Peroxisome proliferator activated receptor γ is not necessary for the development of LPS-induced tolerance in macrophages
B. Zingarelli, Division of Critical Care Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Avenue, Cincinnati, OH 45229, USA. Email: [email protected]
James A. Cook, Ph.D., Department of Neurosciences, Medical University of South Carolina, Charleston, 173 Ashley Avenue, BSB Room 403, Charleston, South Carolina 29425, USA. Email: [email protected]Senior author: Basilia Zingarelli
Summary
Peroxisome proliferator activated receptor-γ (PPARγ) has been reported to exert anti-inflammatory properties in endotoxic shock and sepsis. One phenomenon that alters the inflammatory response to endotoxin [lipopolysaccharide (LPS)] is endotoxin tolerance, which is caused by previous exposure to endotoxin. Here, we investigate whether changes in endogenous PPARγ function regulate this phenomenon using three different models of LPS-induced tolerance in macrophages. In a first in vitro model, previous LPS exposure of murine J774.2 macrophages suppressed tumour necrosis factor-α (TNF-α) release in response to subsequent LPS challenge. Treatment of J774.2 cells with the PPARγ inhibitor GW9662 did not alter tolerance induction because these cells were still hyporesponsive to the secondary LPS challenge. In a second ex vivo model, primary rat peritoneal macrophages from LPS-primed rats exhibited suppression of thromboxane B2 and TNF-α production, while maintaining nitrite production in response to in vitro LPS challenge. Pretreatment of rats with the PPARγ inhibitor GW9662 in vivo failed to alter the tolerant phenotype of these primary macrophages. In a third ex vivo model, primary peritoneal macrophages with conditional deletion of PPARγ were harvested from LPS-primed Cre-lox mice (Cre+/+ PPARγ−/−) and exhibited significant suppression of TNF-α production in response to in vitro LPS challenge. Furthermore, both LPS-primed PPARγ-deficient Cre+/+ PPARγ−/− mice and wild-type Cre−/− PPARγ+/+ mice exhibited reduced plasma TNF-α levels in response to a high dose of LPS in vivo. These data demonstrate that PPARγ does not play a role in the LPS-induced tolerant phenotype in macrophages.
Abbreviations:
-
- 15d-PGJ2
-
- 15-deoxy-Δ12,14-prostaglandin J2
-
- EDTA
-
- ethylenediaminotetraacetic acid
-
- LPS
-
- lipopolysaccharide
-
- PMSF
-
- phenylmethylsulphonyl fluoride
-
- PPARγ
-
- peroxisome proliferator activated receptor-γ
-
- TBS
-
- tris buffered saline
-
- TNF-α
-
- tumour necrosis factor-α
Introduction
Peroxisome proliferator activated receptor-γ (PPARγ) is a member of the nuclear subfamily of transcription factors with pleiotropic effects on lipid and glucose homeostasis, cell proliferation, and control of inflammation.1 Several in vitro studies have demonstrated that pharmacological activation of PPARγ by its ligands, such as 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) or thiazolidinediones, has anti-inflammatory effects. For example, the PPARγ ligands 15d-PGJ2 and rosiglitazone repressed the expression of several inflammatory response genes in activated macrophages, including the genes encoding inducible nitric oxide synthase, tumour necrosis factor-α (TNF-α), gelatinase B and cyclo-oxygenase 2.2,3 Similarly, 15d-PGJ2 and troglitazone inhibited the production of TNF-α, interleukin-6 and interleukin-1β in activated human monocytes.3 In line with these findings, our studies and those of others have demonstrated that in vivo treatment with 15d-PGJ2 provides beneficial effects in endotoxic shock, improving survival, reducing expression of adhesion molecules and tissue leucosequestration in mice,4 and reducing multiple organ injury in rats.5 Similarly, in vivo treatment with the PPARγ ligands 15d-PGJ2 and ciglitazone improved survival, ameliorated haemodynamic performance and reduced the inflammatory response in rats subjected to peritonitis by caecal ligation and puncture.6 In contrast, it has been shown that pharmacological inhibition of PPARγ with the compound GW9662 augments the inflammatory response and organ injury in an in vivo model of haemorrhagic shock,7 thus supporting the hypothesis that PPARγ is part of an important endogenous anti-inflammatory pathway.
The phenomenon known as endotoxin tolerance is a state of altered responsiveness to bacterial endotoxin [lipopolysaccharide (LPS)] in the host immune cells, which has been described both in experimental animals and humans. Endotoxin tolerance can be induced both in vitro and in vivo.8–10 Immune cells in vitro, especially monocytes and macrophages, are rendered tolerant when they are exposed to low concentrations of endotoxin and exhibit a suppressed proinflammmatory mediator production when they are subjected to a secondary challenge with endotoxin. This phenomenon, also known as ‘cell reprogramming’, is also seen after priming with other bacterial components or with proinflammatory cytokines.11 Experimental animals that have been previously treated with a sublethal injection of endotoxin survive a subsequent lethal dose of bacterial endotoxin and exhibit an attenuation of the proinflammatory response.12 The study of endotoxin tolerance is of enormous clinical importance because patients with sepsis also exhibit prolonged systemic endotoxin tolerance, as evidenced by the fact that their circulating leucocytes have a reduced capacity to produce cytokines compared to cells of healthy controls.13 The molecular mechanisms underlying endotoxin tolerance are complex and remain to be defined. Secretion of soluble mediators, changes in LPS receptor complex expression or function, and alterations in toll-like receptor downstream signalling pathways have all been implicated.12
These observations prompted us to examine whether PPARγ may contribute to the macrophage ‘reprogramming’ during LPS-induced tolerance. We investigated whether inhibition of the endogenous function of PPARγ, by the pharmacological inhibitor GW966214 or by genetic deletion, could alter the development of the cell tolerant phenotype. By using three different models of cell priming, our results clearly showed that PPARγ is not necessary for the development of LPS-induced tolerance.
Materials and methods
In vitro LPS-induced tolerance in murine J774.2 macrophages
Murine J774.2 macrophages (American Type Culture Collection, Rockville, MD) were grown in Dulbecco’s modified Eagle’s medium (Gibco Technologies, Grand Island, NY) containing 10% fetal calf serum (FCS), penicillin (50 U/ml), and streptomycin (100 μg/ml) under standard incubation conditions at 37°C and 5% CO2. To induce tolerance, cells were incubated for 24 hr with a low concentration of LPS (100 ng/ml) from Salmonella enteritidis (Boivin preparation; Sigma/Aldrich, St Louis, MO). Macrophages were then subjected to a second challenge with a higher concentration of LPS (10 μg/ml) for 24 hr. Separate groups of cells were incubated with the PPARγ inhibitor GW9662 (1 μg/ml, Sigma/Aldrich) 24 hr before (pretreatment protocol), and/or at the same time as the secondary LPS challenge (pretreament + post-treatment protocol or post-treatment protocol only). The supernatants were then collected for TNF-α quantification.
LPS-induced tolerance in male rats and peritoneal macrophage harvest
In vivo tolerance was induced in male Long Evans rats (200–250 g b.w., Charles River, Wilmington, MA) by intraperitoneal (i.p.) injection of a sublethal dose of LPS (500 μg/kg). Control animals received a similar volume (0·25 ml) of phosphate-buffered saline. Concomitant with LPS priming, groups of animals were treated with the PPARγ inhibitor GW9662 (300 μg/kg) or an equal volume of vehicle by i.p. injection. At 24 hr after in vivo LPS priming, resident peritoneal macrophages were harvested through ice-cold RPMI-1640 lavage (10 ml). Once collected, the cells were spun down and resuspended in RPMI-1640 medium supplemented with 10% FCS, penicillin (200 U/ml) and streptomycin (200 μg/ml). The cells were seeded at 1 × 106 cells/well into six-well plates and stimulated with LPS (10 μg/ml) for 24 hr (in vivo pretreatment protocol). The supernatants were collected for the quantification of thromboxane B2 (TXB2), TNF-α and nitrite.
LPS-induced tolerance in PPARγ conditional knockout mice and macrophage harvest
PPARγ conditional knockout mice were generated using the Cre-loxP system where exon 2 of the PPARγ gene is removed once induction of the Cre recombinase takes place according to the design of Akiyama et al.15 Briefly, PPARγ deletion was induced through a series of five polyinosinic-polycytidylic acid (pIpC) i.p. injections into the PPARγ conditional knockout (Cre+/+ PPARγ−/−) mice. Cre−/− PPARγ+/+ mice were used as control wild-type mice. The injections were spaced 4 days apart. Three days before induction of tolerance, peritoneal macrophages were elicited by i.p. injections of 1% Biogel solution (Sigma-Aldrich). To induce tolerance, mice were then injected with a sublethal dose of LPS (5 mg/kg) or a similar volume (0·1 ml) of phosphate buffered saline.16 At 24 hr after in vivo LPS priming, peritoneal macrophages were harvested through ice-cold RPMI-1640 lavage (10 ml). Once collected, the cells were spun down and resuspended in RPMI-1640 medium supplemented with 10% FCS, penicillin (200 U/ml) and streptomycin (200 μg/ml). The cells were seeded at 1 × 106 cells/well into six-well plates and stimulated with increasing concentrations of LPS (0·1–1 μg/ml) for 18 hr. The supernatants were then collected for TNF-α quantification.
In a second set of experiments, at 24 hr after in vivo priming with the sublethal dose of LPS (5 mg/kg, i.p.), mice received a high dose of LPS (25 mg/kg, i.p.) and were killed 1 hr later. Plasma samples were obtained for TNF-α measurement by cardiac puncture.
Western blot analysis of PPARγ
Nuclear extracts were prepared from peritoneal macrophages of Cre-lox mice. After peritoneal harvest, cells were resuspended in a cold buffer containing 0·32 mm sucrose, 10 mm Tris–HCl, 1 mm ethyleneglycoltetraacetic acid, 2 mm ethylenediaminetetraacetic acid (EDTA), 0·2 μm NaN3, 50 mm NaF, 20 μm leupeptin, 0·15 μm pepstatin A and 0·2 mm phenylmethylsulphonyl fluoride (PMSF), and centrifuged at 3500 g at 4°C for 10 min. The pellet was resuspended in a buffer containing 20 mm HEPES pH 7·9, 420 mm NaCl,, 0·1 mm EDTA, 1·5 mm MgCl2, 25% glycerol, 1 mm dithiothreitol, 0·5 mm PMSF. After incubation in ice for 60 min, the pellet was recovered by centrifugation at 15 000 g at 4°C for 30 min. The supernatant (nuclear extract) was collected and stored at −70°C. Protein was quantified using a Bradford assay and the nuclear content of PPARγ was then determined by Western blot. Nuclear proteins (25 μg) were boiled in equal volumes of loading buffer (125 mm Tris–HCl, pH 6·8, 4% sodium dodecyl sulphate, 20% glycerol and 10% 2-mercaptoethanol) and separated electrophoretically in gel (10% Tris–glycine). After electrophoresis, proteins were transferred to nitrocellulose membranes. For immunoblotting, membranes were blocked with 5% non-fat dried milk in Tris-buffered saline (TBS) and 0·05% Tween for 1 hr and then incubated with primary antibodies against PPARγ (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hr at room temperature. The membranes were washed in TBS with 0·05% Tween-20 and incubated with secondary peroxidase-conjugated antibodies. Immunoreaction was visualized by chemiluminescence.
Measurement of TNF-α, TXB2 and nitrite levels
The proinflammatory cytokine TNF-α was measured in the supernatants or in plasma samples using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) and the protocol recommended by the manufacturer. The stable metabolite of TXA2, TXB2, was measured in the supernatant by radioimmunoassay.17 Nitrite production, an indicator of NO synthesis, was measured in the supernatant by the Griess reaction.16
Statistical analysis
All values in the figures and text are expressed as mean ± SEM of three or four separate experiments performed in triplicate for the in vitro studies, or four to six animals for each group for the in vivo studies. The results were examined by analysis of variance followed by the Bonferroni’s correction post hoc t-test. A P-value < 0·05 was considered significant.
Results
Pharmacological inhibition of PPARγ does not alter the induction of tolerance in J774.2 cells
Stimulation of control non-tolerant J774.2 cells with LPS (10 μg/ml) induced TNF-α production. As expected, previous incubation of J774.2 cells with 100 ng/ml LPS resulted in a significant decrease in TNF-α production in response to a subsequent LPS challenge (Fig. 1). To test whether PPARγ was involved in the development of the tolerant phenotype, groups of tolerant cells were incubated with the PPARγ inhibitor GW9662 (1 μg/ml) 24 hr before (pretreatment protocol), and/or at the same time as the secondary endotoxin challenge (pretreament + post-treatment protocol or post-treatment protocol only). Neither pretreatment, post-treatment or pre- and post-treatment with GW9662 significantly affected the development of LPS-induced tolerance (Fig. 1).
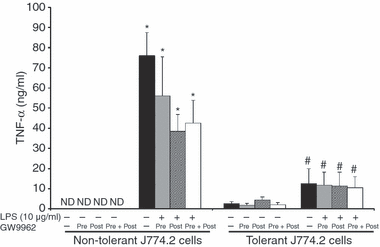
Effects of pharmacological inhibition of PPARγ on induction of tolerance in J774.2 macrophages. J774.2 cells were primed with LPS (100 ng/ml) for 24 hr. Cells were then challenged with a subsequent high concentration of LPS (10 μg/ml). Cells were incubated with the PPARγ inhibitor GW9662 (1 μg/ml) 24 hr before (pretreatment protocol), and/or at the same time as the secondary LPS challenge (pretreament + post-treatment protocol or post-treatment protocol only). Supernatant was collected 24 hr after the secondary LPS challenge for TNF-α measurement. Data are mean ± SEM of three separate experiments performed in triplicate. *Significantly different from non-tolerant cells without LPS (P < 0·05); #significantly different from non-tolerant cells with LPS (P < 0·05).
Pharmacological inhibition of PPARγ does not alter induction of tolerance in rats
In comparative experiments tolerance was induced in vivo in rats by administration of a sublethal dose of LPS (500 μg/kg, i.p.). Twenty-four hours later, peritoneal macrophages were harvested and stimulated in vitro with LPS (10 μg/ml). In peritoneal macrophages that were harvested from control, non-tolerant-rats, LPS significantly stimulated the production of TXB2 (51·9 ± 14·9 ng/ml), TNF-α (2455·9 ± 379·1 pg/ml) and nitrate (43·4 ± 6·22 μm). In contrast, in peritoneal macrophages harvested from tolerant rats, the in vitro LPS-induced production of TXB2 and TNF-α was significantly diminished (3·1 ± 1·7 ng/ml and 1328·5 ± 101·1 pg/ml, respectively; P < 0·05), whereas LPS-induced production of nitrite was maintained (51·1 ± 0·1 μm). To test whether PPARγ was involved in the development of the tolerant phenotype in vivo, groups of animals were treated with the PPARγ inhibitor GW9662 (300 μg/kg, i.p.) 24 hr before harvesting peritoneal macrophages (in vivo pretreatment protocol). Peritoneal macrophages were then stimulated in vitro with LPS (10 μg/ml). In vivo treatment with GW9662 did not alter the development of the tolerant phenotype in the primary peritoneal macrophages (Fig. 2).
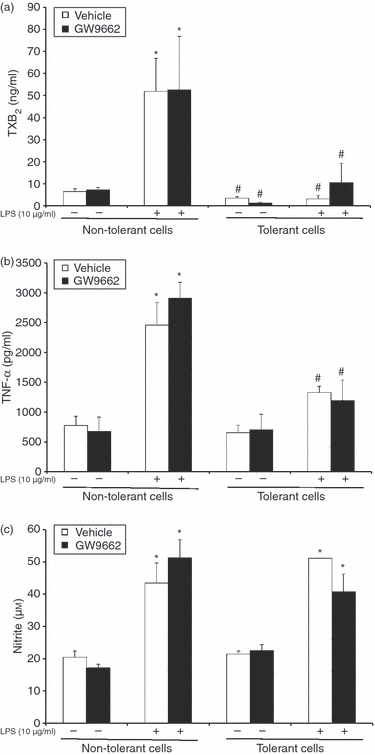
Effects of pharmacological inhibition of PPARγ on LPS tolerance induction in primary rat peritoneal macrophages. Tolerance was induced in vivo in rats by administration of a sublethal dose of LPS (500 μg/kg, i.p.). Groups of animals were treated in vivo with the PPARγ inhibitor GW9662 (300 μg/kg, i.p.). After 24 hr, peritoneal macrophages were harvested and stimulated in vitro with a secondary challenge of LPS (10 μg/ml). Supernatant was collected 24 hr after the secondary LPS challenge for measurement of TXB2 (a), TNF-α (b) and nitrite (c). Data are mean ± SEM of three separate experiments performed in triplicate. *Significantly different from non-tolerant cells without LPS (P < 0·05); #significantly different from non-tolerant cells with LPS (P < 0·05).
Genetic inhibition of PPARγ does not alter induction of tolerance in Cre-lox PPARγ conditional knockout mice
To further examine if PPARγ is involved in LPS-induced tolerance, macrophages elicited from PPARγ conditional knockout mice were used. Mice that are heterozygous (Cre+/−) or homozygous (Cre+/+) for the presence of the Cre enzyme have their PPARγ gene disrupted when treated with pIpC. Mice that are Cre−/− maintain an intact PPARγ gene even when treated with pIpC. Therefore, as evaluated by Western blot analysis, PPARγ protein was barely perceptible in nuclear extracts of peritoneal macrophages from Cre+/+ PPARγ−/− mice, but was expressed in the cells from Cre−/− PPARγ+/+ mice (Fig. 3). Tolerance was induced in the mice by in vivo administration of a sublethal dose of LPS (5 mg/kg, i.p.). Twenty-four hours later, peritoneal macrophages were harvested and stimulated in vitro with increasing concentrations of LPS (0·1–1 μg/ml). Stimulation of macrophages from non-primed (non-tolerant) mice with LPS for 18 hr induced a marked production of TNF-α in a dose-dependent fashion. Interestingly, peritoneal macrophages that had been harvested from LPS-primed (tolerant) Cre+/+ PPARγ−/− mice exhibited suppression of TNF-α production in response to in vitro LPS challenge (Fig. 4).
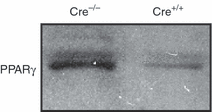
Representative Western blot of PPARγ expression in nuclear extracts of peritoneal macrophages of Cre−/− PPARγ+/+ (Cre−/−, with an intact PPARγ) and Cre+/+ PPARγ−/− mice (Cre+/+, with conditional disruption of PPARγ). Blot is representative of five animals for each group.
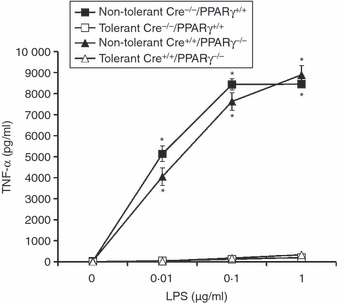
Effect of genetic inhibition of PPARγ on LPS tolerance induction in primary murine peritoneal macrophages. Tolerance was induced in vivo in Cre−/− PPARγ+/+ mice (i.e. mice with an intact PPARγ) and in Cre+/+ PPARγ−/− mice (i.e. mice with conditional disruption of PPARγ) by administration of a sublethal dose of LPS (5 mg/kg, i.p.). After 24 hr, peritoneal macrophages were harvested and stimulated in vitro with increasing LPS concentrations (0·01–1 μg/ml). Data are mean ± SEM of three separate experiments performed in triplicate. *Significantly different from cells taken from LPS-primed mice of the same genotype (P < 0·05).
In a separate experiment, we evaluated whether PPARγ might be involved in LPS-induced tolerance in vivo and whether it might alter the proinflammatory response in mice subjected to a secondary LPS challenge. As shown in Fig. 5, LPS pretreatment reduced the degree of TNF-α release in the plasma caused by a secondary lethal LPS administration both in Cre−/− PPARγ+/+ and Cre+/+ PPARγ−/− mice compared with non-primed animals. These data demonstrate that genetic deletion of PPARγ in macrophages does not play a role in the LPS-induced tolerant phenotype in vivo.
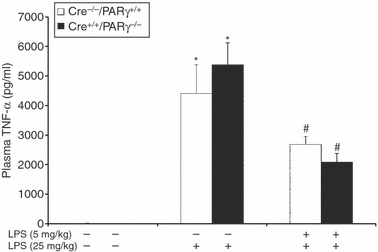
Effect of genetic inhibition of macrophage PPARγ on plasma release of TNF-α in Cre-lox mice subjected to administration of high-dose LPS. Tolerance was induced in vivo in Cre−/− PPARγ+/+ mice (i.e. mice with an intact PPARγ) and in Cre+/+ PPARγ−/− mice (i.e. mice with conditional disruption of PPARγ) by administration of a sublethal dose of LPS (5 mg/kg, i.p.). After 24 hr, mice were injected with a high dose of LPS (25 mg/kg, i.p.). Data are mean ± SEM of four to six animals for each group. *Significantly different from basal levels of naïve mice (P < 0·05); #significantly different from non-tolerant mice of the same genotype subjected to the second challenge of LPS.
Discussion
The generation of the LPS-tolerant phenotype is complex and multifactorial. Although LPS tolerance does not induce global suppression of mediators, a reduction in proinflammatory mediator production, e.g. TNF-α and TXB2, is usually observed; whereas NO production is still maintained and/or potentiated in endotoxin-tolerant animals.16,18–20 This altered mediator release has been linked to changes in molecular mechanisms at the plasma membrane receptor, cytosolic and nuclear levels of the host cell. This report focused on nuclear events during the activation of the nuclear receptor PPARγ. Our results demonstrate that inhibition of PPARγ function does not affect the development of LPS tolerance in macrophages.
We observed that pharmacological inhibition of PPARγ did not suppress the development of LPS-induced tolerance, either in vitro in cultured J774.2 cells or in vivo in rats. In both models, in vitro or in vivo LPS pretreatment effectively induced alteration of inflammatory mediator release to a secondary challenge with LPS in macrophages. In both cases the PPARγ antagonist GW9662 failed to reverse or prevent LPS tolerance, as cells were still hyporesponsive to the secondary LPS challenge.
Our data contrast with those from a recent study demonstrating that the PPARγ antagonists GW9662 and T0070907 reduce the protective effects of LPS preconditioning against renal and hepatocellular injury, and hypotension caused by endotoxaemia.21 However, the authors did not investigate the effects of PPARγ antagonisms in the development of tolerance in the macrophage/monocyte population. Similarly, Collin and colleagues have previously reported that inhibition of PPARγ with GW9662 given before haemorrhage significantly exacerbated the haemorrhage-induced increase in serum aspartate transaminase and alanine transaminase.22 However, in a similar study, GW9662 did not augment endotoxin-induced hepatic injury.5 Although our studies are not directly comparable, we found that in rat peritoneal macrophages and murine J774.2 cells GW9662 did not augment LPS stimulation. On the contrary, the compound appeared to inhibit LPS-induced TNF-α in the J774.2 cells. Although the reported IC50 for GW9662 is 7·6 nm,14 in our in vitro studies, this potent selective PPARγ antagonist was used at high concentrations (1 μg/ml) but did not alter the tolerant phenotype when added during the LPS priming or during the secondary challenging event. Taken together with the previous findings of Collins and colleagues,21,22 our data may suggest that whereas PPARγ may contribute to the tolerance phenomenon in parenchymal cells, it does not participate in the development of the tolerant phenotype in macrophages.
Other studies23 have also suggested involvement of PPARγ in monocyte hyporesponsiveness. Prestimulation of human monocytes and myeloid cell lines with LPS and interferon-γ provoked a profound reduction in the oxidative burst in response to stimulation with a potent oxidant. Using an electromobility shift assay and reporter gene assays, the investigators demonstrated that LPS tolerance activated PPARγ. Blockade of PPARγ function by a decoy DNA abrogated the effect of LPS tolerance on suppressing oxidative burst.23 Several experimental factors may explain the different regulatory mechanism of PPARγ between our models of LPS tolerance and the previous studies. In fact, it must be considered that our study examined LPS homologous tolerance to mediator production, which may differ from the priming events caused by the combination of LPS and interferon-γ, which lead to altered oxygen free radical formation in response to non-specific oxidative stress. Thus, the contribution of PPARγ in cell reprogramming may well be a cell/tissue type- and stimulus-specific event.
Since pharmacological inhibition of PPARγ with GW9662 may produce pharmacologically non-specific effects, we also explored the effects of genetic PPARγ deficiency in LPS tolerance. The PPARγ conditional knockout-derived macrophages from Cre-lox mice have afforded a unique opportunity to examine the exact role of endogenous PPARγ in LPS-induced activation and tolerance. Again, similarly to the results obtained with the pharmacological studies, the genetic absence of PPARγ did not diminish the ability of in vivo LPS pretreatment to suppress TNF-α upon secondary ex vivo LPS stimulation.
During endotoxic shock the excessive release of proinflammatory cytokines by macrophages can be self-destructive for the host and can lead to an uncontrolled inflammatory response, multiple organ failure and, ultimately, death. As the protective effect of LPS tolerance in endotoxin shock is associated with the attenuation of proinflammatory cytokine production, in particular TNF-α,12,13 we compared plasma levels of TNF-α following lethal LPS challenge in tolerant Cre−/− PPARγ+/+ and Cre+/+ PPARγ−/− mice. Induction of LPS tolerance not only blunted the release of TNF-α in Cre−/− PPARγ+/+ mice, i.e. those with a functional PPARγ in the macrophage population, but also reduced plasma levels of TNF-α in the Cre+/+ PPARγ−/− mice, i.e. those with a macrophage deletion of PPARγ, after challenge with lethal LPS.
In summary, we have demonstrated that PPARγ is not necessary for the development of LPS-induced tolerance in rodent macrophages. Whether PPARγ may contribute to the development of LPS tolerance in cell types other than macrophages and to development of heterologous tolerance to other micropathogen stimuli remains to be further explored.
Acknowledgements
This investigation was supported by grants from the National Institutes of Health to Dr Basilia Zingarelli (R01 GM-67202) and to Dr James A. Cook (R01 GM-27673).




