Genetic aspects and research development in haemostasis
Introduction
The science of clinical haemostasis continues to advance on a broad front. This selection of contributions from well-known workers in the field offers a range of studies addressing issues that can affect all the patients with bleeding disorders.
Inhibitors are now the most important complication of replacement therapy. Ingerslev has studied a remarkably large cohort of patients with factor VII deficiency – a very rare disorder, for this complication. Happily the incidence of inhibitors in this group is low.
Peyvandi has also looked at a remarkably large cohort of patients with rare bleeding disorders to establish the types of mutation that underlie these conditions. It is only on this basis that we can begin to address wider issues such as phenotype-genotype correlation and the chances for developing novel strategies to tackle the basic patho-physiology of these states.
One approach that is much researched and discussed, although not yet a clinical reality, is gene therapy. Waddington has developed the ability to introduce gene therapy vectors into foetal mice in order to address the issues of safety, efficacy and lifetime phenotype correction in these animals. This work forms the basis of the clinical protocols that are being introduced with the aim of correcting any congenital bleeding disorder from the earliest age.
Berntorp is taking a genome-wide approach for uncovering the underlying predisposing factors that contribute to the risk of developing inhibitors. These are not limited to the mutation in the gene for the relevant clotting factor but are already known to extend to immune response genes of various types. This study promises to further enhance our understanding of a very complex phenomenon and it is to be hoped that this will help us to devise new ways of preventing this life-threatening problem.
Functional inhibitors to Factor VII:C in patients suffering Factor VII deficiency. Results from a multi-national screening programme
Factor (FVII) deficiency is classified as a rare bleeding disorder. Heterozygous mutations causing mild FVII deficiency are widespread amongst all populations and most often represent a non-bleeding abnormality, randomly found on the basis of a spontaneous deviation in a PT/INR screen. However, founder-based enclaves with a high prevalence of FVII deficiency have been detected in a few countries in Mid-eastern Europe where severely affected patients are more prevalent than elsewhere. A severe bleeding phenotype is typically associated with homozygous or double heterozygous conditions, [1] which is quite rare with an estimated prevalence of 1–2 cases per million inhabitants in most countries. Amongst several interesting aspects related to FVII deficiency, attention has recently been focussed on the likelihood of inhibitor formation amongst those FVII-deficient patients who require substitution therapy. Only a few examples of inhibitors can be found in existing literature [2,3]. In order to explore the possibility that persons with FVII deficiency may develop functional inhibitors against FVII, an open label clinical programme has been established in which patients suffering FVII deficiency are tested for the presence of inhibitors before and 30 days after exposure to FVII concentrate. The test procedure adopted comprise recording of FVII:C of the baseline sample and the 30-day sample, together with measurement of FVII:C inhibitors by means of a Bethesda type of inhibitor assay. As of February 2008, a total of 59 patients have been tested. One case of a ‘de novo inhibitos’ was identified.
Materials and methods
Ethics Committee approval and consent from each patient for this study was obtained locally. Citrated whole blood was collected prior to infusion of FVII concentrate, and platelet-poor plasma obtained. The brand of FVII concentrate as well as the dose administered was determined by the respective investigator. Thirty days later, a second blood sample was aspirated and platelet-free plasma recovered for analysis. Plasma test material that had been frozen and kept at −80°C was sent to our laboratory on dry ice. The entire cohort of patients originated from two separate sources: (i) In accordance with the STER protocol as published on the official website (http://www.targetseven.org), and (ii) From the Greifswald Registry on rare bleeding disorders gathering patient samples exclusively from German centres following the same principles as proposed by STER.
An inhibitor test was constructed following the original protocol of the Bethesda assay. Since factor VII displays no signs of spontaneous degradation at 37°C, there was no need for inclusion of the changes reported as the Nijmegen modified method.
Results
At the time of the present study-update, a total of 68 treatment episodes in 59 patients have been investigated through the STER programme and 31 treatment episodes recorded in 31 patients submitted from the Greifswald Registry.
The outcome so far has been that one patient presented with an inhibitor against factor VII. In this patient the pre-dose sample had an inhibitor level at <0.4 BU mL−1 whereas the 30-day post-infusion sample showed 10.0 BU mL−1. An inhibitor assay in the laboratory of the local investigator revealed a level of 8.9 BU mL−1 [4].
Discussion and conclusion
Patients suffering from FVII deficiency who require FVII substitution are quite rare and bleeds calling for treatment appear to be variable. Hence, recruitment of patients for a study of inhibitors is a quite difficult and time-consuming task. This formal study has analyzed 99 treatment episodes in 90 patients and disclosed the complication of inhibitors in one patient. The study outcome points to the likelihood that inhibitors amongst FVII-deficient patients receiving substitution with FVII concentrate are not common events. In comparison, inhibitors against factor IX (FIX) in haemophilia B show a relatively low prevalence of a few percent, and most, if not all, occur in patients with mutations abolishing expression of FIX. However, estimates are not very accurate. While the prevalence of severe haemophilia B is probably in the range of 1:150 000 inhabitants, severe FVII deficiency is even less common, probably at 1:500 000 inhabitants. The background population required to identify a sufficient number of severely affected FVII-deficient patients at high risk of inhibitor formation would be quite immense.
At present no guidance exists on how to optimally manage bleeds in FVII-deficient patients with inhibitors. Two patients have been subject to follow-up for some time. We published on one patient [3] who subsequently has been seen on a regular basis, and the inhibitors in this patient have shown a tendency to decline as long as the patient was not re-exposed to FVII. A similar type of course has been observed on follow-up with the inhibitor patient identified in this study [4]. Despite the presence of inhibitors, some gain in FVII:C has been observed following infusion of FVII concentrate [3,4].
Continued surveillance is required to further investigate and characterize FVII inhibitors in patients with FVII deficiency, and colleagues wishing to participate in the study are advised to consult the official website of the study at http://www.targetseven.org.
Genetics of rare bleeding disorders
Rare bleeding disorders (RBDs) represent 3–5% of all the inherited deficiencies of coagulation factors [5,6]. Patients affected by RBDs have a wide spectrum of clinical symptoms that vary from a mild or moderate bleeding tendency to potentially serious or life-threatening haemorrhages, caused by the deficiency or dysfunction of a clotting factor. Their distribution is variable in the world, in the general population ranging from approximately 1 in 2 million for prothrombin (factor II, FII) and factor XIII (FXIII) deficiency (the rarest) to 1 in 500 000 for FVII deficiency (the most common) [5,6]. Exceptions are countries with large Jewish communities, where FXI deficiency is much more prevalent. In Muslim countries and Southern India, with a higher rate of consanguineous marriages, RBDs occur more frequently, representing a significant clinical and social problem [7]. RBDs are recessively inherited and are, in general, attributable to a defect in the actual genes encoding the different coagulation factors. Exceptions are the combined deficiency of factor V (FV) and FVIII [8], caused by defects in genes encoding proteins involved in the intracellular transport of these factors, and the combined deficiency of vitamin-K-dependent proteins (FII, FVII, FIX and factor X, FX) [9], on account of mutations in the enzymes involved in post-translational modifications of these factors and in vitamin K metabolism.
Multiple mutations for each coagulation defect have been reported and the majority of them are private mutations, unique for any given patient. The unique nature of the mutations complicates the management of RBDs through prenatal diagnosis in families with at least one affected child, particularly in countries with low economic resources, where the patients rarely live beyond childhood.
In the last 10 years, our centre, which is an International Reference Centre for diagnosis and treatment of RBDs, performed the phenotypic and genotypic characterization of 400 patients coming from 19 different countries with various deficiencies. The major cohorts of patients were from Iran (155) and Italy (153); the remaining were from Europe (44), other Middle-East countries (8), Asia (37) and America (3). The most frequent was FVII deficiency (25%), followed by FX (16%), fibrinogen and FV (14%), FXIII (11%), FV + VIII (8%), factor XI (FXI) (7%) and FII (5%).
On the basis of coagulant activity, the conditions of 41% of the patients were severe, 20% moderate and 39% mild. Seventy-seven percent of patients had been fully characterized and a total of 166 different mutations have been identified including 86 missense mutations, 34 small insertion/deletion, 21 nonsense mutations, 20 splice-site alterations and 5 mutations in the 5′ untranslated region (5’UTR) (Fig. 1); 27% of the mutations that were examined are novel. As shown in the Table 1, nonsense mutations were not identified on FII and FX genes confirming previous data from FII and FX knock-out mice showing that the complete lack of FII and FX is not compatible with life [10,11]. In 5% of cases, no putative mutations have been found. These cases may be on account of defects in non-coding regions or in genes coding for regulators of intracellular transport and post-translational modifications of coagulation factors.
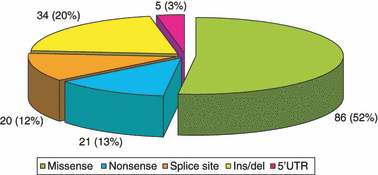
Distribution of mutations in patients.
| Deficiency type | Gene | Missense | Nonsense | Splice site | Ins/del | 5′UTR | Total different mutations |
|---|---|---|---|---|---|---|---|
| Fibrinogen | FGA | 3 | 5 | 3 | 7 | 0 | 18 |
| FGB | 6 | 1 | 1 | 0 | 0 | 8 | |
| FGG | 3 | 0 | 3 | 1 | 0 | 7 | |
| FII | FII | 13 | 0 | 1 | 3 | 0 | 17 |
| FV | FV | 7 | 6 | 3 | 6 | 0 | 22 |
| FV + VIII | LMAN1 | 3 | 2 | 0 | 2 | 0 | 7 |
| MCFD2 | 1 | 0 | 1 | 2 | 0 | 4 | |
| FVII | FVII | 25 | 3 | 5 | 6 | 3 | 42 |
| FX | FX | 16 | 0 | 2 | 2 | 2 | 22 |
| FXI | FXI | 1 | 2 | 0 | 1 | 0 | 4 |
| FXIII | FXIII-A | 8 | 2 | 1 | 4 | 0 | 15 |
| Total | 86 | 21 | 20 | 34 | 5 | 166 |
In general, the gene mutation associated with recessively inherited coagulation disorders are distinct for each patient, with very few repetitive mutations related to a founder effect. Haplotype analysis is an important tool to confirm the existence of a founder effect for a specific mutation, which is a potential diagnostic tool in the prevention of genetic diseases, particularly in countries with a high prevalence of disease and low socio-economical resources. However, the identification of founder effects requires large groups of patients coming from different regions of the world. Our preliminary data on 400 patients suggest a founder effect for: (i) the LMAN1 gene: the Met1Thr mutation occurring in Italian patients, responsible for combined FV + FVIII deficiency; (ii) the FVII gene: Gln100Arg frequent in Europe; (iii) the FXIII gene: Arg77His frequent in Iran; and (iv) the FX gene: Gly222Asp occurring in Turkish patients coming from Iran, Turkey and Germany. Nonetheless, further studies are necessary to confirm these observations.
Expression studies of mutations in cultured cell lines and characterization of the recombinant proteins have proven to be an invaluable tool to understand the nature of the genetic defect and to unravel the underlying molecular mechanism of the deficiencies.
Laboratory developments for evaluating gene therapy
Conventional approaches to gene therapy of single-gene disorders in adult mammals face various difficulties such as: (i) Correction of the disease phenotype may require such large amounts of protein that prohibitively large quantities of gene delivery vector must be used; (ii) Mature tissues or organs may be refractory to infection by some current vector systems which depend upon cellular proliferation for optimal infection, e.g. gamma-retrovirus vectors; (iii) Immune responses, either pre-existing or developing in response to vector delivery, may obviate expression of transgenic protein and limit the efficacy of future gene- or protein therapy; and (iv) The genetic lesion might have already caused permanent pathological changes.
Foetal or neonatal gene therapy may circumvent many of these problems. First, a much higher vector: cell ratio can be achieved. Second, high rates of cell proliferation provide a conducive environment for infection by several vector classes. Third, as the mammalian womb provides protection from pathogens, the foetus’ own immunity (including the skin barrier, mucosal and adaptive immunity) is relatively immature. Finally, analysis of 923 disease-associated genes has shown that 31% encode enzymes and that defects in these genes are overrepresented in diseases occurring in the first year of life (47%). Therefore these diseases might be preventable if treated early.
A significant group of enzymes and their co-factors are the proteins constituting the coagulation cascade. Although deficiencies of FVIII and FIX are generally not lethal around the period of birth, deficiencies of some of the other coagulation factors are associated with significant perinatal morbidity and mortality. Therefore, early gene transfer might avert these events and may also prevent subsequent development of inhibitory antibodies which arise in a significant proportion of individuals treated later in life.
We have previously demonstrated permanent correction of FIX haemophilia by foetal gene transfer of lentivirus vector carrying human FIX cDNA to haemophilia B mice [12]. Others have shown transient (15 days) expression of human factor VIII (hFVIII) delivered by a first-generation adenovirus vector [13]. We now demonstrate expression of hFVIII for up to at least 1 month after foetal systemic gene transfer using a helper-dependent adenovirus vector, i.e. one devoid of all viral genome sequence.
Twenty microlitre of helper-dependent adenovirus vector carrying hFVIII cDNA was injected into the circulation of out-bred foetal MF1 mice at 16 days gestation. Vector administration was performed by injection into the vitelline vessels of two foetuses per dam following laparotomy and exteriorization of the uterus. We have demonstrated that this combination of injection route and vector predominantly results in hepatic transduction. Citrated blood was collected 5, 19 and 32 days after injection for hFVIII ELISA. Shown in the table below, at 32 days hFVIII antigen concentration exceeded 40 ng mL−1 (≈20% normal human plasma concentration).
| Days after injection | Concentration hFVIII (ng mL−1) |
|---|---|
| 5 | >400 |
| 19 | 88 ± 42 |
| 32 | 44 ± 0.8 |
This demonstrates that helper-dependent adenovirus, which has been shown to provoke a reduced inflammatory response compared with first generation vector, may be useful for prolonged expression of hFVIII and perhaps other clotting factors after in utero delivery. An advantage of helper-dependent adenovirus vector is that it can carry a large genetic payload (>35 kb) therefore more extensive cognate regulatory sequences can be incorporated. However, it will be necessary to study the long-term expression to investigate whether expression stabilizes after initially falling at a dramatic rate in inverse proportion to the increase in liver mass.
Genetic aspects of inhibitor development
The development of antibodies with the capability to neutralize FVIII is a T-cell dependent immune reaction that occurs in 20–30% of people with severe haemophilia A who have been treated with replacement therapy. An example of a complex disease, its cause is multifactorial, involving genetic as well as environmental factors.
Family studies offer advantages when evaluating genetic contribution to risk, as several factors are at least partially adjusted for, thus minimizing the variability introduced by non-genetic factors. Our interest in this avenue of inquiry was piqued in 1991 while treating a set of monozygotic twins exhibiting discordant responses to immune-tolerance induction. Both twins had developed high-responding inhibitors to FIX. One twin responded to ITI using the Malmö protocol on the first attempt, whereas the second twin could not be successfully tolerized after three attempts using the same regimen [14].
This observation led us to initiate, in 1996, the Malmö International Brother Study (MIBS) with the objective of identifying demographic and genetic risk factors for inhibitor development in a cohort of brother-pairs with haemophilia. A total of 460 families were recruited from 20 haemophilia treatment centres located throughout Europe. Most brother-pairs had haemophilia A (388) and the remaining families had haemophilia B (72). The proportion of families with an inhibitor in one or more brothers was approximately 25% in those with haemophilia A, and close to 6% in haemophilia B [15]. Findings from the MIBS showed that 70 to 80% of siblings are concordant with respect to inhibitor status, up to 90% wherever they are monozygotic twins. There is a threefold risk for development of an inhibitor in affected families when compared to unaffected families, and type of mutation alone is not enough to predict the risk for inhibitor formation [16]. In terms of specific genetic factors, the MIBS study has shown that polymorphisms in the TNFA gene, the IL10 gene, and the CTLA-4 gene are associated with inhibitor development in severe haemophilia A [17–19].
The next step in this path was the Hemophilia Inhibitor Genetics Study (HIGS), a family-based and association study to identify genetic factors, environmental factors, and genetic/environmental interactions that might increase the risk for inhibitor development. Initiated in 2004, HIGS has three phases. Phase I consists of brother-pairs, either both with an inhibitor or discordant (one with, one without), and their parents. Phase I will implicate by linkage chromosomal regions linked to the phenotype. Phase II consists of a single person with haemophilia and an inhibitor and his parents. In Phase II, a transmission disequilibrium test (TDT) will be done to test associations between a gene and the phenotype, narrowing the chromosomal regions for causal gene identification. Phase III will confirm the results from Phase I and II family studies in a group of unrelated people with haemophilia, with and without inhibitors.
HIGS is underway in 23 countries. As of 1 January 2008, approximately 750 people from 220 families have entered the study through 38 centres. This represents about one-third of our recruitment target. Separation of families on account of death, divorce, or geography is a very common reason for inability to participate. We believe in the strength of the three-phase study design for identification of genetic risk factors, and will aggressively continue our recruitment efforts. In the interim, however, we are maximizing an opportunity to increase the power and diversity of HIGS through collaboration with two other haemophilia studies: MIBS and the multi-centre Hemophilia Growth and Development Study (HGDS).
The rationale for the HIGS/MIBS/HGDS collaboration is that the cohorts are well-characterized, representative of the population from which they were enrolled, and are complementary in terms of design. The objective of the collaboration is to combine the data and resources of the cohorts to identify genetic factors associated with inhibitor development. The combined cohort study is composed of over 700 people with haemophilia A, about half of whom have inhibitors. There are approximately 160 brother-pairs concordant and discordant for inhibitor status, and 550 unrelated people. DNA and clinical data will be used to conduct an association study. The investigation will include examination of approximately 16 000 single nucleotide polymorphisms (SNPs), allowing an extensive interrogation of over 900 immune response, inflammatory and tolerance pathway genes, including HLA class II genes.
Knowledge of the genome has expanded with the completion of the HapMap project, the annotation of the human genome, and the availability of both structural variation and single nucleotide polymorphisms in public databases. This has allowed us to progress beyond a clinical observation in a pair of twins to the assembly of the HIGS combined study group, focussing the cutting edge of genetics technology on the most significant problem in haemophilia care.
Acknowledgements
The HIGS is funded through an investigator-initiated grant from Baxter BioScience, and in part with federal funds from the National Institutes of Health, National Cancer Institute, N01-CO-12400. The Malmö International Brother Study is funded through grants from Wyeth and the Research Fund at Malmö University Hospital. The Hemophilia Growth and Development Study is funded by the National Institutes of Health, National Institute of Child Health and Human Development, R01-HD-41224.




