Back to the future: a recent history of haemophilia treatment
The authors stated that they had no interests which might be perceived as posing a conflict or bias.
Abstract
Summary. In the last few decades, the management of patients with haemophilia has witnessed dramatic improvements, through the larger availability of safe plasma-derived and recombinant products for replacement therapy. Another important step forward is the progressively larger-scale implementation of primary prophylaxis in children. Currently, the main problem in patients with haemophilia is the onset of antibodies inactivating the infused clotting factor (inhibitors), even though immune tolerance regimens that are able to eradicate inhibitors and the availability of products that bypass the intrinsic coagulation defects have dramatically improved the management of these patients. Cure of haemophilia through gene transfer is being attempted, but relatively, it is far from being implemented on a large scale. It is likely that further improvements in replacement therapy will occur in the near future, through the availability of new-therapeutic tools such as factors VIII and IX with longer half-lives, more potent bypassing agents and factors extracted from the milk of transgenic animals.
Introduction
Before the 1970s, very few countries had implemented effective programmes of treatment delivery for persons afflicted with haemophilia. The main obstacle was the non-availability of the essential therapeutic tools, i.e. plasma fractions containing factor VIII (FVIII) or factor IX (FIX). In 1964, the discovery by Judith Pool that the fraction cryoprecipitated from plasma contained large amounts of FVIII marked a gigantic step forward. Cryoprecipitate was, for instance, the first treatment available in Italy that allowed the institution of a programme of haemophilia care 40 years ago at the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center in Milan. This plasma fraction was also the basis for the development of haemophilia A care programmes in the USA, whereas a few European countries (such as France, Sweden, UK and Netherlands) were already producing low-purity lyophilized plasma fractions enriched in FVIII for haemophilia A and in prothrombin complex coagulation factors for haemophilia B. Even in these relatively advanced countries, however, replacement products available for treatment were far from adequate to meet the needs of persons with haemophilia. At the most, cryoprecipitate and early concentrates helped to control life-threatening haemorrhages and to carry out, not without difficulties and mishaps, emergency surgery. Mortality was still high and the average life expectancy of persons with severe haemophilia was much lower than that of the general population.
The 1970s: the success story of the decade
The main step towards a radical change in the quality of life and life-expectancy of persons with haemophilia was the arrival on the stage of a few pharmaceutical companies (Hyland, Immuno and Cutter) that in the early 1970s produced relatively large amounts of intermediate-purity factor concentrates in lyophilized formulations. Cryoprecipitate and low-purity products had been used only in hospital setting, because they were available in formulations only suitable for slow intravenous infusion and caused relatively frequent allergic reactions. The newer lyophilized products could instead be reconstituted in as little as 20–30 mL of fluid and easily injected with syringes. Starting in 1970, these products made the delivery of treatment possible at home, by the patients themselves or their caregivers. Treatment ‘at the doorstep’ was another gigantic step forward in haemophilia care. Early treatment of bleeding became feasible, in contrast to the previous long delays between the first signs of haemorrhage and arrival at the hospital treatment facilities. Specialized haemophilia centres became less overwhelmed by the burden of providing emergency treatment and could develop programmes of comprehensive care, with the involvement of such specialists as orthopaedic surgeons, physiotherapists, dentists and social workers. It was in the golden era of the 1970s that Inge Marie Nilsson and Ake Ahlberg in Malmo pioneered the regular administration of FVIII, with the goal of temporarily transforming severe into moderate disease and thereby preventing rather than treating the majority of bleeding episodes (primary prophylaxis). Elective surgery, particularly orthopaedic operations, became possible and safe, and helped to correct or minimize the musculoskeletal abnormalities that had developed as a consequence of untreated or inadequately treated bleeding episodes into joints and muscles. In 1977, the discovery of desmopressin provided a new, inexpensive and safe treatment to patients with mild haemophilia A (and von Willebrand disease), who could avoid or substantially reduce the use of plasma-derived products, the corresponding costs and also risks of infection. On the whole, haemophilia became one of the most satisfactory examples of successful secondary prevention of a genetic disease, in that decade.
No rose is without thorns. The treatment of bleeding episodes in patients with inhibitory alloantibodies to coagulation factors was difficult with the limited available therapeutic tools (porcine FVIII, plasmapheresis), even though products that bypassed FVIII deficiency (FEIBA and Autoplex) appeared on the stage in the last part of the decade. Concentrates containing vitamin-K-dependent coagulation factors were used successfully in haemophilia B, but caused thromboembolic complications occasionally. Non-A, non-B hepatitis had occurred in practically all patients treated with factor concentrates produced from pooled plasma, and a large proportion of them had persistent alterations of serum transaminases, an index of chronic liver disease. However, the ominous long-term consequences of the infection with what we now know to be the hepatitis C virus (cirrhosis, hepatocellular carcinoma) were not established at that time, the great majority of patients were asymptomatic and appeared to tolerate well the chronic viral infection. In all, it could not be predicted that infectious hepatitis transmitted by large-pool plasma concentrates did herald the much more threatening and rapidly fatal acquired immunodeficiency syndrome (AIDS). Figure 1 summarizes the chronology of the aforementioned developments of haemophilia care in the decade. For more information and references, see a previous review article [1].
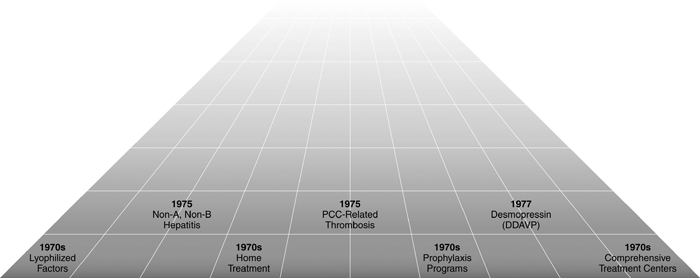
Chronology of the main events in haemophilia care that took place in the 1970s.
The 1980s: the ravages of AIDS
The opinion that the cause of AIDS, first described in two persons with haemophilia in 1982 [2], might be a virus, was held by some of us, but the alternative idea that the immune system was compromised by the continuous exposure of patients to allogeneic plasma proteins through transfusions was also well founded [3,4]. It was only between the end of 1983 and early 1984 that lymphotropic retroviruses were isolated from patients with AIDS [5,6], but definite evidence that they were the cause of the disease rather than a casual association was not acquired before the end of 1984. The industries that manufactured anti-haemophilic products from plasma reacted rapidly to the new scourge of AIDS, through the development of physical and chemical methods meant to inactivate the AIDS virus and other blood-borne pathogens that had escaped detection during the screening of blood donations [1,7]. Virucidal methods, that progressively improved in efficacy and safety in the years between 1983 and 1987, halted first the AIDS epidemic and then that of hepatitis C, caused by a virus much more robust than HIV [1,7]. Unfortunately, progress in the treatment of HIV-infected patients by means of antiretroviral agents developed at a relatively slow pace and the benefit of early available therapeutic agents (azothioprine, didanosine) was very limited. Thousands of persons with haemophilia died of AIDS in the 1980s and 1990s. It was only in the middle of the 1990s that highly active antiretroviral therapy with multiple drugs (particularly protease inhibitors) changed dramatically the course of HIV infection, making it a chronic condition compatible with a prolonged survival and an acceptable quality of life. In the second part of the 1980s, impressive and rapid progress in DNA technology fostered the industrial production of recombinant coagulation FVIII (and subsequently of FIX), with the publication in 1989 of the first report of efficacy in two patients [8].
Figure 2 summarizes the chronology of these important events in the 1980s. On the whole, this pace of progress towards a safe treatment of patients with haemophilia was substantial. Yet, it was not viewed as fast enough by the community of persons with haemophilia, who witnessed a dramatic switch from the golden era of the 1970s to the devastations of the AIDS epidemic.
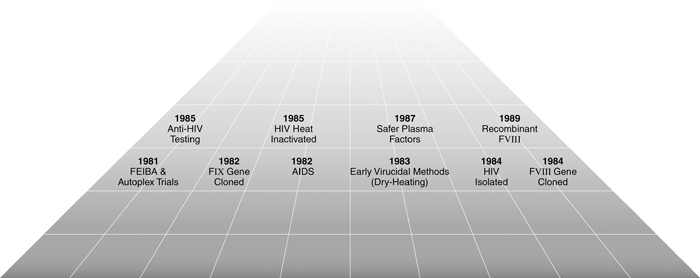
Chronology of the AIDS era.
From the 1990s until now: a new golden era
The progressive improvement of virus-inactivation methods, as well as of those used to screen viruses in blood donations and plasma pools (typically, the adoption of PCR testing) led to an impressive outcome in terms of safety of plasma-derived concentrates: no blood-borne transmission of hepatitis viruses nor of HIV has occurred in the last 15 years [1,7,9]. HIV infection is now controlled by antiretroviral therapy and HCV can be eradicated in at least half of infected persons with haemophilia using combined treatment with α-interferon and ribavirin [10]. A perceived threat was and still is looming large, particularly in the UK: that the abnormal prion protein causing new variant Creutzfeldt-Jakob disease (nvCJD) might be transmitted by antihaemophilic factors concentrated from plasma, or by plasma fractions added to recombinant factors during fractionation or formulation. Now, more than 10 years after the occurrence of the first few cases of nvCJDI [11], the anxiety has diminished. It is not abolished though, because a few cases of nvCJD associated with transfusion of whole blood or red cells have been documented in the UK. On the other hand, the fractionation steps used to purify from plasma coagulation factors and other proteins are capable of removing large amounts of experimentally added prions. This makes it unlikely that prions reach the final product in concentrations high enough to cause spongiform encephalopathies, even in the unlikely event of their presence in source plasma [12].
As a result of the current level of safety of plasma products, factor concentrates are still largely used, in a proportion approximately estimated at 20–30%, even in those high-income countries that can afford the use of the more expensive recombinant factors [13]. The consumption of plasma-derived products is actually increasing in Asia, where consumption has doubled in the years between 2000 and 2006, substantially contributing to the progress in haemophilia care in South-East Asian countries [13,14]. In Western Europe and North America, recombinant factors have definitely become the main product used for treatment, with a constant trend towards increased consumption [13]. The first generation of recombinant products had an impeccable record of efficacy and safety [15–17]. Nevertheless, the manufacturers chose to implement further technological improvements leading to a substantial reduction in the content of proteins other than FVIII or FIX [18–20]. In the last few years, experimental data [21] and two retrospective cohort studies [22,23], carried out in previously untreated boys with severe haemophilia A, have raised the suspicion that the cumulative incidence of inhibitors is higher with recombinant FVIII than with plasma-derived products containing, besides other proteins, large amounts of von Willebrand factor, the carrier and stabilizer of FVIII. There is also a published cohort study that demonstrates comparable immunogenicity of the two forms of treatment [24]; accordingly, this issue is likely to remain unsettled until randomized clinical trials are performed [25].
The high degree of safety of antihaemophilic products, as well as their increased availability (albeit not matched by a decrease in costs) has prompted the more widespread implementation of programmes of primary prophylaxis. The publication of a randomized clinical trial showing the superior efficacy of prophylaxis over on-demand therapy has recently provided the proof of efficacy that, evident to both clinicians and patients, was still lacking according to the principles of evidence-based medicine [26]. In high-income countries that can afford the corresponding costs, regular prophylaxis should be offered to all patients with severe haemophilia, at the time of diagnosis or after the fist few episodes of joint bleeding [27,28]. However, frequently repeated venous access often creates problems in small boys, and indwelling devices are not free from complications [26]. A new approach, based on escalating the frequency of factor infusions depending on whether or not bleeding is satisfactorily prevented, holds the promise of being at the same time less expensive and less demanding on the patient’s veins than infusions given on alternate days, because approximately half of the boys with severe haemophilia had an acceptably low frequency of bleeding episodes with a single weekly dose of 50 U Kg−1 FVIII [29].
There have also been great advances for haemophiliacs with inhibitors. The efficacy of products that bypassed the need for FVIII and FIX in intrinsic coagulation was established in the early 1980s by randomized, double-blind clinical trials [30,31]. However, efficacy of products such as FEIBA and Autoplex in controlling spontaneous bleeding was less than that obtained using FVIII or FIX concentrates in patients without inhibitors (40–60% vs. 80–90%) [15–20,30,31]. In the context of this much improved but still relatively unsatisfactory background, two approaches have substantially changed the picture: the development of recombinant activated factor VII (rFVIIa) as a new bypassing product and the use of immune tolerance to eradicate the inhibitor, a treatment already pioneered in the early 1980. rFVIIa can stop 80–90% of the spontaneous haemorrhages, particularly when administered early in the home setting [32,33], and elective orthopaedic surgery can be carried out safely [34,35]. The advent of rFVIIa also prompted more studies on FEIBA, which was found to be similar in efficacy and safety to rFVIIa in the context of a randomized trial [36]. One of the limits associated with the use of rFVIIa, i.e. the need to give repeated intravenous boluses of 90–120 μg Kg−1 at 2–3 h intervals because of the short plasma half-life, is apparently overcome by the results of two randomized clinical trials, showing that a single larger bolus of 270 μg kg−1 is at least as efficacious as repeated smaller doses [37,38]. This approach to treatment delivery is particularly advantageous in small boys and, in general, in patients with difficult venous access. As to immune tolerance, the demonstration that this expensive and demanding approach to inhibitor eradication on the basis of daily or very frequent infusions of large doses of FVIII is effective in up to 70% of cases, stems mainly from two large international registries [39,40]. Registries have also provided some information on the predictors of positive responses to immune tolerance: low levels of inhibitors at onset and lower historical peak levels appear to be the main determinants. Most importantly, an ongoing randomized study is evaluating whether or not large daily doses of FVIII are truly needed to achieve the eradication of the inhibitor, or if smaller doses given every other day are equally effective albeit perhaps at a slower pace (http://www.itistudy.com).
Correction through gene transfer of the DNA defects underlying the haemophiliacs is the only approach that can lead to cure, the ultimate goal of research and the great expectation of patients. Many efforts have concentrated on this formidable goal in the last 10 years. After excellent results were obtained earlier in animal models of haemophilia [41,42], at the beginning of the third millennium a few phase 1/2 studies of somatic cell gene therapy conducted in patients with FVIII or FIX deficiency appeared to confirm the great expectations. In the majority of patients enrolled in clinical trials using in vivo or ex vivo approaches to transfer the normal gene, measurable levels of VIII or IX were attained in plasma [43–46]. These promising results generated considerable optimism in the haemophilia community, and some of us predicted that cure of haemophilia would become a reality in the first decade of the millennium. Unfortunately, further studies did not fulfil these initial expectations and dampened excessive hype. The problems of transient and therapeutically insufficient factor levels achieved in plasma through the donated normal gene is still unsolved and, most importantly, severe side-effects on account of the reactions of the host immune system to viral vectors [46,47] led to the fact that, at the time of writing, no study is ongoing in humans. There are also concerns about the risk of insertional mutagenesis and transmission of the transgene through the sperm of the host [48]. Nevertheless, studies in animal models are more and more successful, so that newer clinical trials will start soon again in the USA and Europe. Other approaches are being explored, such as the genetic induction of FVIII and FIX expression in megakaryocytes/platelets or endothelial cells [49–51], an approach that has the potential to restore local haemostasis at sites of vascular injury and thus to be effective also in patients with inhibitors. Novel approaches are also the read-through of translation termination codons and site-specific correction of gene defects using zinc finger nuclease technology [47]. Figure 3 shows the main steps of the advances that have occurred up to the present.
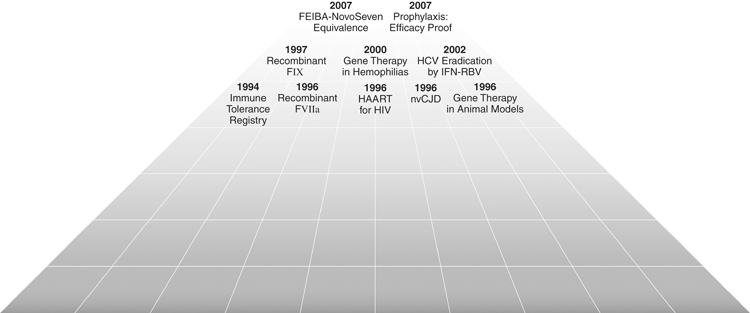
A new golden era: from the 1990s until now.
What next: building on strength
Muscular dystrophy, cystic fibrosis, thalassaemia and sickle cell anaemia are the most frequent and clinically demanding monogenic disorders. None of these diseases are currently treated as efficaciously and safely as haemophilia. Hence, patients and caregivers must be cognizant that any therapeutic advance, including cure through gene therapy, should be devoid of any harm for patients who actually enjoy an excellent treatment. However, this is true only for those living in Europe, North America and in a few high-income Asian countries. The first goal for the future is to deal with the fact that huge and densely populated countries such India and China (having nearly two-thirds of the global population) are far away from having achieved a satisfactory level of haemophilia care. These countries are rapidly developing a high level of technological competence, so that it is probably more appropriate for them to foster DNA technology, and thus the production of recombinant factors and gene therapy, rather than programmes based on plasma fractionation. On the other hand, the industrial production of plasma-derived factors should continue and expand, to meet the increasing needs of South American countries (with populations of nearly half billion), that are rapidly improving their programmes of care delivery to persons afflicted with haemophilia. No information is available for the majority of low-income countries of Africa (nearly one billion). Unfortunately, it is difficult to increase the availability and to decrease the costs of the raw material used in the plasma fractionation industry, also on account of the more-and-more stringent demands of regulatory agents on the quality control of source plasma. Perhaps a newer way to make available large amounts of coagulation factors is their fractionation from the milk of transgenic animals [52]. A US-based biotechnology company is developing the production of human coagulation FIX and FVIII from the milk of transgenic pigs (and has patents that cover the transgenic production of fibrinogen). Commercial development is still in its infancy, but a pharmaceutical company has entered into an agreement with the biotechnology company to enable the development of these products.
Even though the extension of programmes of haemophilia care to the less-privileged countries is, in my opinion, action number one for the immediate future, this strategy does not impinge upon the legitimate eagerness of patients and caregivers to see further progress built on current strength. The first and main goal is to maintain the high levels of treatment that may be jeopardized even in high-income countries by the increase in the costs of the welfare state. Treatment of the haemophiliacs, particularly of those with inhibitors [53], entails usually the highest per capita cost in these countries. On the other hand, in any country the costs of haemophilia are truly a tiny part of the whole budget for health care, and the cost effectiveness of haemophilia care is well proven.
Assuming that the current level of care will be maintained in the near future, which advances in treatment can be foreseen? (Table 1) The most likely is the availability of FVIII and FIX molecules with longer half-lives. This would be a significant step forward, considering that in countries that can afford primary prophylaxis, the main obstacles to its widespread adoption are the difficulties of venous access. Several pharmaceutical companies are currently developing factors with longer half-life, which would require less frequent venipunctures [54–59]. Advances may also materialize through DNA technology that may be able to modify coagulation factor molecules and render them more active and/or less antigenic [60]. Several approaches have been attempted in the laboratories, but at the moment those that are undergoing clinical trials are based upon the PEGylation of factors, or of factor vehicles such as liposomes [54–59].
| Wider factor availability |
| Longer acting rFVIII |
| Improved rFIX |
| Longer acting rFVIIa |
| Improved rFVIIa |
| Recombinant porcine FVIII |
| FIX and FVIII from transgenic animals |
| Cure of haemophilia: gene therapy |
| Professional Scientific Societies (HTRS–EAHAD) |
| More and younger haemophilia and thrombosis specialists |
Patients with inhibitors remain, despite recent progress, one of the main challenges. Newer preparations of rFVIIa endowed with higher haemostatic potency or more-prolonged half-life are currently undergoing clinical trials [60–66]. Porcine FVIII, originally derived from animal plasma and endowed with low cross-reactivity to human FVIII inhibitors, was recently produced by recombinant DNA technology as a B-domainless molecule and tested in a phase 2 trial in haemophilia A patients with inhibitors [67]. This preparation was effective in controlling the bleeds that occurred in the study and was well tolerated [67].
Regular prophylaxis is being considered also in patients with inhibitors, because a randomized study has shown that the prophylactic use of rFVIIa reduces the frequency of bleeding in patients with pre-existing joint damage [68]. The costs involved are exorbitant, but cost-effectiveness may be more favourable in small infants, particularly if less frequent dosing than that used so far to treat bleeding (for instance, once daily or every other day) is efficacious. The hypothesis underlying these results, totally unexpected considering the short-life of rFVIIa, is that the molecule maintains a longer haemostatic activity extravascularly compared with that in plasma. In terms of immune tolerance, the results of the Immune Tolerance Induction (ITI) randomized trial will hopefully establish whether or not the much cheaper regimen promoted in the Netherlands is at least equally effective as the more expensive and demanding classical Bonn regimen. In the context of the ITI study, two sub-protocols are meant to answer two additional important questions: whether or not immune tolerance can also be achieved in patients considered at high risk of not being tolerized, and whether or not in those who initially failed to achieve tolerance using a given FVIII product, salvage can be obtained switching to another product. It is currently difficult to implement immune tolerance in patients with FIX inhibitors, because many of them develop life-threatening anaphylactoid reactions and renal complications. The mechanism of these reactions is still poorly understood, so that multicentre collaboration is warranted to elucidate the causes of this rare but severe complication.
The need of international collaboration in clinical research on haemophilia must be emphasized. Very few of the aforementioned cogent research questions can now be solved by studies done in single, albeit large, haemophilia centres. The adequacy of the sample size, long understood in clinical trials carried out in cardiovascular disease, has not been understood until recently in the field of haemophilia. This failure does not apply only to randomized trials, but also to observational studies. Single-centre study can be at the most only hypothesis-generating. The awareness of this need was certainly one of the triggers for the creation of the Haemophilia and Thrombosis Research Society in the USA and the European Association for Haemophilia and Allied Disorders [69]. These professional organizations should join their research efforts, using as a model the ITI study that is successfully running on both sides of the Atlantic. Another important goal for these physician organizations is to maintain interest and expertise in the field of haemophilia, because we have the impression of a decreasing interest among newer generations of physicians [70]. The problem cannot be easily tackled, except by offering curricula in broader fields of medicine, different from but close to haemophilia, such as venous and arterial thromboembolism, or in chronic diseases that like haemophilia warrant a multidisciplinary approach (examples are rare genetic diseases that are treatable with replacement therapy, such as Fabry and Gaucher disease, thalassaemia and cystic fibrosis).




