Human dendritic cells transfected with allergen-DNA stimulate specific immunoglobulin G4 but not specific immunoglobulin E production of autologous B cells from atopic individuals in vitro
Summary
Atopic/allergic diseases are characterized by T helper 2 (Th2)-dominated immune responses resulting in immunoglobulin E (IgE) production. DNA-based immunotherapies have been shown to shift the immune response towards Th1 in animal models. In further studies we showed that human dendritic cells (DC) transfected with allergen-DNA are able to stimulate autologous CD4+ T cells from atopic individuals to produce Th1 instead of Th2 cytokines and to activate interferon-γ (IFN-γ)-producing CD8+ T cells. The aim of this study was to analyse whether DC transfected with allergen-DNA are also able to influence immunoglobulin production of B cells from atopic donors. For this purpose, human monocyte-derived DC from grass-pollen allergic donors were transfected with an adenovirus encoding the allergen Phleum pratense 1 and cocultured with B cells, autologous CD4+ T cells, and CD40 ligand-transfected L-cells. B cells receiving help from CD4+ T cells stimulated with allergen-transfected dendritic cells produced more allergen-specific IgG4 compared to stimulation with allergen protein pulsed DC or medium, while total IgG4 production was not affected. In contrast, specific IgE production was not enhanced by stimulation with allergen-DNA transfected DC compared to medium and inhibited compared to allergen protein-pulsed DC with similar effects on total IgE production in vitro. Allergen-DNA transfected dendritic cells are able to direct the human allergic immune response from Th2-dominance towards Th1 and Tc1 also resulting in decreased IgE and increased IgG4 production.
Abbreviations:
-
- CD40L
-
- CD40 ligand
-
- DC
-
- dendritic cell
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- IFN
-
- interferon
-
- IL
-
- interleukin
-
- MHC
-
- major histocompatibility complex
-
- PBMC
-
- peripheral blood mononuclear cells
-
- Phl p1
-
- Phleum pratense 1
-
- SIT
-
- specific immunotherapy.
Introduction
Atopic/allergic diseases such as allergic asthma and rhinitis are characterized by T helper 2 (Th2)-dominated immune responses against harmless antigens (i.e. allergens), like grass-pollen.1 Th2-cells also provide help for immunoglobulin E (IgE) production of B cells.2 Cross-linking of IgE bound to mast cells by high-affinity Fcε receptors triggers the release of preformed vasoactive mediators, synthesis of prostaglandins and leukotrienes and the transcription of cytokine genes.3,4
The only curative therapy of the diseases mentioned above, allergen-specific immunotherapy (SIT), was introduced in 1911.5 It is based on the administration of increasing amounts of allergens in order to achieve tolerance. Clinical efficacy of SIT has been demonstrated in many clinical trials. Additionally, it has been shown that allergen specific immunotherapy specifically modifies a pre-existing allergic Th2 dominated immune response shifting it towards a Th1 response and inducing interleukin-10 (IL-10) producing regulatory T cells.6,7
However, the potential risk of adverse effects such as anaphylactic reactions and the intention to increase efficacy drives the search for alternatives and/or modifications of specific immunotherapy. The features of DNA vaccines, including the possibility to induce Th1-biased immune responses, led to the development of new approaches for protective and therapeutic allergy treatments.8 The anti-allergic principles of DNA vaccines could be clearly attributed to the recruitment of interferon-γ (IFN-γ)-producing CD4+/– and CD8+/– T cells and the establishment of a balanced Th1/Th2-type cytokine milieu.9
Apart from that dendritic cells (DC) are potent tools for the antigen-specific modulation of immune responses, including allergic immune responses.10,11 Therefore we employed DC transfected with adenovirus encoding allergen-DNA to investigate the effect on the human allergic immune response in vitro. We used an adenoviral vector because of much higher transfection rates compared to other transfection methods like electroporation or lipofection.12 An additional advantage of allergen-DNA-transfected DC compared to allergen protein-pulsed DC may be better responses from the CD8+ T-cell compartment because of major histocompatibility complex (MHC) class I antigen presentation.
In earlier studies we showed the influence of allergen-DNA-pulsed human DC on autologous T cells from sensitized atopic patients in vitro. We demonstrated that CD4+ T cells produce more Th1-cytokines after stimulation with autologous adenovirus transfected mature DC and that allergen-DNA-transfected DC are also able to activate IFN-γ-producing CD8+ T cells.13 In the present study we analysed the influence of allergen-DNA transfected DC on total and allergen specific immunoglobulin production of autologous B cells from allergic (already sensitized) donors, a very important aspect with regard to therapeutic considerations.
Materials and methods
Blood samples
Heparinized blood was obtained from atopic donors (mainly with allergic rhinitis) sensitized to Phleum pratense who had not undergone specific immunotherapy. Specific sensitization was documented by positive skin prick test to the allergen and detection of allergen-specific IgE in the sera (CAP class ≥2 measured by ImmunoCAP® specific IgE blood test, Phadia AB, Uppsala, Sweden).
Cell culture reagents and cell line
Iscove's modified Dulbecco's medium with l-glutamine and 25 mm Hepes (IMDM; Life Technologies GmbH, Karlsruhe, Germany) supplemented with 3024 mg/l sodium bicarbonate, 100 µg/ml streptomycin, 100 U/ml penicillin, and 1% heat-inactivated autologous plasma was used for the culture of DC and with 5% autologous plasma for the coculture of T cells and DC.
Human recombinant IL-4, IL-1β and tumour necrosis factor-α (TNF-α) were purchased from Strathmann Biotech GmbH (Hannover, Germany) and granulocyte–macrophage colony-stimulating factor (GM-CSF; Leukine®) was obtained from Immunex Corp. (Seattle, WA) and prostaglandin E2 (Minprostin®) from Pharmacia & Upjohn GmbH (Erlangen, Germany). Natural Phleum pratense 1 (Phl p1) was isolated from pollen extract according to the chromatographical separation by Suck et al.14 Phl p1 allergen-DNA was taken from clone pPhlp1.p4.15
Mouse fibroblastic L cells stably transfected with human CD40L were kindly provided by Dr Pierre Garrone (Schering-Plough, Dardilly, France). L cells were cultured in RPMI-1640 medium (Gibco BRL, Life Technologies, Paisley, UK) supplemented with 10% fetal calf serum (FCS; Sigma, Deisenhofen, Germany) and expanded when confluent with Versene 1 : 5000 (Gibco, BRL).
Generation of monocyte-derived human DC
PBMC were isolated from heparinized blood by Ficoll-Paque 1.077 (Biochrom, Berlin, Germany) density centrifugation. To enrich CD14+ monocytes 1 × 107 peripheral blood mononuclear cells (PBMC) per well were incubated for 45 min in a six-well-plate (Costar, Bodenheim, Germany) in IMDM supplemented with 3% autologous plasma at 37°. After washing the non-adherent cells with prewarmed phosphate-buffered saline the remaining monocytes (purity >90%) were incubated in 3 ml/well IMDM supplemented with 1% heat-inactivated autologous plasma, 1000 U/ml IL-4 and 200 U/ml GM-CSF. Cells were fed with fresh medium every other day. On day 7 the resulting immature DC were pulsed with 5 µg/ml of nat Phl p1 or transfected with Ad-Phl p1 and further stimulated with 1000 U/ml TNF-α, 2000 U/ml IL-1β and 1 µg/ml prostaglandin E2 to induce their full maturation. Mature DC expressing high levels of CD80, CD83, CD86 and MHC class II molecules controlled by flow cytometry (>90%) were harvested 48 hr after stimulation, washed twice and used for T-cell stimulation assays.
Purification of T and B cells
Autologous CD4+, CD8+ T cells and CD19+ B cells were obtained from PBMC using antibody-coated paramagnetic MicroBeads (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany) according to the protocol of the manufacturer. Separation was controlled by flow cytometry (purity >98% CD4+ T cells, >95% CD8+ T cells, >90% B cells).
Coculture of T cells and autologous allergen-pulsed or allergen-DNA transfected DC
For proliferation assays, 1 × 105 CD4+ or CD8+ T cells were cocultured in 96-well-plates (Costar) in triplicates with 1 × 104 autologous allergen-pulsed or allergen-DNA transfected DC, in 200 µl IMDM supplemented with 5% heat-inactivated autologous plasma. After 5 days, the cells were pulsed with 37 kBq/well of [3H]TdR ([methyl-3H]thymidine, ICN, Irvine, CA) for 6 hr, and [3H]TdR incorporation was evaluated in a beta counter (1205 Betaplate, LKB Wallac, Turku, Finland).
For cytokine production assays, 5 × 105 CD4+ or CD8+ T cells were cultured in 48-well-plates in the presence of 5 × 104 autologous allergen-pulsed or allergen-DNA transfected DC in 1 ml IMDM supplemented with 5% heat-inactivated autologous plasma. On day 7, T cells were restimulated with 5 × 104 newly generated autologous allergen-pulsed or allergen-DNA transfected DC, and supernatants were collected 24 hr later.
Coculture of B cells, T cells, autologous allergen-pulsed-or allergen-DNA transfected DC and CD40L-transfected L cells
To measure immunoglobulin production of B cells 1 × 105 B cells, 5 × 105 T cells 5 × 104 DC and 5000 irradiated CD40L transfected L cells were cocultured in IMDM supplemented with 5% heat inactivated autologous plasma and 1000 U/ml IL-4. After 12 days supernatants were collected and amount of total IgE, allergen specific IgE, total and allergen specific IgG4 were measured by enzyme-linked immunosorbent assay (ELISA).
Quantification of cytokine and immunoglobulin production by ELISA
Human IL-4, IL-5, IL-10, IFN-γ, total IgE and IgG4 were measured by ELISA according to the instructions of the distributors of the employed pairs of antibodies (BD PharMingen) as described previously.16 Detection limit was 8 pg/ml for IL-4 and 32 pg/ml for all other cytokines and 1 ng/ml for IgE and IgG. For specific IgE and specific IgG4 plates were coated with 20 µg/ml nat Phl p 1, amount of specific immunoglobulins are given as percentage of optical density.
Recombinant adenovirus and transduction of DC
Recombinant adenoviral vectors encoding Phl p 1 (Ad-Phl p 1) were purchased from QBioGene (Heidelberg, Germany), and expanded in 293 cells, purified by cesium chloride gradient density centrifugation and subsequently dialysed according to the instructions of the manufacturer. Adenoviruses were added to DC cultures on day 7 at an multiplicity of infection of 300 (i.e. 3 × 108 plaque forming units/106 DC per well) resulting in optimal transfection efficiency of 92% as shown earlier.13
Results
Allergen-DNA-transfected DC stimulate proliferation of human autologous CD4+ and CD8+ T cells from allergic donors
As shown in Fig. 1 allergen-DNA-transfected mature DC were effective in inducing T-cell proliferation. Allergen protein-pulsed DC served as positive control, whereas unpulsed DC were used as negative controls. DC transfected only with adenovirus which is not encoding allergen-DNA (Psi 5) are not able to induce proliferation in T cells. The induction of the stimulation of CD4+ T cells by allergen-DNA transfected DC was as high as with allergen-pulsed (nat Phl p1)-DC. The lowest proliferation was measured with T cells stimulated with unpulsed DC (medium) (Fig. 1a). As expected CD8+ T cells showed much higher proliferation rates after stimulation with allergen-DNA transfected DC compared to T cells stimulated with allergen protein pulsed DC. The negative controls (medium and Psi 5) did not induce proliferation in CD8+ T cells (Fig. 1b).
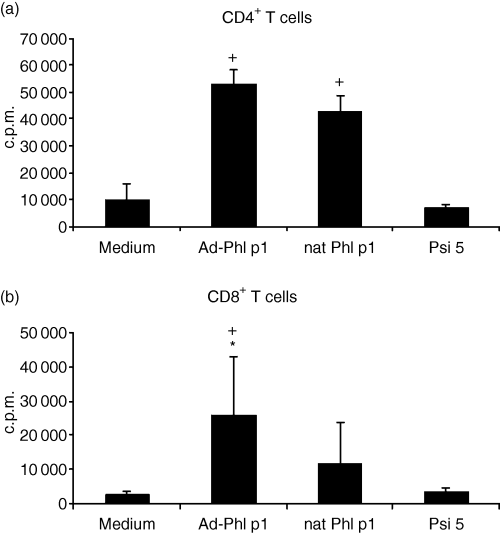
Proliferation of T cells from allergic donors. (a) CD4+ and (b) CD8+ T cells were stimulated with autologous allergen (nat Phl p1)-pulsed, allergen-DNA-transfected or control (medium) DC or DC transfected with virus without encoding allergen-DNA (Psi 5). After 5 days, cells were pulsed with 1 µCi of tritiated thymidine. Results are expressed as the mean ± SD from eight atopic donors. + Indicates statistically significant differences (P < 0·04) to control (medium) DC. * Indicates statistically significant differences (P < 0·04) to allergen-protein pulsed DC.
Allergen-DNA-transfected DC preferentially induce Th1 and Tc1 cytokine production
Furthermore we studied the cytokine pattern of CD4+(Fig. 2a) and CD8+ T cells (Fig. 2b) cocultured with transfected autologous mature DC. To achieve measurable amounts of cytokine production, T cells were restimulated with newly generated transfected autologous mature DC after 7 days. As shown in Fig. 2 allergen-DNA-transfected DC induced higher amounts of the type 1-cytokine IFN-γ in CD4+ T cells as well as in CD8+ T cells than allergen (nat Phl p1)-pulsed DC, while the production of the Th2 cytokines IL-4, IL-5 and IL-10 was lower than in T cells stimulated with allergen (nat Phl p1)-pulsed DC. Production of IL-4, IL-5 and IL-10 was not detectable in CD8+ T cells. DC transfected with adenovirus not encoding allergen-DNA (Psi 5) were not able to induce cytokine production neither in CD4+ nor in CD8+ T cells.
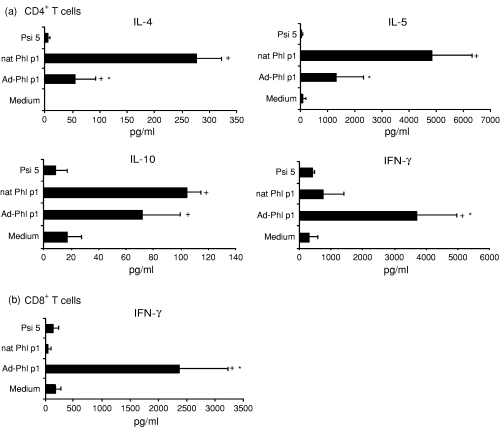
Cytokine production of T cells from allergic donors. (a) CD4+ and (b) CD8+ T cells were stimulated twice with allergen (nat Phl p1)-pulsed, allergen-DNA transfected or control (medium) DC or DC transfected with virus without encoding allergen-DNA (Psi 5) and cytokines were measured by ELISA. Results are expressed as means ± SD from eight atopic donors (which were the same donors as in Fig. 1). + Indicates statistically significant differences (P < 0·04) to control (medium) DC. * Indicates statistically significant differences (P < 0·04) to allergen-protein pulsed DC.
Allergen-DNA transfected DC stimulate allergen specific IgG4 production of B cells from allergic donors more efficiently than allergen protein-pulsed DC
We analysed the production of total IgG4 and allergen specific IgG4 which has been linked to successful SIT.17 For this purpose, human mature allergen-DNA-transfected DC from allergic donors were cocultured with autologous CD4+ T cells, autologous B cells and CD40L-transfected L cells for 12 days. As shown in Fig. 3 total IgG4 production was not affected by stimulation of T cells with allergen-DNA-transfected DC. However, specific IgG4 production of B cells was significantly enhanced when cells were stimulated with allergen-DNA-transfected DC, even compared to cells stimulated with allergen (nat Phl p1)-pulsed DC. Psi 5-transfected DC did not induce immunoglobulin production.
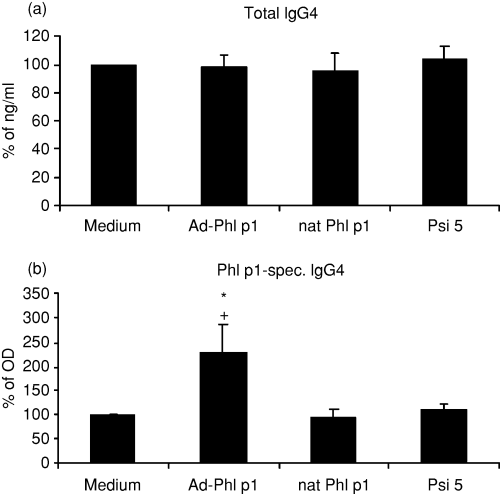
Immunoglobulin G4 production of B cells from allergic donors (a) total and (b) specific IgG4. B cells and T cells were stimulated with autologous allergen (nat Phl p1)-pulsed, allergen-DNA-transfected or control (medium and Psi 5) DC, CD40L-transfected L cells and 1000 U/ml IL-4 for 12 days and immunoglobulin production was measured by ELISA. Results are expressed as means ± SD from 14 atopic donors. + Indicates statistically significant differences (P < 0·04) to control (medium) DC. * Indicates statistically significant differences (P < 0·04) to allergen (nat Phl p1)-pulsed DC.
Allergen-DNA-transfected DC do not stimulate total or allergen specific IgE production of human autologous B cells
Finally, we analysed the production of IgE by B cells after coculture with allergen-DNA-transfected DC. Therefore, human mature allergen-DNA-transfected DC were cocultured with autologous CD4+ T cells, B cells and CD40L-transfected L cells for 12 days. As shown in Fig. 4 total and allergen-specific IgE production were decreased compared to stimulation with allergen protein-pulsed DC reaching the same level as observed for unpulsed or Psi 5-transfected DC. Without addition of IL-4 or autologous CD4+ T cells as well as without additional CD40 stimulation IgE production was too low to detect differences induced by allergen-pulsed- or allergen-DNA transfected DC.
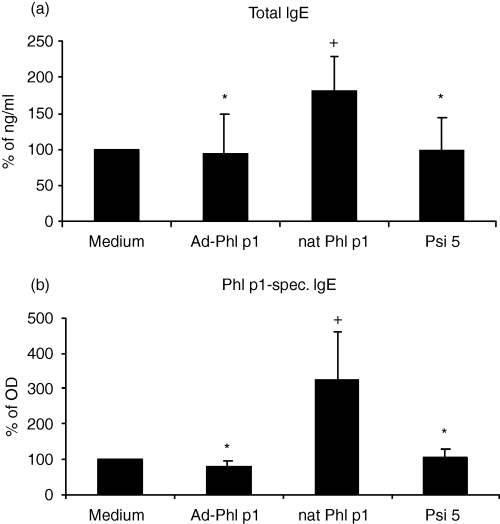
Immunoglobulin E production of B cells from allergic donors (a) total and (b) specific IgE. B cells and T cells were stimulated with autologous allergen (nat Phl p1)-pulsed, allergen-DNA-transfected or control (medium and Psi 5) DC, CD40L-transfected L cells and 1000 U/ml IL-4 for 12 days and immunoglobulin production was measured by ELISA. Results are expressed as means ± SD from 14 atopic donors. The donors were the same as in Fig. 3 and included the eight donors from 1, 2. + Indicates statistically significant differences (P < 0·04) to control (medium) DC. * Indicates statistically significant differences (P < 0·04) to allergen (nat Phl p1)-pulsed DC.
Discussion
In the present study, we extended our earlier findings demonstrating that human allergen-DNA-transfected DC are not only efficient in stimulating autologous CD4+ and CD8+ T cells but also influence immunoglobulin production of B cells. As expected, CD8+ T cells were activated more strongly by allergen-DNA-transfected DC than by allergen-pulsed DC, but CD4+ T cells were also activated. The latter indicates that DC not only produce the respective protein endogenously and present it utilizing the MHC class I pathway after transfection with allergen-DNA, but also shuttle the protein into the MHC class II pathway, potentially after secondary uptake of secreted allergen protein produced by themselves.18 We were not able to detect Toll-like receptor 9 on DC (data not shown), so this seems not to be the mechanism that induces our observed effects. Allergen-DNA-transfected DC are also able to induce a strong IFN-γ production in CD4+ and CD8+ T cells and decrease the production of Th2 cytokines IL-4 and IL-5.13 In the control and regulation of IgE production the induction of CD8+ T cells and the activation of CD4+ T cells with a Th1-polarized phenotype play a prominent role.19 The finding that transfection of DC with adenovirus not encoding allergen-DNA do not induce any T-cell response shows that the effects we observed with allergen-DNA-transfected DC are not responses induced by antigens of the virus itself. Here, we additionally show that transfection of human DC from atopic donors with allergen-DNA prevents the development of specific IgE responses, while specific IgG4 production is induced more efficiently when T cells were stimulated with allergen-DNA-transfected DC compared to allergen protein-pulsed DC. In non-sensitized non-atopic control donors, where significant Th2 immune responses and IgE-production could be detected, similar effects could be observed by stimulation with allergen-DNA-transfected DC concerning the other aspects: proliferation, enhancement of IFN-γ and IgG production (data not shown).
Atopic/allergic diseases are referred to as immediate or type I hypersensitivity reactions, with IgE as a critical factor20 depending on Th2 responses.3 After allergen exposure, CD4+ T cells are activated and differentiated by antigen-presenting cells to produce Th2 cytokines such as IL-4, IL-5 and IL-13, which are responsible for strong antibody responses including IgE synthesis by B cells. The release of histamine, leukotrienes and prostaglandins following cross-linking of allergen-specific IgE on the surface of mast cells and basophils elicits allergic reactions of the immediate type.21 Therefore, modulation of the Th2 immune response is an attractive target for immunological therapies. So far, SIT is the only curative therapy for type I allergy, which modifies a pre-existing allergic Th2 immune response.6 SIT induces a shift in the dominance of allergen-specific T helper cells from Th2 to Th1 (among other changes). This shift in the cytokine production from IL-4 and IL-5 dominance to IFN-γ-dominance also appears to be the reason for the subsequent shift in allergen-specific immunoglobulin production from IgE towards IgG4, as IgE production is enhanced by IL-4 and IgG production by IFN-γ.17,22,23 Modifications of SIT may yield enhanced efficacy and safety of this form of therapy. Higher allergen doses are able to improve the clinical efficacy of SIT, but with higher allergen doses the risk of adverse effects also increases.24 Therefore, modifications of SIT based on the use of higher immunogenic and lower allergenic allergen preparation are desirable.25 In animal models it has been shown that DNA-based vaccinations are significantly more effective than protein-based vaccinations concerning protection against the development of Th2-biased immune responses and lethal anaphylactic reactions.24 Raz et al. showed in rodents that protein immunization induces a Th2-dominated immune response, whereas plasmid DNA-immunization induces a Th1 immune response.26 They also reported that splenic CD4+ T cells isolated from plasmid DNA-immunized mice secreted IFN-γ, but not IL-4 and IL-5, while protein injected mice secreted IL-4 and IL-5 but not IFN-γ.26 The first evidence for the anti-allergic effect of DNA vaccines with clinically relevant allergen was achieved by intramuscular injection of rats with plasmid DNA encoding the dust mite allergen Der p 5.27,28 A variety of model allergens and clinically relevant allergens have been used for DNA-based immunization against allergy. Studies using the major birch pollen allergen Bet v 1 demonstrated, that DNA-based immunization with a construct encoding Bet v 1 increased Th1 immune responses and protected against allergic sensitization.9,29,30 Sudowe et al. have shown that vaccination of mice with replication-defective Ad-CMV-βgal prevents the development of specific IgE responses, shifting the immune response from a Th2- to a Th1-dominated response and generating IFN-γ producing CD8+ T cells.31 In the present study we demonstrate, that after stimulation of CD4+ T cells with allergen-DNA-transfected DC the production of allergen specific IgE was reduced compared to allergen-protein pulsed DC while production of specific IgG4 was increased like in SIT, accompanied by decreased IL-4 and IL-5 and increased IFN-γ-production, indicating a shift from Th2 to Th1 immune response. IFN-γ acts as an inhibitor of IgE production32,33 and induces IgG4 antibodies to allergen in activated memory B cells.17,22 This may be the reason for decreased total and specific IgE-production of B cells after stimulation with allergen-DNA-transfected DC that we observed in this study. Because of the duration of cultures it was necessary to use autologous serum for the cell culture. It is possible that this autologous serum may cause a background of immunoglobulin and specific antibodies. This background is the same in all conditions, also in ‘medium’ (unpulsed DC) and can be assessed this way. Higher amounts of immunoglobulin than in the medium condition must be caused by active production in the culture. It has also been described that immunotherapy with inhalant allergens is associated with increase in serum allergen-specific IgG1 and IgG4 levels.34,35 IgG4 does not activate complement and has little or no inflammatory activity. IgG antibodies have also been proposed as blocking antibodies by competing with IgE for allergen binding to mast cells, basophils and other IgE-receptor-expressing cells. For example, IgG4 induced by means of immunotherapy blocks allergen-induced IgE-dependent histamine release by basophils.36
The use of DC may be of particular advantage for DNA-based immunotherapy because gene transfer to DC ensures that gene products are endogenously processed leading to the generation of long lasting MHC class I-restricted CD8+ T-cell responses.37,38 Viral vectors were used in our study because they allow the achievement of high transfection rates and may also enhance the immunogenicity of a vaccine because of the adjuvant properties of some of the viral products.39 Also the problem of development of neutralizing anti-adenoviral antibodies following injection of viral vectors may be more limited by the use of DC, because the adenoviral proteins are expressed inside the DC and this reduces the exposure of the host to proteins of the virus, and hence weakens the possible neutralization of transfected cells by antiviral antibodies.12 Nevertheless, a non-viral transfection may be preferable avoiding the potential pathogenicity and immunogenicity of viral vectors. However, non-viral-gene transfer systems like electroporation are limited by their low efficiency for transfection of DC.40
Taken together, transfection of human DC with allergen-DNA represents a potential new form of immunotherapy that is already used for immunotherapy in cancer with tumour DNA.37 The fact that allergen-DNA transfected DC induce not only type 1 cytokine production but also subsequent changes in immunoglobulin production in an allergen-specific manner makes than particularly appealing for the treatment of Th2-based IgE-mediated diseases that may be especially ameliorated by inhibition of IgE and induction of IgG4 responses.




