLE disease, we investigated the detailed signalling effects of anti-P mAb on IL-10 expression under LPS treatment in macrophages. In the present study, we demonstrated that anti-P mAb and LPS produced synergistic effects in the induction of IL-10 expression. In addition, we showed that anti-P mAb enhanced IL-10 production via activation of Syk and PI3K in LPS-activated macrophages, while these effects were not found in resting cells. In IL-10 promoter experiments, the downstream transcription factor binding sites cAMP-enhanced activation protein 1 (AP-1), serum response element (SRE) and cyclic AMP response element (CRE) were shown to be required for the enhancement of IL-10 expression by anti-P mAb. Taken together, these data indicate that anti-P autoantibody-induced effects may play a role in the immunological manifestations of SLE, and therapies targeting relevant signalling molecules may provide an opportunity to design a new strategy for the treatment of SLE.
Materials and methods
Cells
The mouse macrophage cell line RAW 264.7 was obtained from the American Type Culture Collection (ATCC, Rockville, MD). These cells were cultured in Dulbecco’s modified Eagle’s minimal essential medium (DMEM; JRH Biosciences, Lenexa, KS) supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin (GibcoBRL Life Technologies, New York, NY). Experiments involving human material were approved by the Institutional Review Board (IRB) of National Yang-Ming University. Human monocytes from peripheral blood were isolated using the adherence of Ficoll-Hypaque (GE Healthcare Bio-Sciences, Uppsala, Sweden) prepared mononuclear cells to the culture dishes. Adherent monocytes were cultured overnight in RPMI-1640 (JRH Biosciences) supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin (GibcoBRL Life Technologies). These cells were maintained at 37° in a humidified incubator containing 5% CO2.
Reagents
LPS from Escherichia coli O26:B6 was obtained from Sigma-Aldrich (St Louis, MO). The p38 MAPK inhibitor (SB202190), the mitogen-activated protein kinase (MEK)/external-signal regulated kinase (ERK) inhibitor (PD98059), the c-Jun NH2-terminal kinase (JNK) inhibitor (SP600125), the PI3 kinase inhibitor (wortmannin), the protein kinase C (PKC) inhibitor (calphostin C), the NF-κB inhibitor (NF-κB activation inhibitor), the IκB kinase (IKK)-2 inhibitor, the Syk inhibitor (piceatannol) and the MAPK inhibitor analogue (SB202474) were purchased from Calbiochem (San Diego, CA). Anti-p38 (phospho-T180/Y182), anti-JNK (phospho-T183/Y185), anti-phospho GSK3α/β (serine-21 and serine-9; inactivating residues), anti-β-actin, and anti-phospho IκBα antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti-ERK1/2 (phospho-T202/Y204) and anti-Akt (PKB; protein kinase B) (phospho-Ser 473) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The jetPEI™ transfection system (PolyPlus-transfection, Illkirch, France) was obtained from Poster. The luciferase assay system was supplied by Promega (Madison, WI).
Preparation of anti-P mAb (9B6-4; 9B6 mAb)
Anti-P mAb was produced by a standard hybridoma procedure as previously described.8,22 The anti-P mAb (9B6 mAb) was raised against the proteins P0, P1 and P2 and identified as being in the immunoglobulin G1 (IgG1) subclass. Culture supernatants of hybridoma and control mouse IgG1 (MOPC21; Sigma-Aldrich) were purified by protein A-sepharose affinity chromatography (Amersham Pharmacia Biotech, Little Chalfont, UK). Any endotoxin contaminants in the purified antibodies were removed using polymyxin B-agarose (Sigma-Aldrich) affinity column chromatography. The concentrations of the anti-P mAb and the control mouse IgG were determined using an enzyme-linked immunosorbent assay (ELISA) kit (Roche, Sandhofer, Germany).
RNA isolation and real-time quantitative polymerase chain reaction (qPCR)
Total RNA was isolated using the RNeasy kit from Qiagen (Hilden, Germany) according to the manufacturer’s instructions. Reverse transcriptions were performed using the First Strand cDNA Synthesis kit (Promega) according to the manufacturer’s instructions. Five micrograms of total RNA was transcribed to cDNA in a 30-μl reaction volume. For transcript quantification by real-time PCR, the SYBR Green Mix containing Thermo-Start DNA Polymerase (Bio-Rad, Hercules, CA) was used according to the manufacturer’s instructions. The forward and reverse primers for IL-10 were 5′-GCT CCT AAG AGA GTT GTG AAG AAA CTC-3′ and 5′-AGC TGC TGC AGG AAT GAT CA-3′. The forward and reverse primers for β2 microglobulin were 5′-CCG GAG AAT GGG AAG C-3′ and 5′-GTA GAC GGT CTT GGG C-3′. A hot-start phase was applied at 95° for 10 min for all primers. cDNA was amplified for 45 cycles (IL-10) at 95° for 10 seconds, 60° for 10 seconds, and 72° for 25 seconds. In each cycle, accumulation of PCR products was detected by monitoring the increase in fluorescence of double-stranded DNA (dsDNA)-binding SYBR Green. The levels of IL-10 were adjusted to the levels of housekeeping β2 microglobulin gene. Data were analysed using the comparative Ct method with the following formula: ΔCt = Ct(target, IL-10) − Ct(normalizer, β2 microglobulin). The fold increase in the expression of IL-10 mRNA in 9B6 experimental groups compared with the mouse IgG1 (mIgG1) control was calculated as 2−(ΔΔCt).
ELISA for cytokines
After overnight culture, RAW 264.7 cells or human macrophages (5×104 cells/ml) were incubated with LPS (1 μg/ml) and affinity-purified anti-P or mouse IgG1 (100 μg/ml) at the indicated time-points. The supernatants were collected and the concentration of IL-10 was determined using a mouse (BioSource Europe S.A., Nivelles, Belgium) or human (Endogen, Rockford, IL) IL-10 ELISA kit. In the inhibition experiments, RAW 264.7 cells were treated with one of the specific chemical inhibitors or dimethyl sulphoxide (DMSO; vehicle control) for 1 hr and then incubated with LPS (1 μg/ml) and affinity-purified anti-P or mouse IgG1 (100 μg/ml) for 48 hr before the IL-10 concentration was determined.
Western blotting
Cell extracts (60 μg/lane) were separated on 13% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) gels and then transferred to a nitrocellulose membrane (Milipore, New Bedford, MA) by electroblotting. The membrane was then blocked and probed with antibodies. The immunoblot analysis was conducted using goat anti-mouse IgG conjugated to horseradish peroxidase (HRP) or HRP-conjugated anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA). An ECL kit (PerkinElmer, Boston, MA) was used for signal detection after blotting.
Transfection of RAW 264.7 cells with jetPEI™
RAW 264.7 cells were transfected with luciferase reporter plasmids containing the NF-κB, AP-1, SRE, CRE (Clontech, Palo Alto, CA) or murine IL-10 promoter region (−553/+64 bp) (the plasmid was a generous gift from Dr Wen-Chang Chang, Department of Pharmacology, Medical College, National Cheng Kung University, Tainan, Taiwan) using jetPEI™ transfection system (PolyPlus-transfection). These cells (in 2 ml of fresh medium) were seeded for 24 hr in a six-well plastic dish before transfection. For transfection, jetPEI™ was first incubated with plasmid DNA (6 μl of jetPEI™/3 μg of total plasmid DNA) in 0·2 ml of a serum-free medium at room temperature for 25 min. The DNA/jetPEI™ mixture was then added dropwise to the cells. The cells were incubated in a humidified atmosphere of 5% CO2 at 37° for 12 hr. After 12 hr, the transfected cells were treated with mIgG1 or 9B6 mAb (100 μg/ml) for 24 hr and then stimulated with LPS (1 μg/ml) for 12 hr. The cell lysates were then used for the luciferase assays.
Assay of luciferase activity
Luciferase activity was measured using a Promega luciferase assay system. After washing with phosphate-buffered saline (PBS), the transfected cells grown in six-well plastic dishes were lysed with luciferase lysis reagent (100 μl/well) and incubated at room temperature for 15 min. The lysates were spun at 7200 g for 15 seconds and the cleared cell lysate (20 μl) was mixed with luciferase assay substrates (100 μl). Activity was measured using a Berthold Lumat LB 9506 luminometer (Berthold, Bad Wildbad, Germany). According to references, all the relative luciferase activities were normalized to the same protein concentration in RAW 264.7 cells.28–30 Briefly, the cells were co-transfected with a β-gal expression vector as a control and we measured β-gal activity (i.e. β-gal activity equivalent to the amount of protein) to ensure the expression of luciferase in all subsequent experiments.
Data analysis
All experiments were repeated independently at least three times. The results are expressed as mean ± standard deviation (SD) and statistical significance was determined by Student’s t-test.
Results
Anti-P mAb (9B6 mAb) augments IL-10 secretion in LPS-activated RAW 264.7 cells and primary human macrophages
To investigate the effects of anti-P Ab on IL-10 production, LPS-activated RAW 264.7 macrophages were treated with anti-P monoclonal antibody (9B6 mAb) or control mouse IgG1 (mIgG1) for 0, 6, 12, 16 or 24 hr. The expression of IL-10 was determined by real-time PCR. Incubation of cells with 9B6 mAb and LPS increased the expression of IL-10 mRNA from 6 hr and lasted up to 24 hr, and induced a time-dependent increase in IL-10 mRNA compared with the control mIgG1-treated group (Fig. 1a). In addition, these data were confirmed using an IL-10-specific ELISA kit. We also found that the 9B6 mAb dramatically enhanced IL-10 production in a dose- (Fig. 1b) and time-dependent (Fig. 1c) manner in the LPS-activated RAW 264.7 cells. This augmentation effect of anti-P mAb on IL-10 production was also observed in LPS-activated human primary macrophages from two healthy subjects (Fig. 1d). However, it was noted that treatment with 9B6 mAb without LPS stimulation was not able to induce IL-10 production in RAW 264.7 cells (data not shown). In addition, monoclonal anti-dsDNA mAb (9D7) did not enhance IL-10 production in LPS-activated RAW 264.7 cells (data not shown). These data suggest that anti-P mAb specifically enhances IL-10 production in LPS-activated RAW 264.7 cells.
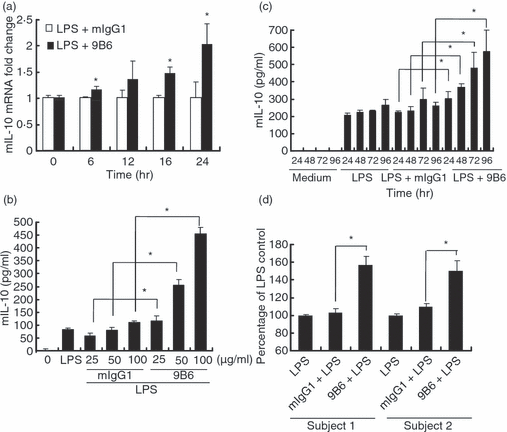
The anti-ribosomal phosphoprotein monoclonal antibody (anti-P mAb) 9B6 mAb enhances interleukin (IL)-10 production in lipopolysaccharide (LPS)-activated RAW 264.7 cells and human primary macrophages. (a) RAW 264.7 cells (1 × 106 cells/ml) were treated with LPS (1 μg/ml) and 9B6 mAb (100 μg/ml) or mouse immunoglobulin G1 (mIgG1; 100 μg/ml) for 0, 6, 12, 16 or 24 hr. Expression of the IL-10 mRNA was determined by real-time quantitative polymerase chain reaction (RT-qPCR). The mouse IL-10 (mIL-10) mRNA fold change was calculated using the formula (IL-10 mRNA fold increase at the indicated times)/(IL-10 mRNA fold increase at 0 hr). (b) RAW 264.7 cells (5 × 104 cells/ml) were incubated with LPS (1 μg/ml) and mIgG1 or 9B6 mAb (25, 50, 100 μg/ml) for 48 hr. IL-10 production in the culture supernatant was determined using a mouse enzyme-linked immunosorbent assay (ELISA) kit. (c) RAW 264.7 cells (5 × 104 cells/ml) were stimulated with LPS (1 μg/ml) and mIgG1 or 9B6 mAb (100 μg/ml) for 24, 48, 72 or 96 hr. IL-10 in the culture supernatant was quantified. (d) Human macrophages (5 × 104 cells/ml) were incubated with LPS (1 μg/ml) and mIgG1 or 9B6 mAb (100 μg/ml) for 48 hr. IL-10 production in the culture supernatant was determined using a human ELISA kit. The percentage of the LPS control was calculated using the formula (IL-10 production in the 9B6 mAb- or mIgG1-treated group)/(IL-10 production in the LPS control) × 100. IL-10 production in the LPS control was 45 and 100 pg/ml in the two healthy subjects, respectively. The mean and error bar were calculated based on triplicate experiments (*P < 0·05).
AKT, ERK and JNK are activated, but GSK3 and IκBα are inhibited, by anti-P mAb (9B6 mAb) in LPS-activated macrophages
As LPS-induced TLR signals activate mitogen-activated protein kinases (MAPKs) and phosphatidylinositol 3-kinase (PI3K),23,31 we next investigated the effects of anti-P mAb on the activation of ERK, JNK, p38 MAPK and PI3K/AKT. Because LPS induces signals rapidly and anti-P mAb requires a longer incubation time for its signalling effects, we pretreated the cells with anti-P mAb for 24 hr prior to addition of LPS (Fig. 2). Increased levels of activated AKT (pAKT), ERK (pERK) and JNK (pJNK) were observed at as early as 10 min in LPS-activated RAW 264.7 cells in the 9B6 mAb treatment. However, the levels of phospho-p38 in the presence of LPS or 9B6 mAb + LPS were similar to that of the control mIgG1-treated group (Fig. 2a). In the PI3K/AKT pathway, it is known that Akt attenuates the activity of GSK3 through Ser21/Ser9 phosphorylation, which in turn regulates the function of the AP-1 and cAMP response element binding protein (CREB) transcription factors in the control of IL-10 production.32–34 Therefore, we examined the activation of GSK3 in the anti-P mAb plus LPS treatment. We used imagequant 5.2 software (Molecular Dynamics, Sunnyvale, CA) to confirm the quantification of the image the density of each band was normalized to loading control (β-actin). Our data showed that 9B6 mAb treatment slightly increased phosphorylated GSK3 (pGSK3, inactive form) in LPS-activated macrophages (2·5-fold increase in pGSK3 in 9B6 + LPS treatment versus 1·5-fold increase in treatment with LPS alone) (Fig. 2b). We then used the constitutively active GSK3S9A to examine the role of GSK3 in anti-P mAb-triggered IL-10 secretion. Our results showed that anti-P mAb (9B6 mAb) was unable to augment IL-10 expression in LPS-stimulated macrophages under the constitutive activation of GSK3 (data not shown). Next, we investigated the effects of anti-P mAb on NF-κB activation in LPS-activated RAW cells. Activation of the NF-κB pathway was measured using the phosphorylation of IκBα as a surrogate marker. The increase in p-IκBα was slightly reduced in the presence of 9B6 mAb and LPS (0·8-fold increase in expression levels of pIκBα in the 9B6 + LPS treatment versus 1·5-fold increase in the treatment with LPS alone) (Fig. 2c). We also found that nuclear translocation of NF-κB was suppressed in LPS-activated macrophages under treatment with 9B6 mAb (data not shown). These data demonstrate that anti-P mAb enhances phosphorylation of AKT, ERK1/2 and JNK1/2. Furthermore, anti-P mAb decreases GSK3 activity and reduces the phosphorylation of IκBα in LPS-activated macrophages.
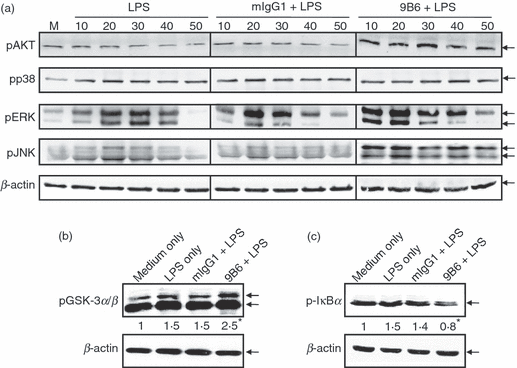
The anti-ribosomal phosphoprotein monoclonal antibody (anti-P mAb) 9B6 mAb activates Akt (PKB; protein kinase B), extracellular signal regulated kinase (ERK) and c-Jun NH2-terminal kinase (JNK) but inhibits glycogen synthase kinase 3 (GSK3) and IκBα in lipopolysaccharide (LPS)-activated RAW 264.7 cells. (a) RAW 264.7 cells (1 × 106 cells/ml) were treated with 9B6 mAb or mouse immunoglobulin G1 (mIgG1; 100 μg/ml) for 24 hr. After incubation, the cells were stimulated with LPS (1 μg/ml) for 0, 10, 20, 30, 40 or 50 min. Whole-cell lysates (60 μg/lane) were analysed by western blotting using phospho-specific antibodies against AKT, p38, ERK1/2 and JNK1/2. β-actin was used as a loading control. (b) RAW 264.7 cells (1 × 106 cells/ml) were treated with 9B6 mAb or mIgG1 (100 μg/ml) for 24 hr. After incubation, the cells were stimulated with LPS (1 μg/ml) for 30 min. Whole-cell lysates (60 μg/lane) were analysed by western blotting using phospho-specific antibodies against GSK3α/β (serine-21 and serine-9; inactivating residues). (c) RAW 264.7 cells (1 × 106 cells/ml) were treated with 9B6 mAb or mIgG1 (100 μg/ml) for 24 hr. After incubation, the cells were stimulated with LPS (1 μg/ml) for 30 min. Whole-cell lysates (60 μg/lane) were analysed using phospho-specific antibodies against IκBα. The results shown are representative of three independent experiments. M, medium only. *imagequant 5.2 software was used to quantify the image the density of each band was normalized to loading control (β-actin). Treatment of 9B6 alone had similar effects to that of mIgG1 control without activation of the signalling cascade (data not shown).
Anti-P mAb (9B6 mAb) enhances IL-10 production via the activation of Syk through the Fc γ receptor (FcγR) in LPS-activated RAW 264.7 cells
To determine whether the effect of anti-P mAb on the IL-10 production of macrophage cells was mediated through FcγR, RAW cells were pretreated with mIgG1 for 1 hr prior to stimulation with LPS and mAbs. We found that competition for FcγR binding produced by mIgG1 pretreatment decreased the IL-10 production induced by anti-P mAb (Fig. 3a). FcγR engagement has been shown to be linked to activation of PI3K via coupling of the protein kinase Syk.35,36 The FcγR-mediated coupling of activated Syk was further examined using a Syk inhibitor (piceatannol). Our results showed that IL-10 secretion augmented by anti-P mAb was obliterated in the presence of piceatannol (the Syk inhibitor) (Fig. 3b). The data indicate that anti-P mAb (9B6 mAb) enhances IL-10 production mainly through FcγR via the activation of Syk in LPS-activated RAW 264.7 cells.
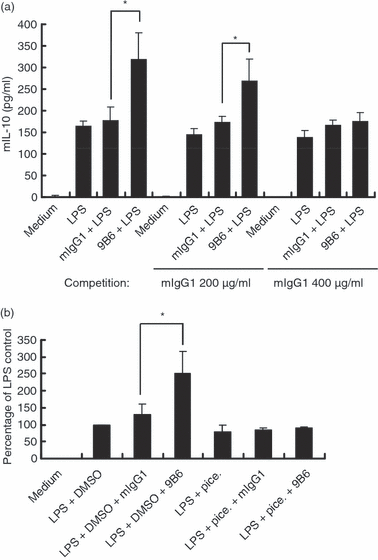
The anti-ribosomal phosphoprotein monoclonal antibody (anti-P mAb) 9B6 mAb enhances interleukin (IL)-10 production via Fc γ receptor (FcγR)-mediated activation of spleen tyrosine kinase (Syk) in lipopolysaccharide (LPS)-activated RAW 264.7 cells. (a) RAW 264.7 cells (5 × 104 cells/ml) were preincubated with mouse immunoglobulin G1 (mIgG1; 0, 200 and 400 μg/ml) for 1 hr. After incubation, the cells were incubated with LPS (1 μg/ml) and mIgG1 or 9B6 mAb (100 μg/ml) for 48 hr. IL-10 production in the culture supernatant was determined using a mouse enzyme-linked immunosorbent assay (ELISA) kit. (b) RAW 264.7 cells (5 × 104 cells/ml) were treated with piceatannol (10 μm) or dimethyl sulphoxide (DMSO; control) for 1 hr. After incubation, the cells were stimulated with LPS (1 μg/ml) and mIgG1 or 9B6 mAb (100 μg/ml) for 48 hr. IL-10 production in the culture supernatant was determined by ELISA. The percentage of the LPS control was calculated as (IL-10 production in each group)/(IL-10 production in the LPS + DMSO control) × 100. The results shown are representative of three independent experiments. The mean and error bar were calculated based on triplicate experiments (*P < 0·05).
IL-10 production triggered by anti-P mAb (9B6 mAb) in LPS-activated macrophages is positively regulated by the Syk, PI3K, PKC, JNK and ERK pathways, but negatively regulated by NF-κB
To further determine whether changes induced by 9B6 and LPS, shown in Fig. 2, are truly involved in the signal transduction pathways underlying IL-10 production, chemical inhibitors of the various key enzymes were used (Fig. 4). Overall responses were influenced by the addition of inhibitors; in particular, IL-10 secretion augmented by anti-P mAb was obliterated in the presence of wortmannin (the PI3K inhibitor) or calphostin C (the PKC inhibitor) (Fig. 4a). We confirmed this result using another PI3K inhibitor (LY294002) (data not shown). In contrast, the increase in the level of IL-10 caused by 9B6 mAb was unaffected by treatment with an NF-κB or IKK-2 inhibitor (Fig. 4b). For stimulation with LPS and 9B6, we used relative expression levels of IL-10 to represent and compare IL-10 secretion levels after the addition of various inhibitors. As shown in Fig. 4(c), compared with the group treated with SB202474 (an inhibitor analogue without effects on MAPK activity), relative IL-10 expression in cells treated with the Syk (piceatannol), PI3K (wortmannin), PKC (calphostin C), JNK (SP600125), or ERK (PD98059) inhibitor was significantly reduced. Consistent with the results shown in 2, 3, the data indicate that Syk, PI3K, PKC, JNK and ERK are involved in signalling effects induced by anti-P mAb. However, the relative IL-10 induction was enhanced by treatment with the NF-κB inhibitor or the IKK-2 inhibitor, but remained unchanged when the p38 MAPK inhibitor SB202190 was used. Thus, the above results suggest that p38 signalling may not directly participate in IL-10 induction by 9B6 and LPS. In addition, NF-κB had negative regulatory effects on anti-P mAb-triggered IL-10 secretion.
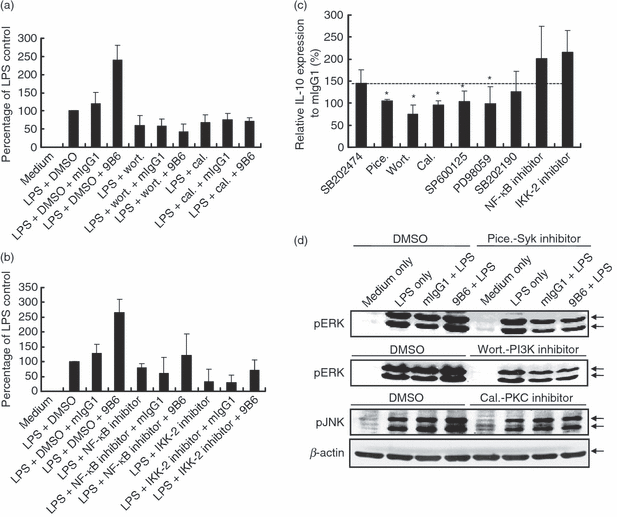
Interleukin (IL)-10 secretion is positively regulated by the spleen tyrosine kinase (Syk), phosphatidylinositol 3-kinase (PI3K), protein kinase C (PKC), c-Jun NH2-terminal kinase (JNK) and extracellular signal regulated kinase (ERK) pathways but negatively regulated by nuclear factor (NF)-κB in the signalling effects induced by the anti-ribosomal phosphoprotein monoclonal antibody (anti-P mAb) 9B6 mAb. (a, b) RAW 264.7 cells (5 × 104 cells/ml) were treated with wortmannin (wort.; 10 μm), calphostin C (cal.; 1·5 μm), NF-κB activation inhibitor (20 nm), IκB kinase (IKK)-2 inhibitor (30 μm) or dimethyl sulphoxide (DMSO; control) for 1 hr. After incubation, the cells were stimulated with lipopolysaccharide (LPS; 1 μg/ml) and mouse immunoglobulin G1 (mIgG1) or 9B6 mAb (100 μg/ml) for 48 hr. IL-10 production in the culture supernatant was determined by enzyme-linked immunosorbent assay (ELISA). The percentage of the LPS control was calculated as (IL-10 production in each group)/(IL-10 production in the LPS + DMSO control) × 100. (c) RAW 264.7 cells (5 × 104 cells/ml) were treated with SB202474 [a negative control for the mitogen-activated protein kinase (MAPK) cascade; 10 μm] or various specific pharmacological inhibitors including piceatannol (pice.; 10 μm), wortmannin (wort.; 10 μm), calphostin C (cal.; 1·5 μm), SP600125 (15 μm), PD98059 (20 μm), SB202190 (10 μm), NF-κB activation inhibitor (20 nm) or IKK-2 inhibitor (30 μm) for 1 hr. After incubation, the cells were stimulated with LPS (1 μg/ml) and mIgG1 or 9B6 mAb (100 μg/ml) for 48 hr. IL-10 production in the culture supernatant was determined by ELISA. IL-10 expression relative to mIgG1 (%) was calculated as (IL-10 production in LPS + individual inhibitor + 9B6)/(IL-10 production in LPS + individual inhibitor + mIgG1) × 100. (d) RAW 264.7 cells (1 × 106 cells/ml) were pretreated with piceatannol (pice.; 10 μm), wortmannin (wort.; 10 μm), calphostin C (cal.; 1·5 μm) or DMSO (control) for 1 hr. After incubation, the cells were treated with mIgG1 or 9B6 mAb (100 μg/ml) for 24 hr and then stimulated with LPS (1 μg/ml) for 30 min. Whole-cell lysates (60 μg/lane) were analysed by western blotting using phospho-specific antibodies against ERK1/2 or JNK1/2. β-actin was used as the loading control. The results shown are representative of three similar experiments (*P < 0·05).
To investigate the role of Syk on 9B6-induced ERK activation, we examined the effect of the Syk inhibitor in detail. As shown in Fig. 4(d), in LPS-activated macrophages, piceatannol decreased 9B6-induced ERK phosphorylation in comparison with that of the control mIgG1-treated group. Furthermore, we used other inhibitors to define the potential roles of PI3K and PKC in 9B6-induced activation of ERK and JNK. Our results showed that 9B6-induced phosphorylation of ERK and JNK was decreased when cells were pretreated with wortmannin or calphostin C (Fig. 4d). Taken together, these results confirm that Syk, PI3K and PKC are involved in 9B6-induced activation of ERK and JNK in LPS-activated macrophages.
AP-1, SRE and CRE in the IL-10 promoter are required for the anti-P mAb (9B6 mAb) response in LPS-activated macrophages
The promoter region of the IL-10 gene contains several potential binding motifs for NF-κB, AP-1, SRF and CREB.28 These transcription factor binding sites appear to be important for the induction of IL-10. To further investigate which of these transcriptional factors may be affected by 9B6 mAb in LPS-activated macrophages, we transiently transfected RAW 264.7 cells with plasmids containing the nuclear factor binding sites of NF-κB, AP-1, SRE or CRE, followed by a reporter (luciferase) gene. Under treatment with 9B6 mAb, activation of the AP-1, SRE and CRE reporters occurred in LPS-activated macrophages despite the fact that NF-κB reporter activity did not change (Fig. 5a).
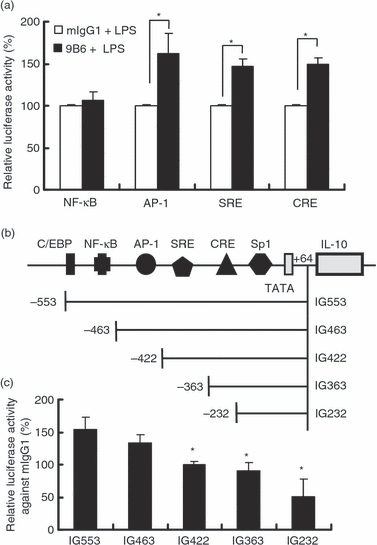
The interleukin (IL)-10 promoter regions covering cAMP-enhanced activation protein 1 (AP-1) binding sites, serum response element (SRE) and cyclic AMP response element (CRE) are required for transcriptional activation of the IL-10 gene by the anti-ribosomal phosphoprotein monoclonal antibody (anti-P mAb) 9B6 mAb. (a) RAW 264.7 cells (6 × 105 cells/ml) were transiently transfected with luciferase reporter constructs containing nuclear factor (NF)-κB, AP-1, SRE or CRE. After 12 hr, the transfected cells were treated with mouse immunoglobulin G1 (mIgG1) or 9B6 mAb (100 μg/ml) for 24 hr and then stimulated with lipopolysaccharide (LPS; 1 μg/ml) for 12 hr. Relative luciferase activity (%) was calculated as (luciferase activity in the mIgG1 control or 9B6 mAb treated group)/(luciferase activity in the mIgG1 control) × 100. (b) The potential consensus binding sites in the 5′-flanking region of the IL-10 promoter are indicated. (c) RAW 264.7 cells (6 × 105 cells/ml) were transiently transfected with reporter plasmids bearing various lengths of the IL-10 promoter. The transfected cells were treated with mIgG1 or 9B6 mAb (100 μg/ml) for 24 hr and then stimulated with LPS (1 μg/ml) for 12 hr. Relative luciferase activity against mIgG1 (%) was calculated as (luciferase activity in each group treated with 9B6 mAb)/(luciferase activity in each group treat with mIgG1 control) × 100. The mean of three independent experiments in each group was calculated (*P < 0·05). C/EBP, CCAAT/enhancer binding protein; Sp 1, stimulatory protein-1.
Sequence analysis suggested that several potential DNA response elements are present in the 553-bp promoter region of the mouse IL-10 gene (Fig. 5b). To study the transcriptional regulation of the IL-10 gene, luciferase reporter vectors bearing various lengths of the 5′-upstream region of the IL-10 promoter were constructed (Fig. 5b). As shown in Fig. 5(c), the augmentation effect of anti-P mAb on IL-10 was absent when the promoter region spanning −463 to −232 bp was deleted. Consistent with Fig. 5(a), this result confirmed that AP-1, SRE and CRE are required for the increase in IL-10 gene expression induced by anti-P mAb in LPS-activated macrophages.
Discussion
Anti-P Abs are SLE-specific and associated with clinical manifestations of SLE, including psychosis, nephritis and hepatitis.9 Previous studies have shown that anti-P Abs can bind to and penetrate cells, alter protein synthesis of hepatocytes,37 induce T-cell apoptosis8 and cause autoimmune depression in vivo.38 Furthermore, anti-P Abs bind to various cells via the P0 protein as well as FcγR on the cell surface of T cells and monocytes. This binding can induce the production of pro-inflammatory cytokines such as IL-6 and TNF-α in human monocytes.39 In our present and previous studies, anti-P mAb has been demonstrated to enhance IL-10 production in LPS-activated macrophages.22 The results suggest that the high levels of autoantibody produced in the later stage of SLE may promote Th2 responses and exacerbate the clinical manifestations of the disease, particularly susceptibility to bacterial infections. Typically, lupus patients have a variety of clinical symptoms, and patient characteristics of multiple organ involvement can vary among individuals. The reason for these variations is not fully understood; however, the specificity of autoantibodies and variations in the sensitivity of different tissues may influence progression of SLE.40
High levels of IL-10 have been found to be associated with human and murine lupus.18,20 Administration of anti-IL-10 neutralizing antibodies to humans and mice results in significant clinical improvements in SLE.41,42 These results suggest that IL-10 is directly involved in lupus pathogenesis. Earlier reports have shown that immune complexes from SLE sera induce IL-10 production by resting peripheral blood mononuclear cells and this involves an FcγR-dependent mechanism.43 9B6 mAb treatment alone was not able to induce IL-10 production in resting macrophages (data not shown). However, 9B6 mAb was able to enhance IL-10 production in the context of macrophage activation by LPS. Thus, the effects of anti-P mAb may not depend on formation of immune complexes. Our studies suggest that anti-P Abs play a role in the pathogenesis of lupus in a manner distinct from the direct effects produced by SLE immune complexes.
The polyreactivity of disease-related autoantibodies is one basic characteristic in which they differ from general monoclonal antibodies.44–46 In addition to reacting with P0, P1 and P2 proteins, 9B6 mAb may cross-react with other antigens. Following the mass spectrometry analysis, a 9B6-interacting candidate, glucose-regulated protein 78 (GRP78), was identified and verified by co-immunoprecipitation and western blotting (data not shown). GRP78 is also known as the immunoglobulin-binding protein BiP. Interestingly, it has been reported that BiP stimulates the secretion of large amounts of IL-10 from human peripheral blood mononuclear cells.47 The property of polyreactivity of 9B6 mAb may account for the autoantibody-induced IL-10 overproduction. A recent study has shown that ova-anti-ova immune complexes as well as immobilized anti-FcγR antibody (2.4G2) in combination with LPS enhance IL-10 production in dendritic cells (DCs).48 Both the ova-anti-ova immune complexes and immobilized anti-FcγR antibody (2.4G2) can cross-link to FcγR. This important work demonstrates that enhanced IL-10 expression is induced under LPS stimulation as well as FcγR ligation. In view of this finding, although we did not use immobilized antibody, we cannot exclude the possibility of cross-linking of FcγR in treatment with 9B6 in combination with LPS in our study.
Compelling data indicate that autoantibodies and their effector mechanisms are central players in the development of autoimmunity.49 Autoantibodies have been clearly identified as the primary cause of morbidity, and Fc receptors (FcRs) are the major mediators of autoantibody-mediated inflammation in SLE.50In vitro and in vivo, activation of cells by autoantibodies depends on interactions between activating and inhibitory FcγRs. The net effects of signalling events mediated by activating and inhibitory FcγRs govern the development of many human autoimmune diseases.43,49,50 In this study, anti-P mAb was dependent on FcγR for augmentation of IL-10 production in activated macrophages, as pretreatment with a competing mIgG1 attenuated the enhancing effects of anti-P mAb on IL-10 expression (Fig. 3a). Recent findings indicated that Syk is a novel modulator of LPS-mediated TLR4 responses including IL-10 production by human monocytes.26 In addition, FcγR engagement has been shown to be coupled functionally to activation of PI3K via the protein kinase Syk.35,36 Therefore, anti-P mAb may enhance IL-10 production via FcγR activation of Syk in LPS-activated macrophages (Fig. 3b). FcγR may be involved in the anti-P mAb (9B6 mAb)-mediated increase in IL-10 production in macrophages. In addition to FcγR, it is possible that anti-P Abs may bind P0 proteins on the cell surface of T and other cells, and then be internalized within cells to influence cellular functions.8,22
Our study shows that anti-P mAb-enhanced IL-10 production in LPS-activated macrophages is regulated by PI3K-dependent signalling cascades. Other reports indicate that ligation of FcγR in combination with LPS stimulation of murine macrophages leads to an increase in IL-10 production.51 In addition, inhibitors of the PI3K pathway decrease IL-10 production in LPS-stimulated monocytes.51 In this study, we also found that inhibition of Syk, PI3K or PKC decreased anti-P mAb-induced activation of ERK and JNK (Fig. 4d), which contributed to the reduced production of IL-10 in LPS-activated macrophages (3, 4). These results suggest that PI3K activation upstream of signalling cascades may be central to the effects of anti-P mAb on augmentation of IL-10 production in LPS-activated macrophages.
GSK3 has been shown to differentially regulate TLR-mediated production of pro- and anti-inflammatory cytokines.27,34 Stimulation of monocytes through TLR2 or TLR4 induces activation of PI3K which results in subsequent GSK3 inhibition, which leads to a substantial increase in IL-10 production.27,34 However, stimulation of monocytes with other pathological stimuli can lead to a distinct pro-inflammatory response with predominant activation of NF-κB pathways.27 In such a situation, PI3K is barely activated and as a result GSK3 remains constitutively active. GSK3 can inactivate CREB to regulate NF-κB signalling, in which CREB and NF-κB compete for co-activator protein (CBP) binding. It has been shown that activation of GSK3 is usually accompanied by increased NF-κB activity.52 Inhibition of GSK3 allows CREB to sequester CBP from NF-κB, which causes suppression of NF-κB activity.27,53 Therefore, inactivation of GSK3 may lead to promotion of CREB-dependent IL-10 production and suppression of NF-κB-dependent inflammatory cytokine expression. In accordance with these findings, in the present study anti-P mAb enhanced IL-10 production via the inactivation of GSK3, the activation of CREB, and the inhibition of nuclear translocation of NF-κB (2, 5).
IL-10 is a key cytokine in the control of infection and pathogenesis in SLE. Our data show that PI3K and Syk play crucial regulatory roles in anti-P mAb-induced IL-10 overproduction in LPS-stimulated macrophages. On the basis of the above results, we propose a molecular model to illustrate the mechanism underlying the augmentation of IL-10 production by anti-P autoantibody in LPS-activated macrophages (Fig. 6). LPS induces IL-10 production in macrophages through binding to the TLRs. After binding to the TLRs, LPS initiates signals via the mitogen-activated protein kinase (MAPK) kinase, PI3K and NF-κB pathways, which induce transcription of the IL-10 gene. Anti-P mAb further augments LPS-induced IL-10 production via binding to FcγR and then activates Syk and PI3K. Subsequently, the PI3K-dependent signalling cascades activate AKT, ERK and JNK, but decrease GSK3 and NF-κB activity. We found that increases in JNK and ERK activities, accompanied by decreased GSK3 activity, result in activation of transcriptional factors of AP-1 sites, SRE and CRE. These effects contribute to the increase in IL-10 production.
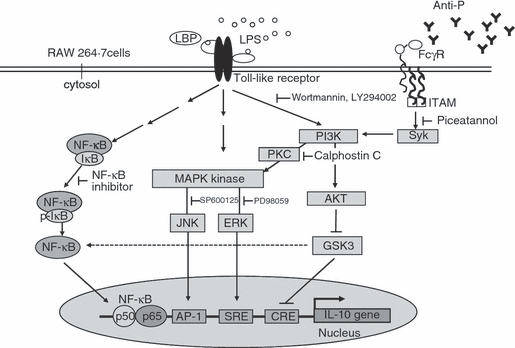
The molecular model illustrates the augmentation of interleukin (IL)-10 expression by anti-ribosomal phosphoprotein monoclonal antibody (anti-P mAb). In macrophages, anti-P mAb (9B6) augments lipopolysaccharide (LPS)-induced IL-10 production via binding to the Fc γ receptor (FcγR) and then activates spleen tyrosine kinase (Syk) and phosphatidylinositol 3-kinase (PI3K). Subsequently, anti-P mAb activates Akt (PKB; protein kinase B), extracellular signal regulated kinase (ERK) and c-Jun NH2-terminal kinase (JNK), while it decreases glycogen synthase kinase 3 (GSK3) and nuclear factor (NF)-κB activities. Increased activity of JNK and ERK, accompanied by decreased GSK3 activity, results in activation of cAMP-enhanced activation protein 1 (AP-1), serum response element (SRE) and cyclic AMP response element (CRE). These signalling effects contribute to the increase in IL-10 production. LBP, LPS-binding protein; ITAM, immunoreceptor tyrosine-based activation motif; PKC, protein kinase B.
In conclusion, as illustrated in Fig. 6, these data clearly demonstrate the roles of Syk, PI3K and GSK3 in mediating the effects of anti-P autoantibody on IL-10 expression, in which promoter regions covering AP-1, SRE and CRE are required. Taken together, our data suggest that anti-P autoantibodies can be directly involved in the immunological manifestations of SLE. Recently, a B-cell-targeted therapy has shown promise for treatment of SLE patients.54 In addition to available therapies, we believe that harnessing activities of relevant signalling molecules may provide alternative therapeutic opportunities to develop a new approach for the treatment of SLE.
Acknowledgements
We thank Dr Wen-Chang Chang for providing reporter plasmids bearing various lengths of the IL-10 promoter. This work was supported by grants from the National Science Council (95-2320-B-010-022-MY3), VGHUST Joint Research Program, Tsou’s Foundation (VGHUST94-P7-40), Yen Tjing Ling Medical Research Foundation (CI 95-8) and Taipei City Hospital, China.




