Age of regrowth as a factor affecting the nutritive value of hay of kikuyu grass (Pennisetum clandestinum) offered to lambs
Abstract
The changes in chemical composition of hay of kikuyu grass (Pennisetum clandestinum) harvested at 50, 70 and 90 d of regrowth, and its effect on intake, digestibility, fermentation and microbial protein synthesis in the rumen (Experiment 1) and oxygen uptake by portal-drained viscera (PDV) (Experiment 2) were evaluated. The experiments were carried out with Polwarth × Texel crossbreed lambs with a mean live weight (s.e.m.) of 35(3·0) kg housed in metabolic cages. Organic matter (OM), neutral-detergent fibre (NDF) and nitrogen (N) intake, as well as rumen ammonia-N concentration, decreased linearly with age of regrowth (P < 0·05). Acid-detergent fibre (ADF) and indigestible NDF intakes were similar for all treatments. Apparent digestibility of organic matter (OM), NDF and N, as well as true digestibility of OM, microbial protein synthesis in the rumen, N retention, pH of rumen fluid and sugars, amino acids and peptide concentrations in rumen fluid were similar for all treatments. Age of regrowth also did not affect the kinetics of passage of the particulate phase through the digestive tract nor oxygen uptake by PDV. Heat production by PDV represented an average of 0·039 of digestible energy (DE) intake. Increasing the age of regrowth of kikuyu grass from 50 to 90 d did not affect digestibility nor the efficiency of DE use by PDV tissues of lambs but it reduced the nutritive value of the hay due to a lower intake of OM. Intake of hay appeared to be most limited by the ADF and indigestible NDF concentrations of the hay.
Introduction
The efficiency of systems of ruminant production, based on forages as the main source of metabolizable protein and energy, is strongly influenced by the maturity of forages, which is considered a primary factor in reducing their nutritive value (Nelson and Moser, 1994). Normally, as maturity of a forage plant increases, the concentrations of cell-wall constituents increase and the concentrations of total and soluble nitrogen (N) decrease (Merchen and Bourquin, 1994). These changes have frequently been observed at different stages of development of the forage plant, e.g. vegetative vs. reproductive or mature, but there are limited and conflicting data regarding the influence of maturity within the vegetative stage of tropical grasses, particularly when no significant changes in the leaf:stem ratio occur (Wilson, 1994). For example, digestibility of hay of dwarf elephant grass (Pennisetum purpureum Schum. cv. Mott), produced from plants cut between 30 and 60 d of regrowth and offered to cattle, was similar (Kozloski et al., 2003). However, digestibility of hay, when offered to lambs, was negatively affected by age of regrowth when harvests were made between 30 and 90 d of regrowth (Kozloski et al., 2005).
Digestive, absorptive and metabolic functions carried out by gastrointestinal tissues have a considerable energetic cost and are influenced by a variety of factors such as forage quality (Seal and Reynolds, 1993). The use of oxygen by portal-drained viscera (PDV), as a proportion of digestible energy (DE) intake, was higher in sheep offered hay of tropical grasses than hay of temperate grasses (Goetsch and Ferrel, 1995; Patil et al., 1995; Goetsch et al., 1997). Kozloski et al. (2003) showed that the energy expenditure associated with digestion by cattle increased as the age of regrowth of dwarf elephant grass hay increased from 30 to 60 d. However, it is not known if this effect is similar among all tropical grasses. The objective of this study was to evaluate if age of regrowth within the vegetative stage of kikuyu grass (Pennisetum clandestinum) affects intake, digestion and energy use by PDV tissues of lambs offered hay of different ages of regrowth.
Material and methods
Feeds, sheep and experimental design
Kikuyu grass hay was prepared by cutting the plants at a height of 10 cm from the soil surface after 50, 70 and 90 d of regrowth, between December 2004 and February 2005, from a pasture previously established in Lages, Santa Catarina, Brazil [27°49′S, 50°35′W; 937 m above sea level (asl)]. Cut material was field-dried, baled and stored in a barn. Data on rainfall, temperature and light conditions during the periods of growth of the herbage were obtained from local weather stations and are shown in Figure 1. The chemical composition of the hays is shown in Table 1.
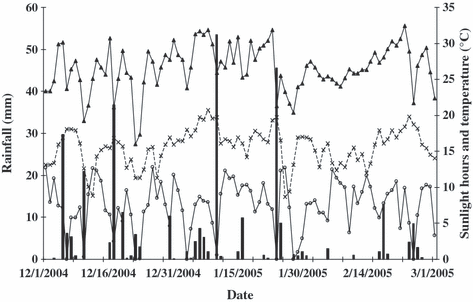
Sunlight hours (––), rainfall ( , mm) and maximum (––) and minimum (–×–) temperatures (°C) from December 2004 to March 2005 at the Experimental Station of Lages, SC, Brazil. Mean values of sunlight, rainfall, maximal and minimal temperatures were 7·4 h, 4·00 mm, 26·6 and 15·6°C respectively.
, mm) and maximum (––) and minimum (–×–) temperatures (°C) from December 2004 to March 2005 at the Experimental Station of Lages, SC, Brazil. Mean values of sunlight, rainfall, maximal and minimal temperatures were 7·4 h, 4·00 mm, 26·6 and 15·6°C respectively.
| Composition | Age of regrowth (d) | ||
|---|---|---|---|
| 50 | 70 | 90 | |
| Dry matter content (g kg−1) | 887 | 881 | 890 |
| Non-nitrogenous compounds (g kg−1 DM) | |||
| Organic matter | 899 | 908 | 915 |
| Neutral-detergent fibre | 654 | 642 | 675 |
| Acid-detergent fibre | 314 | 332 | 357 |
| Acid-detergent lignin | 62 | 49 | 51 |
| Ether extract | 21 | 18 | 15 |
| Non-fibre carbohydrate | 76 | 129 | 126 |
| Nitrogen (N, g g−1 DM) | 27 | 22 | 18 |
| Nitrogenous compounds (g kg−1 N) | |||
| Neutral-detergent insoluble N | 547 | 391 | 486 |
| Acid-detergent insoluble N | 116 | 113 | 138 |
| Non-protein N† | 187 | 255 | 184 |
| Soluble amino-N‡ | 0 | 54 | 0 |
- †Calculated as the difference between total and residual N of the samples treated with water and trichloroacetic solution.
- ‡Calculated as the difference between N soluble in borate-phosphate buffer solution and NPN.
Two experiments were carried out with Polwarth × Texel crossbred lambs housed in metabolic cages. In Experiment 1 six lambs with a mean live weight (LW) of 35 kg (s.e.m. 3·0) were used in a replicated 3 × 3 Latin square design to evaluate intake, digestibility, rumen microbial protein synthesis and N retention. The three treatments were the three ages of regrowth of the hay, which was offered ad libitum. Three of these lambs were fitted with a permanent rumen cannula (siliconized PVC, 35 cm in length, 10 mm in external diameter and 7 mm in internal diameter) to measure fermentation variables in the rumen and the other three were used to estimate the kinetics of passage of the particulate phase through the digestive tract. Each of these sets of variables was studied using a single 3 × 3 Latin square. Experiment 2 was carried out in a 3 × 3 Latin square design with three lambs with a mean LW of 33 (3·0) kg, surgically implanted with permanent indwelling catheters in the portal and mesenteric veins to measure oxygen uptake by PDV. Surgical procedures were carried out under general anaesthesia and followed those described by Katz and Bergman (1969a). For arterial blood collection, one of the carotid arteries was surgically elevated in order to be placed closer to the skin. In all experiments diets consisted of hay only and were offered at 08:00 and 17:00 to provide 100 to 200 g of refusals. A commercial mineral mixture containing per kg: Ca: 100 g, P: 45 g, S: 4·12 g, Na: 205 g, Co: 25 mg, Cu: 450 mg, Fe: 1500 mg, I: 50 mg, Mn: 1000 mg, Se: 9 mg, Zn: 2520 mg and F: 450 mg, was fed at 10 g kg−1 of the dry matter (DM) intake of hay. Animals were treated in accordance with the guidelines of the Animal Care and Ethics Committee of the Universidade Federal de Santa Maria (Santa Maria, RS, Brazil).
Experiment 1
Prior to the start of the experiment, lambs received hay of kikuyu grass ad libitum for approximately 21 d. Thereafter, the experiment was conducted in three 15-d periods, each including a 10-d adaptation period and a 5-d period for data and sample collection. The hay offered and refusals, and faeces output, were recorded daily during the 5-d collection periods. Samples of refusals and faeces were collected daily and those of hay once in each collection period. All samples were dried at 55°C for at least 72 h in a forced-draught oven, ground through a 1-mm screen and stored until analysis. All urine was collected daily during each 5-d collection period in buckets containing 100 mL of 200 mL H2SO4 L−1 H2O. The volume was measured and a sample of 10 mL L−1 of the total volume was stored at −20°C until analysis. Samples of refusals, faeces and urine were pooled on a 5-d basis within each experimental period. Supply of microbial N to the small intestine was calculated from the urinary output of purine derivatives, according to Chen and Gomes (1995).
The estimation of passage of the particulate phase through the digestive tract was performed by the use of chromium-mordanted fibre (CMF) prepared for each hay type, according to the method of Udén et al. (1980). After giving each animal a dose of CMF (approximately 20 g of CMF containing 7 g chromium kg−1 DM), faeces samples were collected directly from the rectum at 0, 12, 24, 30, 34, 38, 42, 48, 60, 72, 96, 120 and 144 h, oven-dried at 55°C for at least 72 h in a forced-draught oven, ground through a 1-mm screen and stored until analysis. Parameters describing the rate of passage of CMF were estimated by the analysis of individual curves of excretion of chromium in the faeces according to the mathematical model of Grovum and Williams (1973). proc nlin of SAS (2002) with the iterative process of Marquardt was used for these calculations.
On day 15 of each period, samples of rumen fluid were collected at 0, 1, 2, 3, 4, 6 and 8 h after the morning meal and filtered through a 50-μm nylon filter. The pH was immediately measured and two 18-mL samples were taken. Two millilitres of 7·2 n H2SO4 was added to one sample and 2 mL of 500 g L−1 trichloroacetic acid (TCA) to the other. Samples were centrifuged (4000 × g for 20 min) at room temperature and the supernatants collected and stored frozen at −20°C. Pellets were discarded. The supernatant of TCA-acidified samples was assumed to contain free amino acids and short-chain peptides (<10 units) while the pellet was assumed to comprise protein and long-chain peptides (Greenberg and Shipe, 1979).
Experiment 2
After a period of approximately 3 weeks before and 1 week after surgery, when the sheep were already housed and receiving experimental hay ad libitum, Experiment 2 was carried out in three periods of 8 d. The first 7 d were used for adaptation to the hay, and the last day of each experimental period was used for sample collection. In the morning of the last day of each experimental period, a temporary catheter [Insyte 20 gauges, 3 cm in length × 1·1 mm internal diameter (i.d.); G.A. Becton Dickenson, Maylar, France], attached to an extension with a three-way valve, was introduced into the carotid artery. Hay was offered and kept available for the sheep for only 1 h. After this period, refusals were removed and weighed. Immediately thereafter, portal blood flow (PBF) was measured by the downstream dilution of p-aminohippurate (PAH) solution (15 g L−1, pH 7·4) initially primed with 10 mL followed by continuous infusion at a rate of 1 mL min−1. Arterial and portal blood samples were simultaneously taken in heparinized syringes hourly from 30 min after PAH infusion. Between sampling intervals, catheters were kept heparinized with a physiological solution containing 20 IU mL−1 of heparin. One sample (10 mL) was used for analysis of packed cell volume (by micro-centrifugation), and for analysis of haemoglobin and PAH concentrations. The other sample (2 mL) was obtained anoxically for immediate measurement of oxygen concentration by an automatic system of blood gas analysis (AVL 990; AVL Co, Graz, Austria).
Portal blood flow was estimated by the portal dilution of PAH (Katz and Bergman, 1969b). The concentration of oxygen in the blood indicated the sum of the oxygen associated with haemoglobin plus the oxygen dissolved in the blood aqueous phase, calculated according to Huntington and Tyrell (1985). Net PDV oxygen flux was calculated by multiplying portal blood flow by the difference between portal and arterial concentrations of oxygen. The amount of aerobic heat produced by the PDV was estimated from the resulting use of oxygen, taking into account a heat equivalent of 20·4 kJ L−1 of oxygen uptake (Huntington and Tyrell, 1985).
Chemical analyses
Dry matter content was determined by drying samples at 105°C for at least 8 h. Ash was determined by combusting the samples at 550°C for 2 h. Total N was assayed by the Kjeldahl method (Method 984.13; AOAC, 1997), modified as described by Kozloski et al. (2003). Analysis of the concentration of neutral-detergent fibre (NDF) included ash but did not include either alpha amylase or sodium sulphite. This analysis was performed according to Mertens (2002) except that samples were weighed in polyester filter bags (Komarek, 1993) and treated with neutral detergent in an autoclave at 110°C for 1 h. Concentrations of acid-detergent fibre (ADF) and sulphuric-acid lignin (ADL) were analysed according to Method 973.18 (AOAC, 1997) except that asbestos was not included. Acid-detergent insoluble N (ADIN) and neutral-detergent insoluble N (NDIN) were analysed according to Licitra et al. (1996). Concentration of ether extract (EE) was determined in a reflux system with ethyl ether, at 180°C for 2 h (Soxtherm, Gerhardt, Germany). Non-fibre carbohydrate concentrations (NFC) were calculated as: 1000 − [(NDF − (NDIN × 6·25)) + (N ×6·25) + EE + ash], according to Van Soest et al. (1991). True digestibility of organic matter (OMTD) was estimated according to Mulligan et al. (2001), considering that only the NDF fraction of the faeces originated from the feed (Van Soest, 1994). Heat of combustion (H) was measured, using a bomb calorimeter (Parr Adiabatic Calorimeter, Moline, IL, USA). Intake of digestible energy (DE) was calculated as: DE (kJ d−1) = (DM intake (g d−1) × hay H (kJ g−1 DM)) − (faecal DM (g d−1) × faecal H (kJ g−1 DM)). Concentration of chromium in samples of faeces was determined by atomic absorption spectrometry. For this analysis, samples were previously combusted at 550°C for 2 h and chromium was solubilized with a sulphuric acid and perchloric acid solution at 200°C (Czarnocki et al., 1961).
In urine samples, allantoin and uric acid concentrations were determined according to Chen and Gomes (1995). Uric acid was determined using a commercial kit (LABTEST, Lagoa Santa MG, Brazil) after xanthine and hypoxanthine were converted to uric acid with xanthine oxidase. Thus, the uric acid values were the sum of uric acid, xanthine and hypoxanthine and the total purine derivatives (PD) were the sum of uric acid and allantoin.
Rumen fluid samples acidified with H2SO4 were analysed for ammonia (Weatherburn, 1967) and sugars (Dubois et al., 1956). The TCA-acidified samples were analysed for amino acids (Palmer and Peters, 1969) before and after hydrolysis with 6 n HCl (2 mL of sample and 2 mL of 6 n HCl), at 120°C for 24 h, in an autoclave. Peptide concentrations were calculated as the difference between concentrations of amino acids before and after hydrolysis. In blood samples, haemoglobin was determined using the iron cyanide method (LABTEST kit) and PAH concentration was analysed as described by Huntington (1982).
Statistical analyses
Experiment 1
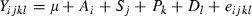
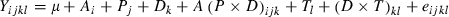
Results
Experiment 1
Intake of components of the hay decreased linearly with increased age of regrowth of kikuyu grass (P < 0·05), except intakes of indigestible NDF and ADF which were similar for all treatments (Table 2). Apparent digestibility of DM, OM and NDF, as well as OM true digestibility, was similar for all treatments. Nitrogen intake and retention decreased linearly (P < 0·05) with increased age of regrowth (Table 3) while apparent and true N digestibility, as well as urinary N excretion, microbial protein synthesis and efficiency of microbial protein synthesis in the rumen were similar for all treatments.
| Age of regrowth (d) | s.e.m. | Level of significance† | |||
|---|---|---|---|---|---|
| 50 | 70 | 90 | |||
| Intake (g d−1) | |||||
| DM | 864 | 786 | 739 | 21 | * |
| OM | 779 | 715 | 675 | 19 | * |
| NDF | 566 | 507 | 499 | 14 | * |
| iNDF | 146 | 139 | 127 | 6 | NS |
| ADF | 268 | 262 | 263 | 9 | NS |
| NFC | 131 | 129 | 123 | 3 | * |
| DOM | 531 | 504 | 474 | 14 | * |
| DE (kJ d−1) | 9631 | 9180 | 8464 | 293 | * |
| DM (g kg−1 LW‡) | 24·9 | 22·6 | 21·3 | 0·6 | * |
| OM (g kg−1 LW0·75) | 54·6 | 50·1 | 47·3 | 1·3 | * |
| Apparent digestibility | |||||
| DM | 0·68 | 0·70 | 0·70 | 1·7 | NS |
| OM | 0·69 | 0·70 | 0·71 | 1·5 | NS |
| NDF | 0·75 | 0·73 | 0·75 | 0·9 | NS |
| OMTD§ | 0·82 | 0·81 | 0·82 | 0·7 | NS |
- *, P < 0·05. NS, not significant.
- †Probability of a linear effect (type I error).
- ‡Live weight.
- §OMTD = (OM intake − NDF faecal output)/OM intake.
| Age of regrowth (d) | s.e.m. | Level of significance† | |||
|---|---|---|---|---|---|
| 50 | 70 | 90 | |||
| Intake of N (g d−1) | 23·7 | 17·5 | 13·9 | 0·6 | * |
| Apparent digestibility of N | 0·644 | 0·661 | 0·638 | 0·018 | NS |
| True digestibility of N | 0·958 | 0·952 | 0·953 | 0·008 | NS |
| Urinary N (g d−1) | 8·0 | 8·2 | 7·0 | 0·7 | NS |
| Retention of N (g d−1)‡ | 7·1 | 3·3 | 1·7 | 0·6 | * |
| Ruminal microbial synthesis of N (g d−1) | 6·0 | 7·1 | 5·4 | 0·7 | NS |
| Efficiency of ruminal microbial protein synthesis of N (g microbial N kg−1 TDOM§) | 7·1 | 9·9 | 8·0 | 1·1 | NS |
- *, P < 0·05. NS, not significant.
- †Probability of a linear effect (type I error).
- ‡N intake − (faecal N + urinary N).
- §True digestible organic matter.
There was no treatment × time interaction for any rumen variable. The pH, sugar, amino acid and peptide concentrations of rumen fluid were similar for all treatments (Table 4) while ammonia-N concentration in rumen fluid decreased linearly with increased age of regrowth (P < 0·05). The rate of passage of the particulate phase through the reticulum–rumen (PRrr) and the caecum–proximal colon (PRcc), and, consequently, the mean retention time of particles in these compartments, were not affected by age of regrowth (Table 5).
| Age of regrowth (d) | s.e.m. | Level of significance† | |||
|---|---|---|---|---|---|
| 50 | 70 | 90 | |||
| pH | 7·1 | 7·1 | 6·9 | 0·05 | NS |
| Concentration (mg L−1) | |||||
| Ammonia-N | 12·6 | 12·1 | 10·9 | 0·30 | * |
| Sugars | 36·7 | 40·3 | 38·2 | 1·00 | NS |
| Amino acids‡ | 19·4 | 19·6 | 18·4 | 0·60 | NS |
| Peptides‡ | 11·4 | 13·2 | 11·0 | 0·80 | NS |
- *, P < 0·05. NS, not significant.
- †Probability of a linear effect (type I error).
- ‡Serine was used as the standard.
| Age of regrowth (d) | s.e.m. | Level of significance† | |||
|---|---|---|---|---|---|
| 50 | 70 | 90 | |||
| PRrr (h−1) | 0·029 | 0·032 | 0·032 | 0·0020 | NS |
| PRcc (h−1) | 0·083 | 0·068 | 0·065 | 0·0019 | NS |
| RTrr (h) | 35·6 | 32·0 | 31·9 | 1·7 | NS |
| RTcc (h) | 15·2 | 15·8 | 20·3 | 3·1 | NS |
| TRT (h) | 62·6 | 62·4 | 65·6 | 0·02 | NS |
- †Probability of a linear effect (type I error).
- NS, not significant.
Experiment 2
Organic matter intake, portal blood flow, oxygen uptake, heat production and heat production as a proportion of DE intake were not affected by age of regrowth (Table 6). Heat production represented on average 0·039 of daily DE intake.
| Age of regrowth (d) | s.e.m. | Level of significance† | |||
|---|---|---|---|---|---|
| 46 | 72 | 90 | |||
| Organic matter intake (g) | |||||
| In morning meal‡ | 217 | 299 | 246 | 16 | NS |
| Daily mean§ | 690 | 733 | 676 | 113 | NS |
| PBF (L h−1) | 55 | 51 | 42 | 5 | NS |
| Oxygen uptake (mL h−1) | 757 | 547 | 653 | 78 | NS |
| Heat production (kJ h−1)¶ | 15·4 | 11·3 | 13·4 | 1·6 | NS |
| Heat production as proportion of digestible energy intake†† | 0·043 | 0·034 | 0·041 | 0·023 | NS |
- †Probability of a linear effect (type I error).
- ‡Intake observed in the meal immediately before taking blood samples.
- §Daily mean intake observed during the 5 d before sampling day.
- ¶20·4 kJ L−1 of oxygen.
- ††Heat production was extrapolated for 24 h and digestible energy intake was the mean value of the 5 d before sampling day.
- NS, not significant.
Discussion
Maturity is considered to be the primary factor affecting the chemical composition and nutritive value of most forages (Nelson and Moser, 1994). As plant maturity increases, cell wall and lignin concentrations of forage increase and total and soluble N concentrations decrease (Merchen and Bourquin, 1994). In the present study, only ADF and total N concentrations varied in this way. The effect of maturity on chemical composition and nutritive value is usually more evident when forages at different developmental stages are compared, i.e. vegetative vs. reproductive or mature, or if there are changes in the leaf:stem ratio (Wilson, 1994, 1997). Moreover, chemical composition of forages is also affected by weather conditions (Van Soest, 1996; Jouven et al., 2006). Chemical differences, however, seem to be modest among green leaves of tropical grasses at different ages of regrowth (Kozloski et al., 2003, 2005).
In ruminants fed forage-based diets it is assumed that intake is regulated by rumen fill which is determined by NDF intake (Van Soest, 1994). Based on this assumption, DM intake of forages with different NDF concentrations should be different but NDF intake should be similar. Results of this study showed that intakes of ADF and the indigestible fraction of NDF, but not of NDF, were similar for all treatments, indicating that either of these fibre fractions could limited forage intake. Compared with others feed fractions, indigestible fibre has a higher retention time in the reticulum–rumen and thus it has a stronger association with rumen fill and forage intake (Allen, 1996).
Maturity and subsequent changes in the chemical composition of forages are usually closely associated with a decrease in digestibility (Nelson and Moser, 1994). Van Soest (1994) observed a negative curvilinear relationship between lignin concentration and NDF digestibility but not with DM digestibility of forages. Theoretically, lignification is the principal factor influencing the extent of degradation of fibre but it has no inhibitory effect on degradation of the soluble components of forages in the rumen. Van Soest (1996) also observed a negative linear relationship between ADF concentration and fibre digestibility of forages grown during the spring, but not in those grown from mid-summer to autumn. However, in the present study, there was no significant correlation between ADF or ADL concentrations of the hays and DM or NDF digestibility. Perhaps the differences in the ADL concentration in hay were not enough to affect the digestibility of fibre.
The OM digestibility also depends on rumen microbial growth and activity which are dependent, among others factors, on carbohydrate and N availability in the rumen. In the present study there was no significant correlation between OM digestibility and N concentration of hay. Satter and Slyter (1974) observed that growth of cellulolitic bacteria was limited at ammonia-N concentrations of rumen fluid lower than 5·0 mg NH3-N 100 mL−1. In this study ammonia-N concentrations of rumen fluid were higher than this value and sugar concentrations and rumen microbial protein synthesis were not affected by treatments. These results indicate that substrate availability for rumen bacteria did not limit digestion of hays of kikuyu grass by lambs.
The non-degraded material in the rumen generally consists of highly lignified fibres, requiring rumination for its fragmentation (Wilson, 1997). It is known, however, that the amount of fibrous material present in the rumen is one of the main factors stimulating gastrointestinal motility (Van Soest, 1994). Concentrations of ADL and NDF in dwarf elephant grass increased as age of regrowth increased from 30 to 60 d and, at a restricted level of intake, it resulted in increased PRrr by cattle (Kozloski et al., 2003). In this study neither ADL or NDF concentrations decreased nor were the kinetics of the particulate phase in the digestive tract of lambs affected by increased age of regrowth of kikuyu grass.
The PRrr is usually directly related to intake and inversely related to digestibility of forages (Okine and Mathison, 1991; Mertens, 1993). In the present study increasing the age of regrowth of forages did not affect OM digestibility or PRrr. Intake of DM of the hays still decreased linearly, which was not expected. The explanation for the results is not clear. Intake of forages depends on rumen fill, which is inversely related to the rate of passage of undigested residues from the rumen and degradation rates of DM in the rumen. It is possible that the degradation rate of digestible fractions decreased with increased age of regrowth. In vitro gas production rate of kikuyu samples decreased from 0·0282 to 0·0236 h−1 as age of regrowth increased from 50 to 90 d (data not shown) supporting a decreased degradation rate.
Portal-drained viscera metabolism accounts for a high proportion of heat produced by ruminants and it is affected, by among others factors, the level of intake and diet quality. The use of oxygen by the PDV is proportional to the total use of oxygen by the organism and tends to be higher in ruminants offered forage-based diets than in those offered concentrates (Seal and Reynolds, 1993; Huntington, 1999). In addition, the PDV use of oxygen, as a proportion of DE intake, was higher in sheep offered a bermudagrass (Cynodon dactylon) hay, cut at the vegetative stage and with higher NDF and ADL concentrations, than in sheep offered a hay made from a mixture of ryegrass (Lolium multiflorum), cut at the end of the vegetative stage, and wheat (Triticum aestivum), cut at the initial stage of milk grain, where NDF and ADL concentrations were lower (Goetsch and Ferrell, 1995; Patil et al., 1995; Goetsch et al., 1997). Differences in the work of digestion, retention and propulsion of the digesta through the gastrointestinal tract are among the factors affecting oxygen uptake by PDV (Huntington, 1999). In the present experiment, digestibility, kinetics of the passage of the particulate phase and oxygen uptake by PDV were similar for all treatments. It may be that the change in chemical composition of hay with increased age of regrowth of kikuyu grass was not enough to affect the efficiency of energy use by visceral tissues.
Conclusions
Increasing the age of regrowth of kikuyu grass from 50 to 90 d did not affect the digestibility or the efficiency of digestible energy use by visceral tissues of lambs offered hay made from these regrowths, but it reduced the nutritive value of kikuyu grass hay due to a reduction in OM intake. Acid-detergent fibre and indigestible NDF concentrations were considered to be the major components of the chemical composition of the hays responsible for limiting intake.
Acknowledgments
Financial support was provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq no. 471782/03-3).




