Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-β
Summary
Humans and other mammals coexist with a diverse array of microbes colonizing the intestine, termed the microflora. The relationship is symbiotic, with the microbes benefiting from a stable environment and nutrient supply, and the host gaining competitive exclusion of pathogens and continuously maintenance of the gut immune homeostasis. Here we report novel crosstalk mechanisms between the human enterocyte cell line, Caco2, and underlying human monocyte-derived DC in a transwell model where Gram-positive (G+) commensals prevent Toll-like receptor-4 (TLR4)-dependent Escherichia coli-induced semimaturation in a TLR2-dependent fashion. These findings add to our understanding of the hypo-responsiveness of the gut epithelium towards the microflora. Gut DC posses a more tolerogenic phenotype than conventional DC. Here we show that Caco2 spent medium (SM) induces tolerogenic DC with lower expression of maturation markers, interleukin (IL)-12p70, and tumour necrosis factor-α when matured with G+ and Gram-negative (G–) commensals, while IL-10 production is enhanced in DC upon encountering G+ commensals and reduced upon encountering G– bacteria. The Caco2 SM-induced tolerogenic phenotype is also seen in DC priming of naive T cells with elevated levels of transforming growth factor-β (TGF-β) and markedly reduced levels of bacteria-induced interferon-γ production. Caco2 cell production of IL-8, thymic stromal lymphopoietin (TSLP) and TGF-β increases upon microbial stimulation in a strain dependent manner. TSLP and TGF-β co-operate in inducing the tolerogenic DC phenotype but other mediators might be involved.
Abbreviations:
-
- G+
-
- Gram-positive bacteria
-
- G–
-
- Gram-negative bacteria
-
- LAB
-
- lactic acid bacteria
-
- SM
-
- spent medium
-
- IEC
-
- intestinal epithelial cells
-
- TLR
-
- Toll-like receptor
-
- TSLP
-
- thymic stromal lymphopoietin
-
- MFI
-
- mean fluorescence intensity.
Introduction
The intestinal epithelium plays key roles in maintaining immune homeostasis in the gut, not only as an indispensable barrier between the gut luminal content and the underlying immune cells, but also as an active player in both maintenance of tolerance toward the microflora and food antigens and in pathogen combat. Dendritic cells (DC) are considered as gate keepers of the immune system and contact between DC and the gut microflora is essential for a proper immune development and regulation. In the healthy state, subepithelial DC gain direct contact with the microflora through two routes: DC sample luminal bacterial antigens by expressing bacteria-induced tight junction proteins and passing their dendrites between epithelial cells into the gut lumen,1,2 and DC interact directly with bacteria that have gained access via specialized epithelial cells, termed M cells.3 It has been shown in various studies that DC respond differentially to gut-derived lactic acid bacteria (LAB) and Gram-negative (G–) commensals in vitro.4,5 Intestinal epithelial cells (IEC), on the other hand, have been shown to be largely unresponsive to the gut flora;6,7 however, a general agreement regarding the capacity of IEC to influence the responsiveness of DC towards the microflora exists, although the mechanisms behind this crosstalk are largely unknown. The local microenvironment has vast influence on the priming ability of DC and evidence points toward gastrointestinal tract DC as several unique subsets, which tends to prime for T helper 2 (Th2) cells8–10 or T regulatory (Treg) cells.11 It is still unknown whether this phenotype represents ‘specialized’ gastrointestinal tract DC subsets or whether it is simply a consequence of the gastrointestinal tract microenvironment comprising factors produced by IEC, which could modulate DC phenotype. Recently, evidence supporting the latter concept has emerged; Rimoldi et al.12 have shown that monocyte-derived DC resemble human colon DC in promoting Th2 cell differentiation after conditioning with supernatant of Caco2 cells and suggested that the responsible component is thymic stromal lymphopoietin (TSLP). TSLP is constitutively released by IEC and plays a major role in the crosstalk between IEC and DC in allergic diseases both in humans and mice.13 It is likely that TSLP and other IEC-derived factors are critical for conditioning gastrointestinal mucosal DC and maintaining mucosal homeostasis. Whether the production of IEC-derived mediators is affected by the presence and composition of the microflora is unknown, but it is possible that the microflora influences the underlying DC indirectly, through the IEC.
Because the IEC are constantly exposed to the resident microflora, mechanisms of IEC exist to ensure tolerance. On the other hand, IEC is the first line of defence against pathogens and therefore crucial in fast initiation of immune response. IEC express several members of the Toll-like receptor (TLR) family. Literature on the expression of TLR on IEC is controversial. Several studies have reported that IEC are non-responsive towards lipopolysaccharide (LPS) and express low levels of TLR414–16 while other groups have reported LPS responsiveness and the presence of TLR4.7,17,18 Otte et al.7 have shown that IEC gain a cross-hyporesponsive phenotype after long-term stimulation with either LPS or lipoteichoic acid (LTA) because of decreased expression of TLR2 and TLR4. Cario et al.17 elegantly demonstrated that both TLR2 and TLR4 are constitutively expressed apically in IEC but traffic to cytoplasmic compartments after ligand stimulation. Primary IEC express both TLR2 and TLR4 but at low levels both in humans19 and mice.20 The maturation state of the epithelial cells may play a role in TLR expression, as studies have shown that human colonic epithelial crypt cells express TLR2 and TLR4 while expression is lost when they mature and move towards the gut lumen.21 Recently, it has been suggested that ligation of TLR expressed by IEC enhances the induction of DC extension and protrusion through the epithelial barrier.22
In this study, we addressed the mechanisms by which the IEC may influence the DC response towards an array of commensals, comprising both G+ and G– bacteria. Using Caco2 cells alone, cocultured with DC or as a conditioner of DC prior to bacterial stimulation we here demonstrate that distinct bacteria have different potential to influence Caco2 cells, DC and the interaction between them. G– bacteria induce semimature DC through Caco2 cell barrier and this effect can be abrogated by G+ bacteria, demonstrating an involvement of both TLR4 and TLR2 on Caco2 cells. The most profound effect of Caco2 cells on DC was the capacity of soluble factors from Caco2 cells to modulate the DC response towards commensals. Our results indicate that the presence and composition of the microflora affect the production of IEC-derived TSLP and transforming growth factor-β (TGF-β) and thereby influences the underlying DC. Furthermore, we show that the modulatory effects of TSLP and TGF-β are highly dependent on the bacterial stimulus. This novel two-route mechanism, in which the composition of the microflora affects production of TGF-β and TSLP in IEC, and the effect of these mediators differentially affect DC priming depending on the composition of the flora, adds to our understanding of the pivotal interaction of the flora with the host.
Materials and methods
Preparation of ultraviolet (UV)-killed lactic acid bacteria (LAB) and G– bacteria
The bacterial strains used in this study were primarily gut flora-derived (Table 1). Lactobacilli and bifidobacteria were grown anaerobically overnight at 37° in de Man, Rogosa, and Sharpe broth (Merck, Darmstadt, Germany), while strains of Escherichia coli and Klebsiella pneumoniae were grown aerobically in Luria–Bertani broth (Merck). The cultures were harvested, washed twice in sterile phosphate-buffered saline (PBS; pH 7·4) and re-suspended in 1/10 the growth volume of PBS. The bacteria were killed by a 40-min exposure to UV light and stored at −80°. Dry matter content was determined by lyophilization (corrected for buffer salt content). Endotoxin levels were determined with the Pyrochrome kit (Ass. of Cape Cod, East Falmouth, MA) to below 0·05 endotoxin units/ml in the highest concentrations used (100 µg/ml).
| Name | Origin |
|---|---|
| Lactobacillus paracasei CRL431 | Child, faecesa |
| Lactobacillus acidophilus X37 | Adult, biopsyb |
| Lactobacillus paracasei Z11 | Adult, biopsyb |
| Lactobacillus reuteri DSM 12246 : 12002 | Pig, faecesb |
| Lactobacillus rhamnosus GG | Adult, faecesc |
| Bifidobacterium longum Q46 | Adult, biopsyb |
| Bifidobacterium bifidum Z9 | Adult, biopsyb |
| Bifidobacterium infantis 20088 | Child, faecesb |
| Escherichia coli Nissle 1917 O6:K5:H1 | Adult, faecesd |
| Escherichia coli F18 OR: K1:H5 | Adult, faecesd |
| Escherichia coli BJ4 OR:K–:H2 | Rat, faecesd |
| Escherichia coli MG1655 OR:K–:H48 | Laboratoryd |
| Escherichia coli UTI | Urinary tractd |
| Klebsiella pneumoniae T74421-N | Adult, faecesd |
| Klebsiella pneumoniae H79129-N | Adult faecesd |
- a Chr. Hansen A/S, Hørsholm, Denmark.
- b Faculty of Life Science, University of Copenhagen, Denmark.
- c Valio, Helsinki, Finland.
- d Statens Serum Institut, Copenhagen, Denmark.
Caco2 cell culture
Upon 90% confluence of the human colon cancer cell line passage 25–35, Caco2, the cells were trypsinized (Trypsin-ethylenediaminetetra-acetic acid, Gibco, Taastrup, Denmark), re-seeded in 48-well plates and maintained in Dulbecco's modified Eagle's minimal essential medium (DMEM) supplemented with 2 mm l-glutamine, 10% (v/v) heat-inactivated FCS (Cambrex BioWhittaker, Verviers, Belgium), 100 U/ml penicillin, 100 µg/ml streptomycin, and 1% non-essential amino acids. Medium was exchanged every other day, and after 7 days the differentiated confluent monolayer was stimulated with UV-killed or live bacteria at concentrations as indicated for 18 hr. When Caco2 cells were stimulated with live bacteria, bacteria were grown, adjusted to optical density (OD) 1, washed twice in culture media and diluted 100 times in culture media supplemented with 50 µg/ml kanamycin (Fluka, St. Gallan Switzerland) prior to stimulation. Dry weight correlated with OD with some variation between strains, in average 1 OD unit corresponded to 1·0 ± 0·2 mg/ml dry weight for the G– bacteria and 3·0 ±8 mg/ml for G+ bacteria. For coculture experiments with DC, the medium was exchanged to RPMI-1640 supplemented with 2 mm l-glutamine, 10% (v/v) heat-inactivated fetal calf serum (FCS; Cambrex Bio Whittaker), 100 U/ml penicillin, 100 µg/ml streptomycin and 50 µm 2-mercaptoethanol (2-ME; culture medium). Caco2 spent medium (SM) was harvested after 2 days incubation of Caco2 cells in culture medium in culture flask and sterile filtered (0·22 µm pores).
In vitrogeneration of dendritic cells from monocytes
Human PBMC were obtained after Ficoll density-gradient centrifugation of buffy coats from healthy donors from the local blood bank. DC were generated from PBMC as described earlier.4 Ninety to 95% of the cells expressed the DC-marker CD1a measured by flow cytometry and tryphan blue exclusion indicated that cultures contained ≥ 95% viable cells. The immature DC were harvested and re-seeded in 48-well tissue culture plates (Nunc) at 6 × 105 cells/500 µl/well in either culture medium or Caco2 SM. UV-killed bacteria or TLR ligands (E. coli O26:B6 LPS, Staphylococcus aureus LTA (Sigma-Aldrich, St. Louis, MO), Pam3CSK4 and muramyl dipeptide (MDP; Invivogen, San Diego, CA)) were added in 100 µl/well in final concentrations as indicated. DC were incubated for 18 h at 37° in a 5% CO2 humidified atmosphere as the concentration of cytokines was found to peak after 18 hr independent of the presence or absence of Caco2 SM. Neutralization of TGF-β1 and TSLP (antibodies from R & D, Minneapolis, MN) in Caco2 SM was performed 2 hr prior to incubation with DC at 2·5 µg/ml and 1 µg/ml, respectively, while 100 pg/ml rhTSLP and 1000 pg/ml rhTGF-β1 (R & D) were added to DC 1 hr prior to stimulation. Isotype control antibodies to anti-TGF-β1 and anti-TSLP had no effect on DC maturation.
Transwell coculture system
Caco2 cells (passage 25–35) were grown for 21 days on 12-mm transwell polycarbonate membranes with 0·4 µm pores (no. 3401, Costar, Cambridge, MA) in DMEM supplemented with 2 mm l-glutamine, 10% (v/v) heat-inactivated FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, and 1% non-essential amino acids (all Cambrex BioWhittaker). Medium was exchanged every other day. Monocyte-derived DC were generated as described above and added basolaterally as 1 × 106 cells/1000 µl/well in culture medium. Bacteria were added apically in 500 µl culture medium and the coculture system was incubated for 24 hr before DC were harvested for flow cytometric analysis and supernatant was harvested for enzyme-linked immunosorbent assay analysis (ELISA). Neutralization of TLR2 and TLR4 was performed 1 hr prior to stimulation by apical addition of 50 µg/ml of antibodies (eBioscience, San Diego, CA). Isotype-matched control antibodies had no effect on the maturation of the underlying DC.
Immunostaining and flow cytometry
DC were stained and analysed using a BD FACSArray flow cytometer (BD Biosciences, San Jose, CA) based on counting 10 000 cells. The following antibodies were used for staining: phycoerythrin (PE)-conjugated anti-human CD1a, allophycocyanin (APC)-conjugated anti-human CD83, PE-conjugated anti-human TGF-βRI, II, III (BD Biosciences), PE-conjugated anti-human human leucocyte antigen (HLA)-DR, PE-conjugated anti-human CD86, PE-conjugated anti-human CD80, APC-conjugated anti-human CD40 (Southern Biotech, Birmingham, AL), and PE-conjugated anti-human-TSLPR (eBioscience) all with matched isotype controls. The level of expression was expressed as the geometric mean of fluorescence intensity (MFI).
Co-culture of DC with naïve CD4+ T cells
Human naïve CD3+CD4+CD45RA+ T cells were isolated from PBMC by negative selection using magnetic-activated cell sorting naïve CD4+ T-cell isolation kit (Miltenyi Biotech, Bergisch Gladbach, Germany) and cultured at a cell density of 1 × 105 cells/well with allogeneic 2 × 104 DC/well in round-buttoned 96-well tissue culture plates (Nunc) for 6 days in culture medium or Caco2 SM containing 1 µg/ml UV-killed bacteria as indicated or medium alone. As controls, DC and T cells were cultures separately in the presence or absence of Caco2 SM and bacteria.
Cytokine quantification in culture supernatants
The production of interleukin (IL)-12(p70), IL-6, TSLP, TGF-β1, IL-4 and tumour necrosis factor-α (TNF-α) was analysed using commercially available ELISA kits (R & D), interferon-γ (IFN-γ) with ELISA kit from Biosource, Camarillo, CA, and IL-10 and IL-8 with ELISA kits from BD Biosciences. The TGF-β1 assay included an activation step of the latent form of TGF-β1 prior to the assay and culture medium contained 956 ± 49 pg/ml (this background was subtracted from the presented data).
Statistical analysis
Statistical analysis (one-way anova with Tukey post test or two-way anova with Bonferroni post test) was performed using the GraphPad Prism version 4.03 (GraphPad Software, San Diego, CA). Differences were considered significant if P < 0·05 or lower.
Results
Caco2 cells produce IL-8, TGF-β1 and TSLP in response to G+ and G– commensals
To evaluate the epithelial responsiveness to distinct gut-derived G+ and G– commensals we used the Caco2 cell line, which closely resemble epithelial cells of small intestinal villi and crypts forming apical microvilli and prominent tight junctions as a model.23 Commensals induced a highly varied production of IL-8 and TSLP in non-polarized Caco2 cells, whereas less variation was seen for TGF-β1 (Fig. 1). In general, the IL-8 production was highest for the G– bacteria with the strains of Klebsiella being the most potent of the tested strains (800–900 pg/ml), while the IL-8-inducing capability varied between the different strains of E. coli with levels from 500 to 900 pg/ml. The Bifidobacterium genus was more potent (300–500 pg/ml) in inducing Caco2 cell-derived IL-8 production than the lactobacilli genus (100–300 pg/ml). The non-stimulated Caco2 cells produced relatively large amounts TGF-β1, and the level was elevated upon bacterial stimulation from 1000 pg/ml to 1500–2500 pg/ml for 100 µg/ml bacteria in dose-dependent manner. Significant difference between genera was only found between lactobacilli and E. coli. TSLP was produced in highest concentrations in the presence of G– bacteria (75–125 pg/ml) while LAB induced 25–100 pg/ml. For all cytokines, the pattern from stimulation with UV-killed bacteria resembled the pattern obtained with live bacteria, but the live bacteria were more potent in inducing cytokine production, because the concentration corresponded to around 10 µg/ml for the G– bacteria and 30 µg/ml for the G+ bacteria. TNF-α production by Caco2 was below the detection limit in ELISA (<12 pg/ml). For comparison the same experiment was conducted using HT29 cells and the same results were obtained with the only difference being a higher production of IL-8 (data not shown).
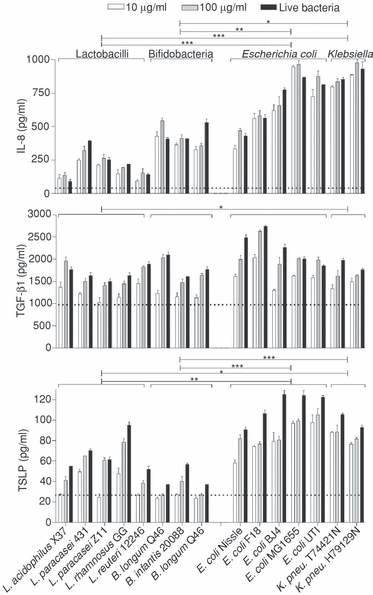
Non-polarized Caco2 cells produce IL-8, TGF-β1 and TSLP in response to G+ and G– commensals. The culture supernatant of Caco2 cells was analysed for IL-8, TGF-β1 and TSLP after 18 hr stimulation with UV-killed or live commensal bacteria. The dotted line represents cytokine production in non-stimulated Caco2 cells. Live bacteria were washed and diluted to 0·01 OD units corresponding to approximately 10 µg/ml for G– bacteria and 30 µg/ml for G+ bacteria. Data are means and SD of triplicate cultures. Data are representative of four experiments. Data were analysed by two-way anova and Bonferroni post-test to compare genera. Significant difference between genera is represented as *P < 0·01, **P < 0·05 and ***P < 0·001.
Only G– bacteria induce semimaturation of DC underlying a Caco2 cell layer in a TLR4-dependent manner
In earlier studies we have seen that gut-derived commensals differentially induce maturation patterns in monocyte-derived DC.4 In the gut, only a very limited number of the commensals are in direct contact with DC, while a substantial part may interact with DC through the epithelium. Accordingly, we studied the effect of selected commensals in a transwell system where polarized Caco2 cells separate the bacteria from the basolaterally located DC. Caco2 cells grown on a porous membrane form tight junctions and develop a strong barrier to diffusion; hence effects of apically added bacteria on DC present basolaterally are a consequence of cross-talk between the epithelial cells and DC. When screening the bacteria included in Table 1, we found that only G– bacteria were able to induce a semimaturation state in the DC through the Caco2 layer, while addition of LAB did not mature the DC (Fig. 2). The induction of maturation markers by E. coli Nissle and Lactobacillus reuteri DSM12246 is shown in Fig. 2(a) as representatives of G– and G+ commensals. The maturation status induced by E. coli was characterized by a modest increase in the expression of CD83, CD80 and HLA-DR while expression of CD86 and CD40 was not increased, along with a low expression of TNF-α (IL-10, IL-6 and IL-12p70 were below the detection limit of the ELISA; <12 pg/ml), while L. reuteri was unable to mature the DC. Since all G– bacteria in Table 1 along with LPS-induced similar maturation patterns, it was tested whether TLR4 was involved, and indeed, blocking of TLR4 almost totally abrogated the maturation induced by E. coli both in relation to surface markers and TNF-α production.
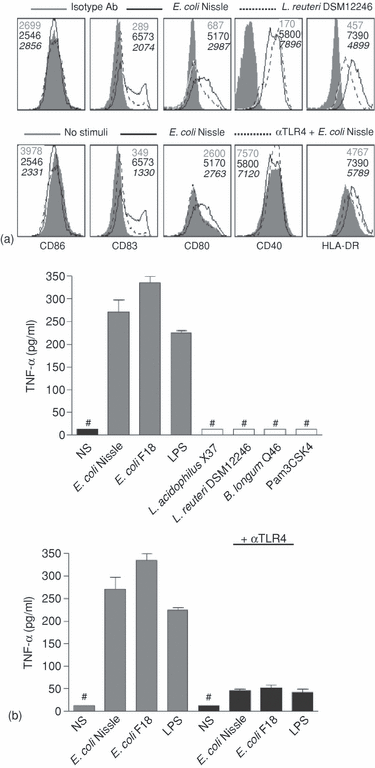
Only G– bacteria induce semimaturation of DC underlying a Caco2 cell layer in a TLR4-dependent manner. Bacteria were added apically to fully differentiated Caco2 cells in a transwell coculture system with monocyte-derived DC present basolaterally and incubated for 24 hr. (a) Expression of surface markers on DC after apical stimuli of Caco2 cells. Filled histograms represent isotype control antibodies in the upper panel and non-stimulated DC in the lower panel, solid lines represent E. coli Nissle-stimulated Caco2 and dotted lines represent L. reuteri 12446-stimulated Caco2 in the upper panel and E. coli Nissle-stimulated Caco2 after neutralization with αTLR4 antibody apically in the lower panel. Numbers in grey represent MFI of the grey histogram, black numbers the black line and numbers in italic refers to the dotted line. (b) Basolateral TNF-α production by DC after apical stimulation of Caco2 cells as indicated with 100 µg/ml bacteria. ‘#’ indicates: not detected. Data are means and SD of duplicate cultures. Data are representative of four experiments with cells from different donors.
LAB abolish E. coli Nissle-induced semimaturation of underlying DC through a Caco2 cell layer in a TLR2-dependent manner
Because data pointing towards a balanced microflora as a major factor for maintaining homeostasis are accumulating, the maturation status of DC was assessed when the Caco2 layer was incubated with LAB and E. coli Nissle or LPS simultaneously. All strains of LAB (Table 1) prevented the E. coli or LPS-induced maturation of underlying DC (Fig. 3) both in relation to expression of surface markers (as an example the inhibitory effect of L. reuteri is shown in Fig. 3a) and cytokine production of DC (Fig. 3b). Because the prevention of E. coli-induced DC-maturation seemed a general feature of LAB, we tested whether TLR2 was involved. Interestingly, neutralization of TLR2 re-established the maturation status induced by E. coli or LPS in the absence of LAB. This finding strongly indicates an involvement of TLR2 in the inhibitory effect of LAB and led us to consider which component of LAB that is responsible, and hence we tested synthetic TLR2 ligands (Pam3CSK4 and MDP) added apically in our coculture system. We found no inhibitory effect of the TLR2 ligands on DC maturation induced by apical E. coli or LPS, implying that only intact LAB were able to induce a TLR2-dependent inhibitory signal in the Caco2 cells (data not shown).
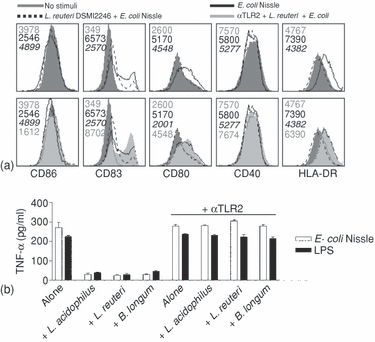
LAB abolish E. coli Nissle-induced semimaturation of underlying DC through a Caco2 cell layer in a TLR2-dependent manner. Bacteria were added apically to fully differentiated Caco2 cells in a transwell coculture system with monocyte-derived DC present basolaterally and incubated for 24 hr. (a) Expression of surface markers on DC after apical stimulation of Caco2 cells. Filled grey histograms represent no stimuli (numbers in grey refer to MFI), solid lines represent E. coli Nissle-stimulated Caco2 cells (numbers in black refer to MFI), dotted lines represent L. reuteri 12246- and E. coli Nissle-stimulated Caco2 cells (numbers in italic refer to MFI), and silver filled histograms represent stimulation with L. reuteri 12246 and E. coli Nissle after apical neutralization of Caco2 cells with anti-TLR2 antibody (numbers in silver refer to MFI). (b) Basolateral TNF-α production by DC after apical stimulation of Caco2 cells with 100 µg/ml bacteria as indicated. Data are means and SD of duplicate cultures. Data are representative of four experiments with cells from different donors.
The maturation pattern of human monocyte-derived DC changes in the presence of Caco2 spent medium
Next we investigated how non-polarized Caco2 spent medium (SM) changes the DC responses towards gut-derived commensals. Butler et al. have characterized the phenotype of immature monocyte-derived DC either in contact with Caco2 cells or only in contact with basolaterally secreted factors from Caco2 cells in a transwell system and concluded that cell contact was not needed for lowering expression of surface markers.24 Hence we chose to study the effect of the spent medium from non-polarized Caco2 on DC maturation. The presence of Caco2 SM more than halved bacterially induced levels of IL-12p70 (Fig. 4a). Caco2 SM had a much stronger inhibitory effect on TNF-α production when DC were matured by LAB or TLR2 ligands than when matured by G– bacteria or LPS. The IL-6 production was reduced only weakly by the Caco2 SM, and this reduction was only significant for about half the strains and TLR ligands tested. Interestingly, Caco2 SM more than halved the production of IL-10 in response to G– bacteria and TLR ligands, while IL-10 produced in response to G+ bacteria was elevated. The expression of CD80, CD83, HLADR and CD40 on immature DC, G– bacteria-matured DC and LAB-matured DC was differentially altered by the presence of Caco2 SM (only one representative strain of each genus is shown in Fig. 4b). In general, the reduced expression caused by the Caco2 SM was most pronounced for HLA-DR, CD83 and CD40, while CD80 was not affected. The effect was more pronounced for DC matured by G– bacteria and bifidobacteria than DC matured by lactobacilli. These novel findings suggest that not only does the presence of epithelial cells change the microenvironment, but these changes in the environment also alters the DC maturation pattern differentially dependent on the bacteria the DC encounter.
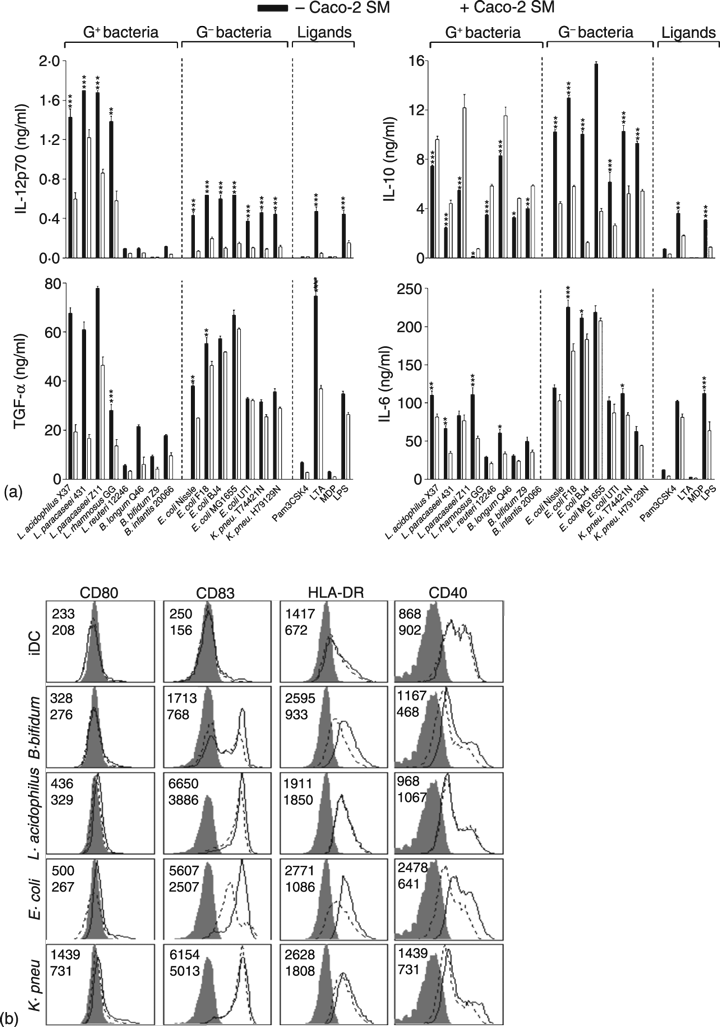
The maturation pattern of human monocyte-derived DC changes in the presence of Caco2 spent medium (SM) (a) Cytokine production by DC upon maturation with commensal bacteria (5 µg/ml) in the presence or absence of Caco2 SM. Data are means and SD of triplicate cultures. Differences in DC responses in the presence or absence of Caco2 SM was tested with one-way anova with Tukey post test, ***P < 0·001, **P < 0·01 and *P < 0·05. (b) Up-regulation of costimulatory molecules and HLA-DR induced by no stimuli (iDC), B. longum Q46, L. acidophilus X37, E. coli Nissle or K. pneumonia H79129N in the presence or absence of Caco2 SM. The filled histogram is the isotype control antibody, while the solid line represents expression on DC without Caco2 SM (upper numbers refer to MFI) and the dotted line with Caco2 SM (lower numbers refers to MFI). Data are representative of four experiments with cells from different donors.
Caco2 spent medium changes DC priming of naïve T cells away from a Th1 response
Because Caco2 SM induced lower expression of maturation markers and reduced the production of IL-12p70 and TNF-α in response to commensals, we investigated whether this modulation would affect the differentiation of naïve allogeneic CD4+ T cells. As shown in Fig. 5, IFN-γ production was markedly reduced in the presence of Caco2 SM, while TGF-β1 was increased (IL-4 was not detectable by intracellular staining or in ELISA). TGF-β1 is produced by both DC and CD4+ cells and therefore the levels depicted in Fig. 5 are not solely T-cell derived. Both immature and bacteria-matured DC (in the absence of T cells) produced TGF-β1 at levels around 200 pg/ml (data not shown), therefore the levels depicted in Fig. 5 in the absence of Caco2 SM are mainly DC derived. Because of donor variation, three DC donors are included in Fig. 5, however, the reduction in IFN-γ production and elevation in TGF-β1 production in the presence of Caco2 SM were evident for all donors and for DC matured by different stimuli. L. acidophilus and E. coli-matured DC were more potent in inducing a Th1 phenotype than L. reuteri-matured DC, which is in line with the levels of IL-12p70, TNF-α, and surface markers induced by these selected strains (Fig. 4). There was no cytokine production by T cells in the absence of DC.
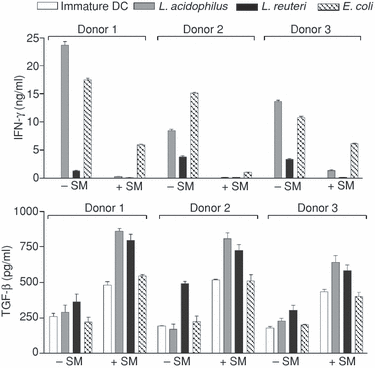
Caco2 spent medium (SM) changes DC priming of naïve T cells away from a Th1 response. IFN-γ and TGF-β1 produced by naïve CD4+ allogeneic T cells incubated for 6 days with immature DC or matured by L. acidophilus X37, L. reuteri 12246, or E. coli Nissle (1 µg/ml) in the presence or absence of Caco2 SM from three donors. CD4+ T cells incubated without DC did not produce measurable amounts of cytokine either in the absence or presence of Caco2 SM. Data are means and SD of triplicate cultures. T-cell cytokine responses were significantly different in the absence and presence of Caco2 SM (P < 0·01) for all donors and for all bacteria tested (except IFN-γ production induced by immature DC). Data are representative of two experiments with DC from six different donors in total.
The Th1-inhibiting effect of Caco2 spent medium is both TSLP and TGF-β dependent
We next speculated as to which Caco2-derived factors could be responsible for the observed effects of Caco2 SM on the DC maturation pattern upon bacterial stimulation. TSLP and TGF-β are known to be secreted by IEC12,25. Indeed, non-polarized non-stimulated Caco2 cells produced both cytokines, and this production was elevated upon stimulation with commensals (Fig. 1). This prompted us to test the roles of TSLP and TGF-β by two approaches: addition of rhTSLP and rhTGF-β1 to DC during maturation in concentrations corresponding to those found in Caco2 SM and neutralization of TSLP and TGF-β1 in the Caco2 SM prior to incubation with DC. Addition of rhTSLP significantly reduced the production of IL-12p70 and TNF-α and induced a small increase in production of IL-6 and IL-10 (Fig. 6). However, this was not true for E. coli-matured DC, where rhTSLP only reduced TNF-α production but not IL-12p70 production, which was slightly increased. Addition of rhTGF-β1 totally abolished IL-12p70 production and markedly reduced TNF-α and IL-6 production, while IL-10 remained unchanged. The combination of the two factors induced the same maturation pattern as seen with rhTGF-β1 alone, but with higher IL-6 levels. Both neutralization of TSLP and TGF-β1 abolished to some extent the effect of Caco2 SM in regard to all four cytokines with the most pronounced effects observed when both TSLP and TGF-β1 were neutralized simultaneously. As shown in Fig. 4, Caco2 SM also inhibited expression of DC surface markers. Addition of rhTGF-β1 reduced expression of CD40, CD80, CD83 and HLA-DR more or at least as effectively as Caco2 SM, while addition of rhTSLP also reduced expression but not consistently for all surface markers (data not shown).
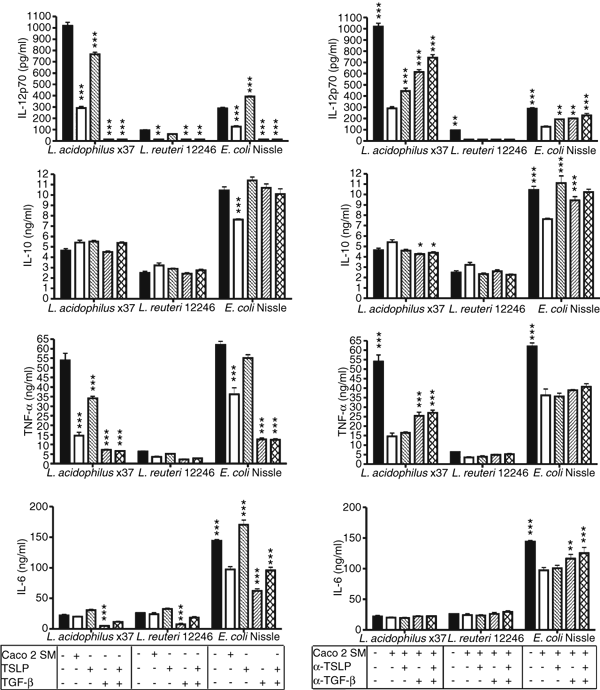
The Th1 inhibiting effect of Caco2 spent medium (SM) is both TSLP and TGF-β dependent. Cytokine production by DC after maturation with L. acidophilus X37, L. reuteri 12246, E. coli Nissle (5 µg/ml) in the presence or absence of: Caco2 SM, human recombinant TSLP (100 pg/ml) and TGF-β1 (1000 pg/ml) and neutralizing αTSLP (1 µg/ml) or αTGF-β1 (2·5 µg/ml) as indicated. Data are means and SD of triplicate cultures. In the left panel, responses of differentially treated DC were tested against DC responses in the absence of Caco2 SM (black bars) with one-way anova and Tukey post test, while they in the right panel were tested against DC responses in the presence of Caco2 SM (white bars), ***P < 0·001 **P < 0·01 and *P < 0·05. This is one representative experiment out of four with cells from different donors.
The expression of TGF-βRI, II, III and TSLP-R is independent of the presence of Caco2 spent medium
Next, we assessed the DC expression of receptors for TSLP and TGF-β after encountering commensals in the presence and absence of Caco2 SM. Caco2 SM, bacterial stimulation and rhTGF-β1 did not enhance expression of the TGF-β-R (Fig. 7a). TSLP interacts with DC through the heterodimeric complex of TSLP-R/IL-7Rα26, which leads to phosphorylation of STAT5. Expression of the TSLP-R was not up-regulated in the presence of Caco2 SM or rhTSLP (Fig. 7b), but interestingly, the TSLP-R was up-regulated when DC underwent bacteria-induced maturation. The up-regulation of TSLP-R followed the up-regulation of costimulatory molecules (Fig. 4b) with E. coli inducing the highest expression.
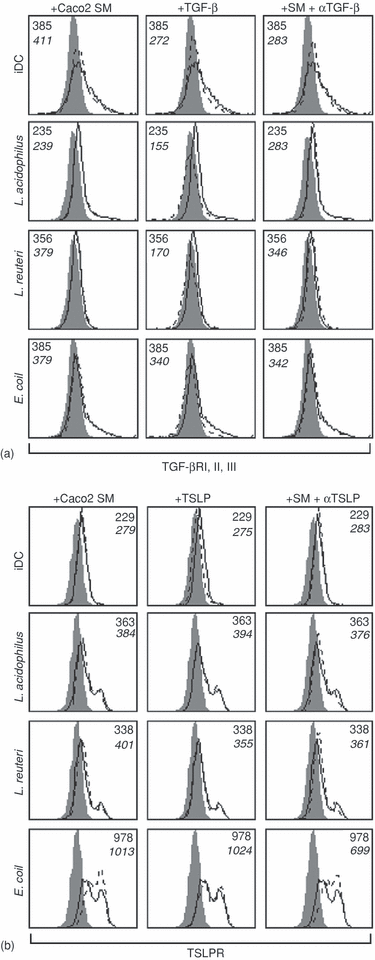
Expression of TGF-βRI, II, III and TSLP-R is independent of the presence of Caco2 spent medium (SM). (a) Expression of TGF-βRI,II,III is independent of the presence of Caco2 SM, but is decreased by addition of rhTGF-β. (b) The expression of TSLP-R is independent of the presence of Caco2 SM, but is enhanced upon bacteria-induced maturation (5 µg/ml of L. acidophilus X37, L. reuteri 12246 or E. coli Nissle). The filled histograms represent an isotype-control antibody, the black lines represent expression in normal culture medium (upper number represents MFI), while the dotted lines represent the expression after treatment of DC as indicated (lower number represents MFI). This is one representative experiment out of three with cells from different donors.
Discussion
We have previously shown that distinct strains of G+ and G– bacteria differentially mature DC in vitro.4 However, in vivo the epithelial barrier is playing an active role in the interaction between lamina propria DC and the gut flora in several aspects. Recently, Chieppa et al.22 have shown that TLR ligation on epithelial cells precedes DC protrusions to the lumen. Several reports suggest a more passive role of IEC, as it has been shown that IEC develop hypo-responsiveness upon repeated exposure to bacterial matter.6,7 Here we present evidence on IEC playing an active role in priming DC toward a tolerogenic phenotype by secreting soluble mediators such as TSLP and TGF-β.
We show that both G+ and G– commensals induce low levels of IL-8 and TSLP in non-polarized Caco2 cells, with G– bacteria being the most potent in elevating production of both cytokines. None of these cytokines were detected in the Caco2 cells cultured in the transwell system, both in the presence and absence of underlying DC, due to a lower concentration of Caco2 cells. The TGF-β1 production was relatively high in non-stimulated Caco2 cells and was further increased by stimulation with both G+ and G– commensals. These findings suggest that the microflora help maintaining tolerance by increasing the production of IEC-derived cytokines known to be involved in priming for tolerogenic DC.27
We used the transwell coculture system with a pore size of 0·4 µm to mimic the in vivo separation of DC from direct contact with commensals. Such a small pore size was chosen because we wanted to study the potential crosstalk between Caco2 cells and DC. Many other studies have used a pore size of 4 µm and after an ‘upside-down’ incubation with DC lying in the upper chamber on the basolateral side of Caco2 cells, DC have been shown to extend protrusions through the membrane.28 Rimoldi et al. showed that both Salmonella typhimurium and L. plantarum in this transwell system induce the same amounts of IL-12 in basolaterally located DC as in DC, which are in direct contact with the bacteria.24,28 We tested whether upside-down incubation increased DC maturation in our system, and found no difference (data not shown), probably because dendrites cannot protrude under these conditions, because the diameter of a protrusion is about 2–4 µm.22 Our finding that only strains of E. coli were able to induce semimaturation in DC, and that LAB abolished this maturation process, might reflect a novel mechanism of how tolerance towards the microflora is maintained, since LAB are more dominant than E. coli in healthy individuals.29,30 We also found that TLR2 was involved in the inhibition of the TLR4-dependent E. coli-induced semimaturation of the DC, revealing a novel TLR-dependent crosstalk between IEC and DC. However coculture with either MDP or Pam3CSK4 were not able to prevent E. coli-induced DC maturation, suggesting that the complexity of structural motifs provided by the LAB can not be mimicked by adding synthetic ligands. TLR2 has been shown to interact with multiple pattern recognition receptors,31 therefore inhibition induced by LAB is likely to depend on ligation of TLR2 in combination with other pattern recognition receptors. IEC only translocate non-invasive E. coli across the epithelium after intestinal injury or upon inflammation both in vitro and in vivo;32,33 however, a novel mechanism of TLR4-dependent phagocytosis of non-invasive E. coli by IEC has recently been described.34 In our study, it is plausible that the abolished crosstalk between Caco2 and DC caused by neutralization of TLR4 could be explained by an abolishment of TLR4-dependent phagocytosis of E. coli. Further studies are needed to examine whether commensal strains of E. coli are phagocytosed by Caco2 cells in the transwell system.
Caco2 cells secrete factors that inhibit IL-12 production in Salmonella typhimurium-matured DC, and it was reported that TSLP plays a role, but is not the only factor involved since silencing of TSLP mRNA did not totally abolish the inhibitory effect.12 Interestingly, the same group also found indices on lower expression of TSLP in primary IEC from Crohn's disease patients than in healthy individuals. These findings led us to investigate the effect of Caco2 SM on DC in response to an array of both G+ and G– commensals. In line with Rimoldi et al.12 we found a marked inhibition of IL-12p70 and a slight decrease in TNF-α (only significant for strains of LAB) while IL-6 was less affected by the presence of Caco2 SM. Interestingly, we found a decrease in IL-10 production for G– matured DC and an increase for LAB-matured DC. This divergent effect of Caco2 SM on IL-10 production, and to some extend on TNF-α production, induced by LAB vs. G– bacteria might help explain the pathology of Crohn's disease, which is characterized by pathogenic T-cell responses to enteric bacteria and loss of tolerance to commensals. In Crohn's disease patients, levels of bifidobacteria are lower;35 hence a flora dominated by G– bacteria might lead to lower IL-10 levels than a LAB-dominated flora. We have also seen an inhibitory effect of Caco2 SM on expression of maturation markers particularly CD40, CD83 and HLADR in commensal-matured DC. Because colonic DC in patients with Crohn's disease are characterized by increased production of IL-12, IL-6 and a TNF-α-dependent enhanced expression of CD40,36 it is likely that beside a different composition of the microflora, lack of tolerogenic IEC-mediators (e.g. TSLP and TGF-β) might further induce the immunopathology. The data presented here suggest that the composition of the microflora influences the levels of tolerogenic IEC-mediators produced.
Caco2 SM has been shown to prime naïve T cells to produce less IFN-γ and slightly more IL-4 in response to S. typhimurium.12 Hence, we tested the effect of Caco2 SM on T-cell priming by DC matured by selected strains and indeed found an inhibition of IFN-γ of about 25-fold for L. acidophilus and L. reuteri, and fourfold for E. coli. This extreme effect on T-cell priming by LAB-matured DC might be a consequence of the effect on DC-derived IL-10, which was elevated for G+ but decreased for G– bacteria in the presence of Caco2 SM. IL-4, IFN-γ and IL-10 were measured intracellularly but only IFN-γ bound specific antibody above the isotype control and levels resembled the results obtained with ELISA (data not shown). IL-4 was also below the detection limit in ELISA (<12 pg/ml) and IL-10 measured with ELISA resembled IL-10 derived from DC alone (data not shown). The Caco2 SM increased production of TGF-β1 by a factor of 2–3 for both immature and bacteria-matured DC. Overall, these data suggest that the Caco2 SM not only possesses a Th1-inhibiting effect but also a Treg-promoting effect in response to commensals, which has not been reported earlier. It is likely that DC become tolerogenic after treatment with Caco2 SM. IL-10 and/or TGF-β have been implicated in the in vitro differentiation of tolerogenic DC.27,37,38 The Caco2 SM contained around 1000 pg/ml TGF-β1 but no IL-10. However, the IL-10 needed for induction of tolerogenic DC could be autocrine, induced by the commensals. Because of the T-cell derived TGF-β1 induced in Caco2 SM-treated cells, it is reasonable to interpret the results obtained with the Caco2 SM as an induction of tolerogenic DC which induce differentiation of tolerogenic T cells and not solely a Th2 skewing effect caused by the presence of TSLP as has been proposed.12
Because of the observed Treg skewing effects of Caco2 SM conditioned DC matured by commensals, we investigated the role played by TGF-β1, which is a pleiotropic cytokine known to inhibit immune responses at several levels including inhibition of T cell proliferation and differentiation39–41 and inhibition of DC maturation.27 Furthermore, the relatively high level of TGF-β1 produced by non-stimulated Caco2 cell supported that this cytokine could play a role in induction of tolerogenic DC. Indeed, addition of rhTGF-β1 during DC maturation radically lowered the production of IL-12, TNF-α and IL-6 but did not influence IL-10 production, which resembled the effects of Caco2 SM. Because it has been reported earlier that Caco2-derived TSLP plays a role in priming DC12 we included TSLP as a possible candidate responsible for the inhibitory effect of Caco2 SM. Both IL-12 and TNF-α was reduced by the addition of rhTSLP but not to levels produced in the presence of Caco2 SM. Furthermore, neutralization of TSLP or TGF-β1 partially abolished the effects of Caco2 SM, with the greatest effects observed when both were neutralized simultaneously. These findings suggest that Caco2-derived TGF-β and TSLP co-operate in priming for the tolerogenic phenotype of the DC, but it is likely that the two cytokines play different roles dependent on the bacterial stimuli. The finding that commensals and especially E. coli increased production of TSLP in Caco2 cells and at the same time enhanced the expression of TSLP-R on DC suggest a novel feedback loop promoting maintenance of gut homeostasis in healthy individuals. The unfavourable strong Th1 response normally induced by the presence of G– bacteria might be abolished by these two mechanisms leading to higher TSLP reactivity and sensitivity and thereby a less Th1-skewed environment.
DC underlying the lamina propria are most of the time not in direct contact with the microflora. Here we show that only G– bacteria have a limited effect on DC through the epithelial layer, but this is counteracted by LAB. When DC do come in contact with commensals they are still under the influence of IEC-mediators promoting a tolerogenic phenotype, especially in the presence of LAB.
Acknowledgements
The authors thank Lisbeth B. Rosholm, Anni Mehlsen, and Marianne K. Petersen whose assistance is greatly appreciated. We thank Dr A. Lanzavecchia, Bellinzona, Switzerland, for providing an IL-4-producing cell line. This work was supported by The Centre of Advanced Food Studies, Copenhagen, Denmark, and the Food of the Future Research Program, Ministry of Food, Agriculture and Fisheries, Denmark.




