Inherited antithrombin deficiency: a review
Abstract
Summary. Antithrombin (AT) is a potent inactivator of thrombin and factor Xa and the major inhibitor of blood coagulation. Inherited AT deficiencies are uncommon, with prevalences in the general population between 1 in 500 and 1 in 5000. They are either quantitative (type I) or qualitative (type II). Type II is subdivided into the more common, but less thrombogenic, type IIb deficiency caused by a defect in the heparin-binding region of AT and the less common, but more thrombophilic, type IIa variant caused by mutations in the thrombin-binding site. A pleiotropic type IIc deficiency also exists. In the evaluation of a thrombophilic individual, a functional AT assay (AT activity) should be used and the diagnosis of AT deficiency only established after acquired causes have been ruled out and repeat AT testing on an additional sample has been performed. A subsequent antigenic AT assay result leads to differentiation between type I and type II deficiency. Further specialized tests help subclassify the type II deficiencies, but this is typically not carried out for clinical purposes, even though it might be helpful to assess thrombosis risk. AT deficiency is associated with an increased risk for venous thromboembolism (VTE) and pregnancy loss. The association with arterial thrombosis is only weak. VTE prophylaxis and treatment management will be discussed in this article and existing treatment guidelines presented. The lack of data surrounding the use of AT concentrates and the resulting ambiguity as to when to use such concentrates will be discussed.
Introduction
Antithrombin (AT) deficiency was first described in 1965 by Olav Egeberg in a Scandinavian family in which several family members had venous thromboembolism (VTE). Egeberg also established the deficiency to be an autosomal dominant disorder.
Materials and methods
A detailed MEDLINE and PubMed search for all English-language articles pertaining to AT deficiency was performed, using the terms antithrombin, antithrombin III, hereditary antithrombin deficiency, acquired antithrombin deficiency, thrombophilia and venous thromboembolism as keywords. The images in Fig. 2 were created in MacPyMOL (http://www.pymol.org) using program database files 1tb6 [1] and 2gd4 [2].
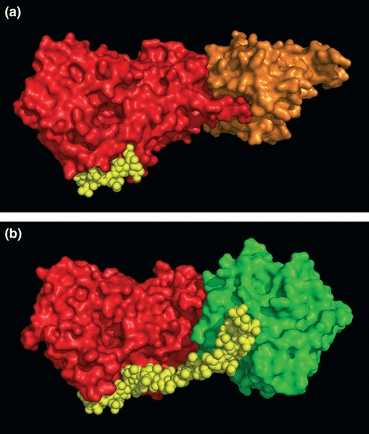
(a) Tertiary structure of antithrombin molecule (red) bound to factor Xa (orange) and a pentasaccharide (yellow). (b) Antithrombin (red) bound to thrombin (green) and heparin (yellow). Images courtesy of Herbert C. Whinna, MD, PhD, Chapel Hill, NC.
Epidemiology
Inherited AT deficiency is an uncommon autosomal dominant disorder. Most cases are heterozygous. Homozygosity for AT deficiency is rare and is almost always fatal in utero. Prevalence rates for AT deficiency of 1 in 500 to 1 in 5000 in the overall population have been reported [3,4]. This wide range of prevalence rates is due to several factors; foremost is the fact that in some studies no repeat AT determinations were performed in individuals found to have low AT activities. Because it is known that many individuals with a low AT value on one occasion will have a normal AT value on repeat testing, it is crucial to repeat testing from a new sample if a low value is found. Another reason for the wide range of reported prevalences may be the fact that many individuals with low activity levels have a type IIb deficiency (Table 1) (i.e. a dysfunctional protein that is not detected when using only antigen assays). Although in the general population the prevalence of type II AT deficiency (Table 1) outnumbers type I, among symptomatic patients type I is much more prevalent, often representing up to 80% of total cases. Type IIb deficiency, a qualitative deficiency caused by mutations in AT’s heparin-binding site, appears to be much less thrombogenic than quantitative (type I) deficiency [5]. Following the rules of autosomal dominant Mendelian inheritance, there is a 50% chance of transmission to a child if one of the parents has AT deficiency. Men and women are equally affected, and there is no racial or ethnic predilection.
| Type of defect | Defect where? | Results of laboratory assays | Prevalence in general population | Prevalence in patients with thrombosis | Prevalence of VTE in persons with this subtype of deficiency (%) | |||
|---|---|---|---|---|---|---|---|---|
| AT activity | AT antigen | |||||||
| Heparin co-factor assay* | Progressive AT assay† | |||||||
| Type I | Quantitative | Low | Low | Low | 12% of all ATD | 60% of all ATD | 53 | |
| Type II | Qualitative | 88% of all ATD | 40% of all ATD | 6–66 | ||||
| IIa | Thrombin-binding domain | Low | Low | Often normal | 58 | |||
| IIb | Heparin-binding domain | Low | Normal | Often normal | 6 | |||
| IIc | Pleiotropic | Low | Varied | Low | 66 | |||
- *Inactivation of thrombin or factor Xa in the presence of heparin.
- †In the absence of heparin or with low concentration of heparin.
Pathophysiology
Role of AT in coagulation
Antithrombin is a serine protease inhibitor (serpin) that physiologically inactivates thrombin (factor IIa) and factor Xa (FXa) (Fig. 1) and, to a lesser extent, factors IXa, XIa, XIIa, tissue plasminogen activator (tPA), urokinase, trypsin, plasmin and kallikrein. AT is an α2-globulin synthesized predominantly in the liver, has a half-life of approximately 2.4 days and a molecular weight of 58 200 Da, and contains 432 amino acids. There are two isoforms of the AT protein in circulation, the α (90–95%) and β (5–10%) isoforms. The β-isoform shows a higher affinity for heparin due to lack of glycosylation at Asn 135; however, its exact physiological role remains to be ascertained.
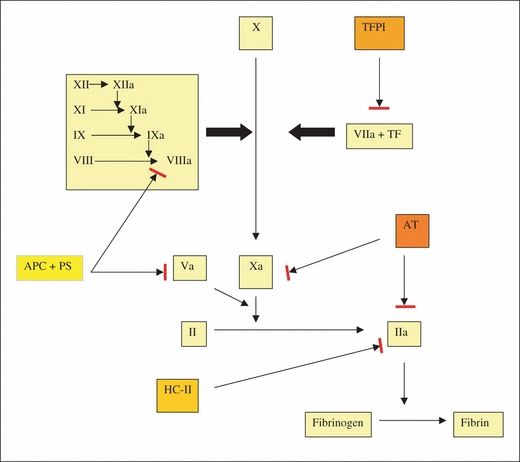
Natural anticoagulants and the coagulation cascade. AT, antithrombin; II, prothrombin; IIa, thrombin; APC, activated protein C; PS, protein S; TFPI, tissue factor pathway inhibitor; HC-II, heparin co-factor II.
Antithrombin physiologically circulates in a form that has a low inhibitory activity. The anticoagulant effect of AT is accelerated at least a thousand times in the presence of heparin and other heparin-like glycosaminoglycans, such as heparan sulphate. As free heparin is not present in the circulation under normal physiological circumstances, it is thought that the heparan sulphate located on the vascular endothelium provides the main backbone for this accelerating mechanism. The therapeutic use of heparin as an anticoagulant works through the potentiation of endogenous AT.
The AT-mediated inactivation process for coagulation factors requires the binding of a unique sequence-specific pentasaccharide domain of heparin to the heparin-binding domain of AT. This interaction induces a conformational change in AT, which accelerates the inhibition of FXa (Fig. 2a). The inhibition of thrombin, in addition, requires heparin to bind to both AT and thrombin, to form a ternary bridging complex (Fig. 2b), so that then thrombin can be inhibited. The proposed sequence of events is that AT first interacts with the pentasaccharide domain, and thrombin then binds to a remote domain of heparin, thus becoming suitably oriented for inhibition. This sequence of events produces tightly bound, irreversible thrombin-AT (TAT) complexes, which are then rapidly cleared from the circulation.
In vivo, normal AT undergoes a slow conversion to a latent form that is not only inactive by itself but also dimerizes with an active AT molecule (with preferential binding to the β-isoform of AT). This reaction, which normally has minimal physiological consequences, is accelerated with an increase in the body temperature, explaining episodes of acute thrombosis in families with conformationally unstable ATs (Rouen-VI AT variant) during febrile episodes.
In addition to its anticoagulant role, AT has been found to have an important anti-inflammatory effect that occurs in relation to its interaction with the endothelium. By inhibiting thrombin and FXa, it reduces the thrombin/FXa-mediated release of proinflammatory cytokines such as interleukin 6 and interleukin 8. By binding to heparan sulphate on the endothelium, AT increases the production of the important anti-inflammatory cytokine prostacyclin, which then mediates smooth muscle relaxation and vasodilatation and inhibits platelet aggregation. The anti-inflammatory effects of AT are closely dependent on its ability to bind with the endothelial glycosaminoglycans and are not observed in its reaction with commercial/free circulating heparin.
Levels of AT associated with development of thrombosis
Normal plasma levels of AT range from 112 to 140 μg mL−1. Because of the presence of interlaboratory variations, most laboratories express AT antigen and activity levels in terms of percentages, with normal ranges being approximately 80–120%, where 100% of AT corresponds to 1 unit of AT in 1 mL of reference plasma. Neonates and infants have lower levels of AT than adults, with levels reaching adult ranges by 6 months of age. Although there are some variations in normal ranges, such as slightly lower levels in women taking birth control pills and elderly men, these variations are only mild and clinically not meaningful. The use of a single reference range has, therefore, been recommended and seems appropriate [6]. Most patients with inherited, heterozygous AT deficiency have AT activity levels in the range of 40–60% [7]. Whether the risk for thrombosis correlates with the degree of AT decrease has, to our knowledge, not been investigated.
Types of AT deficiency
Hereditary AT deficiency. Inherited AT deficiency is divided into type I deficiency (Table 1), in which both the functional activity and antigenic levels AT are proportionately reduced (quantitative deficiency), and type II deficiency, in which normal antigen levels are found in association with low AT activity due to a dysfunctional protein (qualitative deficiency) [7]. Type II deficiencies can be further subclassified into three types (Table 1), depending on the location of the mutations and, consequently, the performance of different AT assays [7]. Although different classification systems have been used for the type 2 subtypes [5,7,8], the Antithrombin Mutation Database system is now often considered the accepted classification system [7,9,10]. This is important to realize to avoid confusion when interpreting publications in which different classification systems were used.
Type IIa is caused by mutations that affect AT’s reactive site (i.e. the region where AT binds to its target protease). Type IIb is characterized by an abnormality of the heparin-binding domain of AT, interfering with AT activity only in the presence of heparin. Type IIc variants are a pleiotropic group of mutations near the reactive loop site, which may interfere with the mobility of the reactive loop site after heparin binding, thus influencing AT’s interaction with thrombin. Type IIc variants show decreased antigen levels of the mutated AT, which may be caused by reduced synthesis and secretion, as well as increased catabolism.
Acquired AT deficiency. Before concluding that a person with low AT levels has an inherited AT deficiency, it is important to rule out acquired causes of AT deficiency (Table 2). More detailed discussions of these disorders are available in the literature [11].
| Decreased AT synthesis |
| Cirrhosis of liver |
| Increased AT losses |
| Nephrotic syndrome |
| Protein loosing enteropathy |
| Enhanced consumption/inactivation of AT |
| Sepsis with disseminated intravascular coagulation |
| Burn injuries |
| Polytrauma |
| Hepatic veno-occlusive disease |
| Thrombotic microangiopathies |
| Cardiopulmonary bypass surgery |
| Large haematomas |
| Metastatic tumours |
| Extra corporeal membrane oxygen therapy |
| Drug induced AT deficiency |
| l-asparaginase therapy |
| Heparin therapy |
Genetics and molecular basis of AT deficiency
The gene for AT is located on chromosome 1q23-25, contains seven exons and six intrones, and spans 13 477 base pairs of genomic DNA [7]. More than 130 different mutations in the AT gene have been reported and are described, in part, in the online AT gene database (last updated in 1998) (http://www1.imperial.ac.uk/medicine/about/divisions/is/haemo/coag/antithrombin). The genetics behind the major types of AT deficiency are as follows.
Type I deficiency
Type I AT deficiencies are most commonly caused by short deletions and insertions and less commonly by single base pair substitutions. The deletions vary from 1 to 30 base pairs in length and are scattered throughout the AT gene. Three preferred regions for these deletions have been identified, codons 244/245, codon 81 and codons 106/107. The insertional aberrations tend to be in the form of single base insertions resulting in premature translational termination. Single base pair substitutions tend to occur as nonsense mutations, also affecting translational capacity.
Type II deficiency
The type II AT deficiencies commonly arise secondary to single base pair substitutions, affecting the reactive domain (type IIa) or the heparin-binding domain of AT (type IIb), resulting in qualitative defects. Among the mutations known to involve the reactive domain, most occur in two distinct clusters: at residues Ala382 and Ala384 in the hinge region of AT, and around the reactive domain at residues 392, 393 and 394. Mutations that involve the heparin-binding domain (type IIb) alter the heparin-binding capacity of AT, but the molecule retains normal AT activity when assayed in the absence of heparin. Most of these mutations are missense mutations, often involving residues 41, 47, 99 and 129.
The type IIc deficiencies include a host of different mutations with pleiotropic effects, especially involving residues 402, 404–407 and 429. These mutations are located in strand Ic of AT, impairing the function of the reactive domain and also giving rise to a reduction in AT levels, because strand Ic is necessary for the structural and functional integrity of AT.
Clinical manifestations
Thromboembolic manifestations
Patients with AT deficiency are at significantly increased risk for thromboembolism, predominantly in the venous circulation. Of the known inherited thrombophilias, AT deficiency leads to the highest risk for VTE. Although some cases of arterial thromboembolism in AT-deficient individuals have been reported, this association is much weaker. VTE typically occurs as deep vein thrombosis of the legs and arms and pulmonary embolism, but can also occur in unusual sites, such as cerebral or sinus, mesenteric, portal, hepatic, renal and retinal veins. Approximately, 60% of VTE in patients with AT deficiency is unprovoked, and 40% is associated with transient risk factors [12]. VTE is uncommon during the first two decades of life [8,13,14], possibly as a result of the protective effects of high levels of another naturally occurring thrombin inhibitor, α2-macroglobulin. The risk increases significantly around the age of 20 years, and by the age of 50 years, approximately 50% of individuals with AT deficiency will have had an episode of VTE [8,13,14]. However, it is noteworthy that individuals with type IIb deficiency have a significantly lower risk for thrombosis than individuals with other types of AT deficiencies [5]. This observation argues for additional testing to subclassify individuals found to have inherited AT deficiency, because this affects the discussion of lifetime risk of VTE in that individual. Patients with AT deficiency may have resistance to therapy with heparin and may require higher heparin doses for achievement of therapeutic activated partial thromboplastin time (aPTT) and protective anticoagulation, which may be the first clue of the underlying defect.
Pregnancy-related complications
VTE during pregnancy. The risk for pregnancy-related VTE in women with AT deficiency is high if no anticoagulation prophylaxis is given [15,16]. Of AT-deficient women who have not had a previous VTE, 31% will develop a VTE during pregnancy, and in women with previous VTE, this rate is 49% [16]. More than half of the episodes of VTE occur postpartum.
Fetal loss. The risk of fetal loss is slightly increased in women with AT deficiency: retrospective data on 260 pregnancies in 108 women with AT deficiency show that 19.2% of pregnancies in women with AT deficiency end in fetal loss compared with 12.2% in women without thrombophilia [17]. Pregnancy loss can occur after week 28 (2.3% and 0.6% of pregnancies in women with AT deficiency and without thrombophilia respectively) or before week 28 (16.9% and 11.6% respectively) [17]. Noteworthy is that these data indicate that the probability for a favourable pregnancy outcome in AT-deficient women is high. Unfortunately, it is not clear whether women reported in this study had received anticoagulant prophylaxis during pregnancy or not. However, relatively good pregnancy outcome even in the absence of thromboprophylaxis is also reported in a subsequent prospective study, which showed successful pregnancy outcome in 80% (four of five) of women with AT deficiency who did not receive anticoagulant prophylaxis [18].
Other pregnancy complications. Currently, data are inadequate to conclude whether there is an association between AT deficiency and intrauterine growth restriction, placental abruption, pre-eclampsia and eclampsia.
Diagnosis
Laboratory diagnosis
The appropriate first test to obtain when evaluating for AT deficiency is an AT functional assay [9,10]. There is no need to perform routinely an AT antigen assay, either as a screening test or as an additional test, if the AT activity is normal. Because a normal AT antigen level does not rule out a type II deficiency and because type II is much more common than type I, a significant number of cases of AT deficiency would be missed if only antigen levels were used for screening purposes. No patient should be diagnosed as having AT deficiency on the basis of a single abnormal test result. An abnormal test result should lead to repeat testing on a new blood sample. AT testing is preferably avoided in the setting of acute thrombosis and is best performed several days after cessation of heparin therapy, as acute thrombosis and heparin therapy may transiently lower AT levels. In patients taking vitamin K antagonists, AT levels may be increased and an AT deficiency may, thus, be masked. All causes of acquired AT deficiency (Table 2) should be excluded before classifying a person with abnormal AT test results as having inherited AT deficiency. At times, it may be necessary to test a patient’s parents to determine whether the AT deficiency is hereditary. If the functional assays are abnormal, an AT antigen level can be obtained to help differentiate between type I and type II deficiencies. The distinction of AT deficiency subtypes may be clinically relevant, because the type IIb subtype has a much lower risk of thrombosis than other subtypes [5].
Functional assays
The functional AT assays are amidolytic (chromogenic) assays. A patient’s plasma is incubated in the presence of heparin with excess thrombin or FXa. The AT in the patient’s plasma reacts with and neutralizes thrombin or FXa, a reaction catalyzed by heparin. The amount of thrombin or FXa that remains non-neutralized is inversely proportional to the patient’s AT activity level, and this remaining thrombin or FXa is then quantified using an automated chromogenic detection system. The assays that use FXa instead of thrombin do so to decrease potential interference by accelerator proteins other than AT, such as HC II. An additional way to overcome the potential interference caused by HC II in thrombin-based functional AT assays is to use bovine thrombin as a substrate, because it does not interact with HC II. Furthermore, newer commercial assays have incorporated protease inhibitors, such as aprotinin, to negate substrate cleavage by other serpins. The amidolytic AT assays are not influenced by the presence of heparin in the patient’s plasma as they are performed in the presence of excess of heparin. Because the FXa inhibition-based assays appear to be the most widely used routine AT activity measuring method, it is important to realize that there is a possibility of not detecting all type II deficiencies with this method [19].
As with any AT deficiency, the functional activity is also low in type IIb AT defects characterized by impaired binding of AT and heparin. This subtype can be identified by (i) two-dimensional counter/crossed immunoelectrophoresis; (ii) an AT activity assay performed in the absence or with only a low concentration of heparin (so-called progressive AT activity assay); or (iii) mutation analysis with gene sequencing. Although identifying the AT deficiency in a patient as being due to the IIb subtype may lead to counselling of a patient’s affected family members that they may have a relatively low risk for thrombosis, this subtyping is typically not performed in clinical practice, because the tests needed are not easily available.
Antigenic assays
The antigenic assays are quantitative assays that measure the amount of AT present in plasma. These tests can be performed once AT deficiency has been detected by a functional assay to define the type of AT deficiency. In the absence of secondary causes, and in the appropriate clinical setting, a low antigenic assay result classifies a patient as having a type I (or type IIc) AT deficiency.
Genetic analysis and prenatal diagnosis
Genetic analysis is typically not performed in routine clinical practice and, due to the large number of different mutations underlying AT deficiency, would require gene sequencing. Because the knowledge of the fetal or newborn’s AT status usually does not influence prenatal and perinatal management, there is typically no indication for prenatal testing. Testing can be considered in the rare cases in which the fetus may be expected to be homozygous or compound heterozygous for coagulation inhibitor defects (AT, protein C, or protein S). Laboratories that offer AT gene sequencing are as follows. Other laboratories, commercial or research, may exist.
- 1
Department of Haematology, UZ Brussel, 1090 Brussels, Belgium. Contact: Dr Kristin Jochmans ([email protected]); research laboratory that also performs crossed immunoelectrophoresis.
- 2
Centre for Haemostasis and Thrombosis, St. Thomas’ Hospital, London, UK. http://www.gstt.nhs.uk/services/managednetworks/oncologyandhaem/chat/homepage.aspx; commercial laboratory.
- 3
Sheffield Molecular Genetics Service, Sheffield, UK. Contact: Dr Ann Dalton ([email protected]).
- 4
Center for Nephrology and Metabolic Disorders, Laboratory for Molecular Diagnostics, Weisswasser, Germany. http://www.moldiag.de/en/gen/107300.htm; commercial laboratory.
- 5
Centro de Genética Humana, Instituto Nacional de Saúde Dr Ricardo Jorge, Lisboa, Portugal. Contact: João Gonçalves, PhD; telephone: (351)-217519320; e-mail: [email protected].
Management
Treatments currently available
Asymptomatic individuals. The risk for developing a VTE event for individuals with AT deficiency depends on an individual’s family history, the presence or absence of other thrombophilias, and probably the subtype of AT deficiency. The risk of VTE is low if individuals’ conditions were diagnosed because they were randomly screened for AT deficiency (such as random blood donors), but higher if individuals’ conditions were diagnosed because a family member was found to have VTE and AT deficiency [12–14,20–22]. In this later group, the VTE incidence has been found to be 0.9–2.9% per year, with a recent large prospective study showing an incidence of 1.7% per year [12]. Although 58% of these episodes occurred spontaneously, 42% were associated with a transient risk factor and, thus, were potentially preventable [12]. These data show that the risk for VTE is relatively low and probably does not outweigh the risk of bleeding if long-term oral anticoagulation therapy was given. In keeping with these findings, the Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology (BCSH) concluded in 2001 that there is no evidence to support a policy of long-term pharmacological primary thromboprophylaxis of asymptomatic family members found to have thrombophilia, because the risk of serious or fatal haemorrhage considerably outweighs the risk of a fatal VTE event, even for patients with the most severe types of thrombophilia, such as type I AT deficiency [10]. However, future anticoagulant regimens with a lower risk for bleeding may change this risk–benefit assessment.
Prophylaxis during surgery and immobility. Because 42% of the episodes of VTE in individuals with AT deficiency occur in the setting of transient risk factors, diligent VTE prophylaxis in risk situations is important [12]. No published evidence suggests that individuals with AT deficiency need to receive more intense or longer duration thromboprophylaxis than other patients in similar clinical circumstances. However, empirically, the authors of the present review tend to recommend somewhat higher prophylactic heparin doses than for non-AT-deficient patients, in view of the potential for individuals with AT deficiency to have some degree of heparin resistance, rendering heparins less effective. It has been argued that fondaparinux, even though AT dependent in its anticoagulant action, might still be fully effective when given in standard doses to AT-deficient patients [23]. Hirudins, given subcutaneously, also appear to be a good choice for VTE prophylaxis, because they do not require AT for their anticoagulant action. The authors of this article empirically tend to recommend somewhat longer postoperative VTE prophylaxis in individuals with AT deficiency compared with non-deficient patients undergoing similar surgical procedures, in view of the thrombogenicity of AT deficiency.
No randomized clinical trials have been performed assessing the need for and efficacy of AT replacement therapy. Its use has been reported in case series only [24,30,31]. No guidelines or consensus statements exist as to when to use AT concentrates and when not. It appears reasonable to consider giving them in surgical procedures with a high VTE risk when anticoagulation prophylaxis cannot be safely given because of the risk for serious bleeding, for example, procedures that involve neuroaxial anaesthesia or cerebral surgery. It may also be appropriate to consider giving them in surgical procedures that have a relatively high rate of VTE despite heparin or fondaparinux VTE prophylaxis, such as knee and hip replacement or major abdominal or pelvic cancer surgery. If given, it is not known for how long they should be given.
Venous thromboembolism. The initial management of VTE in patients with AT deficiency is usually not different from that of VTE in any other patient: (i) consideration of thrombolytics; (ii) initial therapy with heparin or fondaparinux; and (iii) transitioning to a vitamin K antagonist [10]. In individuals with AT deficiency there is, theoretically, a risk of heparin resistance and thrombus progression, no matter whether unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or fondaparinux is used, but this does not seem to be a clinical problem in most patients [25]. In clinical practice, it may simply be sufficient to increase the dose of UFH until a therapeutic aPTT is achieved. Because LMWH and fondaparinux are typically not monitored with anti-Xa assays, subtherapeutic dosing may, theoretically, occur in patients with AT deficiency. It has not been investigated whether certain patients with AT deficiency would benefit from AT concentrate therapy at the time of an acute thrombotic event. It may be worthwhile to consider AT concentrate in the individual with extensive or clinically symptomatic VTE. Even though no randomized trial has been performed on the intensity of oral anticoagulation needed (i.e. the optimal target international normalized ratio), there is no indication from published reports that patients with AT deficiency have a higher VTE recurrence rate while taking standard intensity oral anticoagulants. Thus, an International Normalized Ratio target of 2.0–3.0 appears appropriate.
In determining the length of vitamin K antagonist therapy, the circumstances of the thrombotic event and all the patient’s risk factors for recurrence should be weighed, similar to any other patient who has experienced a VTE. In addition, all risk factors for bleeding, and the patient’s preference regarding long-term vs. time-limited anticoagulation need to weigh into the decision making. The risk of recurrent VTE in patients with AT deficiency not treated with long-term anticoagulants is high (10% and up to 17% per year) [12,26]. Long-term anticoagulation clearly lowers that risk [12]. It is, therefore, typically recommended that a patient who has had a VTE and who has inherited AT deficiency should be considered for long-term anticoagulation [10,27]. It is not known whether the same recommendation applies to patients who have the type IIb subtype. The most recent guidelines of the American College of Chest Physicians do not take reference to presence of antithrombin deficiency as a determinant of length of anticoagulant therapy [33]. It is noteworthy that, despite long-term anticoagulation, a substantial risk of recurrence of 2.7% per year still remains [12].
Arterial thromboembolism. No data exist as to what the best treatment is for the patient with AT deficiency who has had an arterial thrombotic event (i.e. whether anticoagulants or antiplatelet agents should be used). If the patient also has significant underlying arteriosclerosis or arteriosclerosis risk factors, which by themselves could explain the thrombotic event, then antiplatelet therapy may be appropriate, because this would be standard therapy in any other patient with a similar arterial event. However, if the thrombotic event occurred in a younger person and in the absence of any obvious arteriosclerosis, the suspicion that hypercoagulability due to AT deficiency may be the main cause of the thrombotic event is heightened. In this situation, the consideration of longer-term anticoagulation may be appropriate. However, it is important to realize that this approach is not derived from clinical studies, but from the inference that anticoagulants may be more effective than antiplatelet agents based on the known physiological role of AT in the coagulation cascade.
Pregnancy. Because of the absence of clinical studies of good quality, recommendations for pregnancy management in AT deficiency are weak recommendations, reflecting only expert committee opinions and/or clinical experience of respected authorities [10,29]. The uncertainty about best treatment refers to (i) pharmacological agents used for VTE prophylaxis; (ii) doses of drugs given; and (iii) whether AT replacement therapy should be given and, if administered, when, for how long, and at what dose. The absence of a uniformly accepted treatment standard is highlighted by the large variety of treatment approaches used, as reported in a review of 49 pregnancies published in the medical literature [28]. The recommendations from the American College of Chest Physicians (ACCP) and the BCSH are as follows [10,29]:
- 1
In AT-deficient women without previous VTE, the ACCP guidelines recommend ‘antepartum and postpartum prophylaxis’ [29].
- 2
In AT-deficient women with previous VTE not receiving long-term anticoagulants, the ACCP suggests in addition to postpartum prophylaxis, antepartum prophylactic or intermediate-dose low molecular or unfractionated heparin [29].
- 3
In women with previous VTE who are receiving long-term VKA therapy, the ACCP recommends - independent of whether the woman has or does not have antithrombin deficiency - LMWH or UFH throughout pregnancy (either adjusted-dose LMWH or UFH, 75% of adjusted dose LMWH, or intermediate-dose LMWH) followed by resumption of long-term anticoagulants postpartum [29]. This means full-dose heparin.
- 4
The BCSH guidelines recommend that women who have type I or type II reactive site AT defects (whether or not they have had a thrombotic episode) (i) wear graduated compression stockings throughout pregnancy and for 6–12 weeks postpartum and (ii) use adjusted doses of LMWH or UFH. Detailed dosing and anti-Xa target recommendations are provided in the guideline publication [10].
AT concentrates. Two types of commercial AT concentrates exist: (i) plasma human AT concentrates (phAT) and (ii) recombinant human AT (ATryn). Plasma-derived AT is manufactured from random donor pooled plasma. The manufacturing processes differ for the various available phAT concentrates, depending on the manufacturer. The plasma-derived AT concentrates are well tolerated, with minimal adverse reactions, and an extremely low risk for transmission of infectious agents [30,31]. When choosing to supplement an individual with AT, the initial dose is calculated as follows: initial dose = (desired minus current AT level as % of normal level) times weight (kg) divided by 1.4. A peak and trough level should be obtained. Maintenance doses can then be calculated by approximately using 60% of the loading dose to maintain peak and trough AT activity in the range of 120–80%, respectively, given once every 24 h. A daily trough AT plasma level should be obtained.
The transgenic form of AT, also referred to as recombinant human AT (rhAT), is commercially manufactured by GTC Biotherapeutics Inc., (Framingham, MA, USA) and available for routine clinical use in Europe (ATryn) but not the United States. The rhAT is produced in transgenic goats that express rhAT in their milk, under control of the β-casein promoter gene [32]. The rhAT isolated from the milk of transgenic goats is identical to phAT, with the exception of its heparin-binding affinity, which is fourfold higher for rhAT, and the nature of its glycosylation. The major glycosylation differences are the presence of fucose and GalNAc, a higher level of mannose, and a lower level of galactose and sialic acid in rhAT. There is also substitution of 40–50% of the N-acetyl-neuraminic acid with N-glycolyl-neuraminic acid. Compared with phAT, rhAT requires a lower concentration of heparin for inhibition of both FXa and thrombin. However, differences in glycosylation between phAT and rhAT do not appear to elicit immune reactions, as none of the patients treated with rhAT during various clinical studies has developed an antibody response. rhAT is conventionally purified using tangential flow filtration, heparin affinity chromatography, nanofiltration, anion exchange chromatography and hydrophobic interaction chromatography. The purity of rhAT has been found to be >99%, and rhAT and phAT exhibit equivalent activity in in vitro thrombin and FXa inhibition assays. The mean half-life of rhAT is estimated to be 10.49 ± 7.19 h, in comparison to 56.8–68 h for phAT. Since 2006, ATryn has been approved for use in Europe for the prophylaxis of VTE in patients with hereditary AT deficiency, who are undergoing surgical procedures.
Research protocols
A GTC Biotherapeutics-sponsored, multicentre, multinational trial assessing the incidence of thromboembolic events after prophylactic intravenous administration of rhAT to patients with hereditary AT deficiency undergoing surgery or delivery (NCT00110513, http://www.clinicaltrials.gov) was completed in 2008 and publication of results is pending.
A Grifols Inc.-sponsored phase II/III trial is ongoing that evaluates the safety, pharmacokinetic properties, and efficacy of a plasma-derived human AT concentrate in patients with hereditary AT deficiency during surgery, pregnancy and thrombotic events (NCT00319228, http://www.clinicaltrials.gov).
Individuals with an interest in AT
Basic laboratory and clinical researchers with an interest in AT are best identified through searching (i) the most recent peer-reviewed literature via PubMed and MEDLINE and (ii) national grant agency databases, such as the Computer Retrieval of Information and Projects (CRISP) Web site of the National Institutes of Health (http://crisp.cit.nih.gov/crisp/crisp_query.generate_screen). A list is also available on e-mail request from the authors of this review article.
Disclosures
Dr. Patnaik has been a paid consultant for Talecris, Dr. Moll a paid consultant for Talecris and GTC Biotherapeutics.
Links to organizations
Non-profit organizations
- 1
The National Alliance for Thrombosis and Thrombophilia (NATT) –http://www.nattinfo.org. A comprehensive patient information brochure on AT deficiency is downloadable or can be requested as a printed version –http://www.nattinfo.org/natt_publications/antithrombin_def.pdf.
- 2
http://www.fvleiden.org– an educational web site on thrombosis and thrombophilia for patients, to some degree also for health care providers.
- 3
National Organization for Rare Diseases (NORD) –http://www.rarediseases.org.
- 4
The International Society on Thrombosis and Hemostasis (ISTH) –http://www.med.unc.edu/isth.
- 5
The Mediterranean League Against Thromboembolic Diseases –http://www.medleague-thrombosis.org/principal.htm.
- 6
The Thrombosis Interest Group Of Canada –http://www.tigc.org.
Commercial organizations
- 1
Talecris web site: http://www.thrombate.com/2.0.0_about_deficiency.aspx– educational web page with video and animated graphics to educate about AT.
- 2
GTC web site: http://www.atiii.com/index.htm. This is a web page to inform patients and physicians about hereditary AT deficiency and to provide them with a forum for asking questions.




