Four years of experimental climate change modifies the microbial drivers of N2O fluxes in an upland grassland ecosystem
Abstract
Emissions of the trace gas nitrous oxide (N2O) play an important role for the greenhouse effect and stratospheric ozone depletion, but the impacts of climate change on N2O fluxes and the underlying microbial drivers remain unclear. The aim of this study was to determine the effects of sustained climate change on field N2O fluxes and associated microbial enzymatic activities, microbial population abundance and community diversity in an extensively managed, upland grassland. We recorded N2O fluxes, nitrification and denitrification, microbial population size involved in these processes and community structure of nitrite reducers (nirK) in a grassland exposed for 4 years to elevated atmospheric CO2 (+200 ppm), elevated temperature (+3.5 °C) and reduction of summer precipitations (−20%) as part of a long-term, multifactor climate change experiment. Our results showed that both warming and simultaneous application of warming, summer drought and elevated CO2 had a positive effect on N2O fluxes, nitrification, N2O release by denitrification and the population size of N2O reducers and NH4 oxidizers. In situ N2O fluxes showed a stronger correlation with microbial population size under warmed conditions compared with the control site. Specific lineages of nirK denitrifier communities responded significantly to temperature. In addition, nirK community composition showed significant changes in response to drought. Path analysis explained more than 85% of in situ N2O fluxes variance by soil temperature, denitrification activity and specific denitrifying lineages. Overall, our study underlines that climate-induced changes in grassland N2O emissions reflect climate-induced changes in microbial community structure, which in turn modify microbial processes.
Introduction
In recent decades, changes in land use and human activities have had significant impacts on gaseous nitrogen (N) losses and the global cycle of N (Galloway et al., 2004), contributing to regional and global changes in the atmosphere (IPCC, 2007). Emissions of nitrous oxide (N2O) are of particular interest because this trace gas has a strong global warming potential (ca. 310 times greater than that of carbon dioxide) and is the single most important ozone-depleting emission (Ravishankara et al., 2009). The magnitude of N2O emissions depends on both microbial activities (nitrifiers and/or denitrifiers, Bremner, 1997; Wrage et al., 2004) and abiotic factors, including soil temperature, oxygenation, mineral nitrogen, pH, carbon availability and water content (Simek et al., 2002; Smith et al., 2003; Jones et al., 2005). Consequently, understanding the interplay between microbial and environmental variables is critical for the estimation of potential N2O fluxes from soils under climate change.
Despite a large number of studies documenting gaseous N2O emissions from grassland ecosystems, few have focused on impacts of climate change drivers on N2O fluxes and associated microbial processes (Clayton et al., 1997; Flechard et al., 2007; but see Avrahami & Bohannan, 2009). In theory, warming is expected to have positive effects on nitrification and denitrification rates (Gödde & Conrad, 1999), with cascading effects on N2O emissions. However, warming responses of both nitrification and denitrification appear to be highly variable across sites (Emmett et al., 2004; Horz et al., 2004; Malchair et al., 2010; Szukics et al., 2010), which may partly reflect variable soil water content status during experiments (Barnard & Leadley, 2005). Impacts of reduced soil moisture status on microbial processes are well established (Barnard et al., 2004; Barnard & Leadley, 2005; Bateman & Baggs, 2005), typically promoting denitrification at the expense of nitrification via changes in soil aeration and O2 content (Smith et al., 2003). In addition, elevated CO2 may indirectly alter microbial processes by both increasing soil moisture (Smith & Tiedje, 1979) and carbon substrate availability (Luo & Mooney, 1999). Previous study suggests that elevated CO2 may have greater positive effects on denitrification than nitrification (Baggs et al., 2003; Barnard et al., 2004), but considerable variation is observed across studies.
Although information on N2O emissions and microbial activities subjected to individual climate change drivers is becoming increasingly available (Bateman & Baggs, 2005; Kammann et al., 2008; Malchair et al., 2010), data on N2O flux responses to multiple and simultaneous environmental changes remain scarce. In a recent study, examining the impact of co-occurring climatic changes on N2O fluxes in an upland grassland, Cantarel et al. (2011) found that N2O fluxes responded equally strongly to both warming alone and the combination of summer drought or elevated CO2 and warmed conditions. Results from laboratory incubations suggest that interactions between soil moisture and temperature can generate complex patterns of N2O emissions under controlled conditions (Avrahami & Bohannan, 2009), but the importance of multiple climate changes for field N2O emissions remains unclear.
In addition to direct climate-induced changes in microbial activities, climate change drivers can impact N transformations and N2O emissions via indirect effects on the abundance of different microbial populations, and microbial community structure. Variation in soil N2O emissions may reflect differences in terms of abundances and/or composition of AOB (ammonium oxidizing archea seem to be not involved in N2O emission; Di et al., 2010) and denitrifying microorganisms (Avrahami & Bohannan, 2009; Philippot et al., 2010; Brown et al., 2011). To date, only AOB community structure has been studied for grasslands subjected to complex, multiple climate change treatments (Horz et al., 2004). Horz et al. found that abundance of AOB decreased in response to combined elevated CO2 and increased precipitation, but these effects appeared to be buffered under elevated temperature conditions. To our knowledge, no study has yet focused on changes in denitrifiers community structure under climate change. Hence, the potential impact of multiple climatic variables on the microbial community structure, and the respective contributions of AOB and denitrifying microorganisms to N2O fluxes on terrestrial ecosystems remain poorly understood.
In the present study, we investigated the relationship between field N2O fluxes and soil microbial parameters under three key climate change drivers at the Clermont Climate Change Experiment facility (Bloor et al., 2010). This long-term grassland climate change facility manipulates air temperature (+3.5 °C), atmospheric CO2 (+200 ppm) and summer drought (−20% summer rainfall) in an additive experimental design. The aims of our study were to determine impacts of sustained single and combined climate change treatments on N2O fluxes, nitrification, denitrification, abundance of microorganisms (AOB and nitrite reducers), denitrifiers community structure and to estimate the existing relationships between N2O fluxes, abiotic parameters and microbial parameters. Specifically, we asked: (1) How do nitrification, denitrification, abundances and composition of microbial nitrifiers/denitrifiers respond to multiple and simultaneous climate changes? (2) Are variations in field N2O fluxes mirrored by changes in microbial activities, abundance or community structure of specific microbial functional groups?
Materials and methods
Experimental design and climate treatments
The studied ecosystem was an upland permanent grassland in the French Massif Central region (45°43′N, 03°01′E, 850 m a.s.l.), characterized by a Cambisol soil (59.5% sand, 19.7% silt, 20.8% clay, pH 6.2), and a grass-dominated plant community (Festuca arundinaceae, Elytrigia repens, Poa pratensis; described in Bloor et al., 2010). The study area has a mean annual temperature of 8.7 °C and a mean annual rainfall of 780 mm.
The Clermont Climate Change Experiment was established in 2005, manipulating air temperature, summer rainfall and atmospheric CO2 in line with IPCC projections for the study area in 2080 (ACACIA A2 scenario, IPCC, 2007; see Bloor et al., 2010 for full details). In brief, the experimental design consisted of 80 grassland monoliths (0.5 × 0.5 × 0.4 m in size), excavated from the study grassland site and allocated at random to one of four climate treatments; C (control), T (+3.5 °C), TD (+3.5 °C, 20% reduction in summer rainfall) and TDCO2 (+3.5 °C, 20% reduction in summer rainfall, CO2 levels of 600 ppm). Each experimental treatment comprised of five experimental units (or repetitions), formed by grouping four monoliths together in specially prepared cavities in the ground. Elevated temperatures were achieved by transporting monoliths to a nearby lower-altitude site (Clermont-Ferrand, 350 m a.s.l.). Summer drought was established by the use of rain screens and reduced watering regimes during June, July and August. Enrichment of atmospheric CO2 was obtained by Mini–FACE (Free Air Carbon dioxide Enrichment) technology; the target CO2 concentration was only operational during daylight hours.
Meteorological measurements were achieved using a Campbell Scientific automatic weather station and logged to a CRX-10 data logger (Campbell scientific Inc., Utah, USA) at 30 min intervals for both the upland and lowland sites. Volumetric soil moisture (0–20 cm) was recorded hourly using ECH2O-20 probes (Dielectric Aquameter; Decagon Devices, Inc., Pullman, WA, USA). To stimulate the management prior to monolith extraction (i.e., low-intensity sheep grazing and no fertilization), vegetation in all experimental units was cut to a height of 5 cm at 6 month intervals (April and October). Monoliths were not fertilized throughout the study, in keeping with extensive management practices.
N2O flux measurements and soil sampling
N2O fluxes were determined on four dates between May and November 2009, using medium-size, closed and non-vented manual chambers on one monolith per experimental unit (following Cantarel et al., 2011). During each N2O measurement campaign, chambers were fixed onto a permanent base for each target monolith and gas samples were taken at 520 min intervals using a quick release pneumatic connector (TST Tansam Inc, Kocaeli, Turkey) and a PTFE-Teflon tube connected to an INNOVA 1412 photoacoustic multi-gas analyzer (INNOVA AIR Tech Instruments, Ballerup, Denmark). The INNOVA gas analyzer was encased in an air-conditioned box maintained at 20–25 °C to avoid confounding effects of temperature on analyzer measurements. N2O fluxes were calculated by linear regression of N2O in the chamber against time; flux data were rejected if the statistic P-value was above 0.05 and r² < 0.95 (Cantarel et al., 2011). Soil temperature in the topsoil layer (2–5 cm) was recorded by thermocouples (TC S.A., Dardilly, France) during N2O measurement campaigns. Immediately following in situ N2O measurements, three soil cores (diameter 1.5 cm) were taken from the top layer (0–10 cm) of each target monolith, pooled together and sieved at 4 mm. Soils were stored for less than 5 days at 4 °C before carrying out assays for nitrification and denitrification enzyme activity (NEA, DEA respectively). A subsample of ca. 2 g fresh soil was frozen at −18 °C for subsequent molecular analyses.
Denitrifying and nitrifying enzyme activities
Denitrification enzyme activity (DEA) was measured in fresh soils from each monolith following the protocol described in Patra et al. (2006). Two sub-samples (10 g equivalent dry soil) from each soil sample were placed into 150 ml plasma flasks, and 7 ml of solution containing KNO3 (50 μg NO3− N g−1 dry soil), glucose (0.5 mg C g−1 dry soil) and glutamic acid (0.5 mg C g−1 dry soil) were added. Additional distilled water was provided to achieve 100% water holding capacity and optimal conditions for denitrification. The atmosphere was replaced by helium to provide anaerobic conditions and for one flask of each pair, 10% C2H2 was added to inhibit N2O reductase activity. During incubation at 28 °C, gas samples were taken at 2, 3h30, 5, 6h30 and immediately analyzed for N2O quantitation using a gas chromatograph (R3000μGC; SRA instrument, Marcy l'Etoile, France). For the first series of samples without C2H2, we measured N2O accumulation, i.e., potential N2O emission rates of our soil (N2ODEA). The second series of samples with C2H2 allowed the determination of maximal N2O production (N2OTOT). We estimated potential fluxes of N2 (N2DEA) by subtracting N2ODEA from N2OTOT.
Nitrification enzyme activity (NEA) was determined following the protocol described in Dassonville et al. (2011). Briefly, subsamples of fresh soil (3 g equivalent dry soil) were incubated with 6 ml of a solution of N-NH4 (50 μg N-(NH4)2SO4 g−1 dry soil). Distilled water was adjusted in each sample to achieve 24 ml of total liquid volume in flasks. The flasks were sealed with Parafilm® (Pechiney Plastic Packaging, Menasha, WI, USA) and incubated at 28 °C with constant agitation (180 rpm). During incubation, 1.5 ml of soil slurry was sampled at 1, 2h30, 4, 5h30 and 7h, filtered (0.2 μm pore size). Samples were stored at −20 °C until analysis of NO2−/NO3− concentrations on an ionic chromatograph (DX120 Dionex, Salt Lake City, USA). A linear rate of NO2− + NO3− production with time was always observed, and the rates of NEA were determined from the slope of this linear regression. The intercept was used to estimate pools of soil nitrate (NO3−).
Soil DNA extraction and quantitation of AOB, nirK and nosZ abundances
DNA was extracted for each frozen soil subsample (0.5 g equivalent dry soil) using the 96 Well Soil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA, USA) and manufacturer protocols. The quantity of the DNA extraction was checked using the Quant-iT™ PicoGreen® method (Quant-iT™ PicoGreen® dsDNA Assay kit; Molecular Probes Inc., Eugene, OR, USA).
All gene quantitations were obtained by qPCR, using a Lightcycler 480 (Roche Diagnostics, Meylan, France). The abundance of β-proteobacterial AOB, that represented known AOB in soil, and which are potentially implied in N2O emissions (Wrage et al., 2004) was measured by qPCR targeting 16S rRNA gene sequences specific to this group (Hermansson & Lindgren, 2001). The final qPCR reaction volume was 20 μl, with 0.5 μm of a 2 : 1 ratio of primer CTO189fA/B (GGAGRAAAGCAGGGGATCG) and CTO189fC (GGAGGAAAGTAGGGGATGC; Kowalchuk et al., 1997), 0.5 μm of RT1r primer (CGTCCTCTCAGACCARCTACTG; Hermansson & Lindgren, 2001), 0.5 μm of TPM1 probe (CAACTAGCTAATCAGRCATCRGCCGCTC), 0.4 mg ml−1 bovine serum albumin (BSA), 10 ng of sample DNA or standard DNA with known number of copies. The samples were run as follow: 10 min at 95 °C; 45 cycles at 95 °C for 10 s, 58 °C for 20 s, and 1 s at 72 °C; and 30 s at 40 °C.
The abundance of nirK genes was determined using SYBR Green as the detection system in a reaction mixture of 20 μl, with 10 μl of SYBR Green PCR master mix, including HotStar Taq™ DNA polymerase, QuantiTec SYBR Green PCR buffer, dNTP mix with dUTP SYBR Green I, ROX and 5 mm MgCl2 (QuantiTect™ SYBR ® Green PCR Kit; Qiagen, Courtaboeuf, France), 1 μm of nirK876 primer (ATYGGCGGVAYGGCGA), 1 μm of nirK1040 primer (GCCTCGATCAGRTTRTGGTT), according to Henry et al., 2006, 0.4 μg of T4 gene protein 32 (QBiogene, France), 5 ng of soil DNA and Rnase-free water to complete the 20 μl volume. The conditions for nirK qPCR were 15 min at 95 °C for denaturation; 45 cycles at 95 °C for 15 s, 63 °C for 30 s and 72 °C for 30 s for amplification; 1 s at 95 °C and 20 s at 68 °C for acquisition step and 10 s at 40 °C to finish analysis.
For nosZ gene quantitation, the primers nosZ2F (5′-CGCRACGGCAASAAGGTSMSSGT-3′) and nosZ2R (5′-CAKRTGCAKSGCRTGGCAGAA-3′), according to Henry et al. (2006) were used. The final volume 25 μl PCR mix contained: QuantitTect SybrGreen PCR Master Mix 1X (Qiagen), 0.1 μg of T4 gene protein 32 (QBiogene), 1 μm of each primer, and 5 ng of soil DNA extract or 5 μl of ten-fold standard serial dilution ranging from 107 to 102 nosZ copies of genomic DNA from Pseudomonas aeruginosa PA14. Thermal cycling was carried out by an initial enzyme activation step at 95 °C for 10 min followed by 55 cycles of denaturation at 95 °C for 15 s, annealing at 68 °C for 30 s with a touchdown of −1 °C by cycle until reach 63 °C and elongation at 72 °C for 30 s.
Characterization of nirK community by cloning-sequencing
Characterization of nirK community was achieved on DNA extracted from samples taken at the beginning and at the end of flux measurements, i.e., May and November 2009 with/without field N2O fluxes respectively. We used the conditions described by Wertz et al. (2006) to amplify partial nirK gene sequences prior to cloning procedures. Briefly, PCR was performed using the primers Copper 583F (5′-TCATGGTGCTGCCGCGKGACGG-3′) and Copper 909R (5′-GAACTTGCCGGTPGCCCAGAC-3′) according to Liu et al. (2003) and 30 ng of extracted DNA. The final reagent concentrations for PCR were 1 μm primers, 200 μm of each dNTP, 1.75 U of Taq (Qbiogene, Carlsbad, USA), and 0.5 μg of T4 protein in 50 μl of 10 mm Tris-HCl, 50 mm KCl, 0.1% Triton X-100, 1.5 mm MgCl2, pH 9. Thermal cycling was carried out by an initial step at 94 °C for 5 min followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 72 °C for 1 min with a touchdown of −1 °C by cycle until reach 67 °C and elongation at 72 °C for 1 min and a final elongation cycle at 72 °C for 7 min. PCR products were purified using the NucleoSpin® Extract II kit (Macherey-Nagel, Düren, Germany) and were cloned using the pGEM T-Easy vector system (Promega Ltd., Southampton, UK) and DH10B electrocompetent Escherichia coli cells (Fisher Scientific–Invitrogen, Illkirch, France). For each treatment, three clone libraries were constructed from three (out of the four) randomly selected replicates. From each clone library, at least 28 clones were randomly picked and their vector sent for purification and sequencing (LGC Genomics, Berlin, Germany). Nucleotide sequences have been deposited in GenBank under the following accession numbers: JQ770451–JQ771049.
DNA sequence process, phylogentetic assessment and comparison of microbial communities
Vector and primer sequences were trimmed from the raw sequence dataset. Chimera formations were detected using ChimeraCheck (Cole et al., 2005) and sequences shorter than 358 bp were removed from the original dataset. From a total of 631 remaining ‘clean’ sequences random normalization of sample sizes was carried out using the Daisy Chopper tool (Gilbert et al., 2009), based on the smallest sample i.e., 25 sequences. This subsampled dataset was then aligned together, included the outgroup sequence of the nirS gene of Dechloromonas aromatica using MUSCLE (Edgar, 2004) and the resulting alignment was manually checked using Seaview (Galtier et al., 1996). From the resulting optimized alignment, a maximum likelihood phylogenetic tree was inferred using RAxML (Stamatakis, 2006) under a GTR + Gamma + Invariable model of sequence evolution (Appendix 1). The resulting tree was then imported into the UNIFRAC on-line tool (Lozupone et al., 2006) for comparison of community composition and detection of lineages specific to the various treatments. The RAMI tool was used to measure accurate branch lengths and distances between nodes containing (Pommier et al., 2009). Proportional abundances of selected nodes were depicted using the KRONA tool (figure 2, Ondov et al., 2011).
Statistical analyses
Effects of climate treatment on N2O, NEA, NO3−, N2ODEA, N2DEA, and abundance of gene copies (16S rRNA of AOB, nirK, nosZ) were analyzed using mixed model repeated measures analysis of variance (anova) with both treatment and date as fixed factors (Zar 1998). Effects of individual climate change drivers (temperature, drought, and CO2) were analyzed using orthogonal contrasts (Gilligan, 1986). Effects of warming were determined by comparing the C and T treatment; effects of summer drought under elevated temperature by comparing T and TD; effects of elevated (CO2) under elevated temperature and drought by comparing TD and TDCO2; effects of simultaneous application of warming, summer drought, and CO2 enrichment (2080 climate scenario) were investigated by the C vs. TDCO2 comparison. All data used were checked for normality and non-normal data were log transformed to conform with assumptions of normality and homogeneity of variances. Relationships between field N2O fluxes, potential activities and gene abundances were examined using Spearman correlation coefficients. All analyses were carried out using Statgraphics Plus 4.1® (Statistical Graphics Corp., Rockville, Maryland, USA).
We performed a restricted maximum likelihood method (REML) with the software jmp8® (SAS Institute Inc., SAS Campus Drive, NC, USA) considering monoliths as a random factor to determine, which variables (among soil temperature, WFPS, NO3- and NH4+ contents, abundances, activities and composition of denitrifiers) were significantly related to in situ N2O fluxes in May and November (i.e., dates with diversity analyses). To compare field measures of N2O fluxes to denitrification activities measured in the laboratory, which differed in experimental temperature, we linearly transformed the denitrification values (N2ODEA corr) as suggested by their known linear correlation between 4 and 25 °C (Braker et al., 2010).
Structural equation modeling (SEM) was performed using Amos18® (Amos Development Corporation, Crawfordville, FL, USA) with the data from May and November to explore the causal links between denitrification, microbial community structure, abiotic factors and the in situ N2O fluxes, using the following parameters: soil temperature, WFPS, pool of NO3-, N2ODEA, N2ODEA corr, percentage of sequences in nirK lineages A and B (Appendix 2). In a SEM, a χ² test is used to determine whether the covariance structures implied by the model adequately fit the actual covariance structures of the data. A non-significant chi-squared test (P > 0.05) indicates adequate model fit. The coefficients of each path as the calculated standardized coefficients were determined using the analysis of correlation matrices. Paths in this model were considered significant with a P-value <0.1. These coefficients indicate by how many standard deviations the effect variable would change if the causal variable was changed by one standard deviation.
Results
Characteristics of climate treatments
During the study period (May–November 2009), the difference in mean monthly temperature between control and elevated temperature treatments was 3.4 ± 0.03 °C (Appendix 3). In summer (June, July, and August), the drought treatments (TD, TDCO2) were subjected to a 21% reduction in rainfall compared with the no-drought treatments (C, T). Mean daily CO2 differences between the TDCO2 treatment and the ambient CO2 treatments (C, T, and TD) were 193.3 ± 13.1 ppm (data not shown). Meteorological variables (i.e., soil moisture and soil temperature) recorded on days of N2O measurement indicated higher soil temperature in the warmed treatments (T, TD and TDCO2) compared with the control (C). No significant differences in soil moisture between the C, T and TDCO2 treatments were found for the four sampling dates (T-test, Table 1). However, the TD treatment showed lower soil moisture values than the T treatment in July and September. We found no significant difference between soil moisture and air temperature measured on the days of sampling and the averages recorded on the five previous days (all dates and treatments). This consistence between measurements allows considering measurements of each sampling date as representative of the preceding week.
| 29th May | 27th July | 23rd September | 28th November | |
|---|---|---|---|---|
| WFPS (%) | ||||
| C | 31.9 ± 0.0 | 35.9 ± 0.0 | 50.1 ± 0.0 | 52.9 ± 0.3 |
| T | 29.3 ± 1.1 | 32.7 ± 1.6 | 45.2 ± 2.9 | 52.6 ± 2.3 |
| TD | 32.4 ± 0.8 | 29.4 ± 0.3 | 30.6 ± 0.3 | 48.8 ± 0.2 |
| TDCO2 | 29.5 ± 0.8 | 37.3 ± 1.8 | 47.1 ± 1.7 | 51.8 ± 1.5 |
| Soil temperature (°C) | ||||
| C | 17.3 ± 0.4 | 17.3 ± 0.4 | 14.3 ± 0.2 | 4.2 ± 0.3 |
| T | 22.7 ± 1.5 | 23.8 ± 0.6 | 17.1 ± 0.5 | 6.3 ± 0.6 |
| TD | 23.5 ± 1.2 | 24.9 ± 1.1 | 17.3 ± 0.7 | 5.7 ± 0.7 |
| TDCO2 | 22.6 ± 1.2 | 22.9 ± 0.9 | 17.4 ± 0.8 | 6.7 ± 0.6 |
Effects of climate change drivers on in situ N2O fluxes
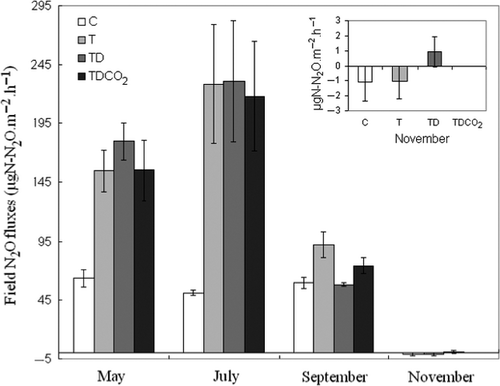
During the four measurement dates, N2O fluxes ranged from −5 to 369 μg N2O-N.m−2.hr−1 across treatments. N2O fluxes showed significant climate treatment effects for measurement dates during the growing season, but no response to climate treatments in November (significant treatment × date interaction; F1,9 = 2.16, P < 0.05; Fig. 1). This significant interaction was driven by very low N2O fluxes in November across all climate treatments. With the exception of the November sampling date, warming had a positive effect on N2O emissions (C vs. T comparison; F1,16 = 23.1, P < 0.001; F1,16 = 6.6, P < 0.05 and F1,16 = 14.6, P < 0.01 respectively for May, July and September). This pattern of response was also found for the combined climate change drivers (C vs. TDCO2) in May and July. Unlike warming and combined climate change, summer drought (T vs. TD) and elevated CO2 (TD vs. TDCO2) had little impact on N2O fluxes. However, drought was associated with a significant negative effect on N2O fluxes in September (F1,16 = 15.5, P < 0.01; Fig. 1).
Changes in nitrifying and denitrifying enzyme activities
Over the course of the study, climate treatments had a significant effect on NEA, N2ODEA and soil nitrate pools (Table 2). Warming and combined climate treatments had a positive impact on nitrification (NEA; F1,38 = 5.7, P < 0.05 and F1,16 = 6.9, P < 0.05 respectively) and on NO3−, which is the product of the nitrification (F1,38 = 10.67, P < 0.01 and F1,38 = 10.85, P < 0.01 for C vs. T and C vs. TDCO2 respectively). In addition, combined warming, drought and elevated CO2 had a positive effect on N2ODEA across all measurement dates (C vs. TDCO2, F1,38 = 5.4, P < 0.05). In contrast, the potential fluxes of N2 (N2DEA) and denitrification product ratio (N2ODEA/[N2ODEA + N2DEA]) showed no response to climate treatments. Neither summer drought under warmed conditions (T vs. TD) nor elevated CO2 in combination with warming and summer drought (TD vs. TDCO2) had any significant effect on nitrifying and denitrifying enzyme activities. Across treatments, NO3−, N2ODEA, N2DEA, and denitrification product ratio showed a significant effect of measurement date (Table 2). NO3− and N2DEA showed a continuous increase over time (r² = 37.7, P < 0.001 and r² = 23.8, P < 0.001 respectively), whereas N2ODEA and denitrification product ratio showed a progressive decrease overtime (r² = 18.3, P < 0.001 and r² = 49.6, P < 0.001 respectively).
| Climate treatments | Repeated measures anova | |||||||
|---|---|---|---|---|---|---|---|---|
| C | T | TD | TDCO2 | P | Treatments | Dates | Treatments × dates | |
| (a) NEA (μg N(NO2 + NO3) g−1h−1) | ||||||||
| May | 0.51 ± 0.09 | 0.82 ± 0.09 | 0.87 ± 0.16 | 0.89 ± 0.16 | * | 0.003 | 0.238 | 0.807 |
| July | 0.53 ± 0.06 | 0.70 ± 0.16 | 0.89 ± 0.14 | 0.91 ± 0.08 | ||||
| September | 0.72 ± 0.10 | 0.94 ± 0.13 | 0.82 ± 0.09 | 0.92 ± 0.12 | ||||
| November | 0.81 ± 0.10 | 0.91 ± 0.13 | 0.82 ± 0.08 | 1.06 ± 0.18 | ||||
| (b) NO3− (μg N-NO3−.g−1) | ||||||||
| May | 3.1 ± 0.5 | 2.8 ± 0.6 | 2.5 ± 0.6 | 3.2 ± 0.6 | *** | 0.002 | <0.001 | 0.080 |
| July | 4.3 ± 0.4 | 5.5 ± 0.9 | 6.2 ± 1.9 | 7.2 ± 0.8 | ||||
| September | 6.8 ± 1.1 | 14.7 ± 3.9 | 15.5 ± 3.2 | 10.3 ± 2.3 | ||||
| November | 5.2 ± 0.8 | 13.3 ± 2.8 | 14.3 ± 3.6 | 14.9 ± 2.9 | ||||
| (c) N2ODEA (μg N-N2O g−1h−1) | ||||||||
| May | 1.17 ± 0.04 | 1.39 ± 0.13 | 1.51 ± 0.22 | 1.39 ± 0.01 | *** | 0.022 | <0.001 | 0.712 |
| July | 1.05 ± 0.07 | 1.15 ± 0.09 | 1.15 ± 0.01 | 1.30 ± 0.09 | ||||
| September | 1.19 ± 0.06 | 1.15 ± 0.06 | 1.21 ± 0.13 | 1.28 ± 0.10 | ||||
| November | 0.98 ± 0.04 | 1.03 ± 0.07 | 1.03 ± 0.06 | 1.19 ± 0.1 | ||||
| (d) N2DEA (μgN-N2 g−1h−1) | ||||||||
| May | 0.07 ± 0.04 | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.17 ± 0.09 | ** | 0.555 | <0.001 | 0.557 |
| July | 0.08 ± 0.04 | 0.19 ± 0.05 | 0.20 ± 0.06 | 0.09 ± 0.03 | ||||
| September | 0.08 ± 0.03 | 0.27 ± 0.11 | 0.25 ± 0.15 | 0.19 ± 0.04 | ||||
| November | 0.29 ± 0.04 | 0.29 ± 0.05 | 0.38 ± 0.09 | 0.37 ± 0.03 | ||||
| (e) Denitrification product ratio (% N2ODEA/[N2ODEA + N2DEA]) | ||||||||
| May | 93.1 ± 2.2 | 97.7 ± 2.2 | 96.8 ± 2.1 | 95.7 ± 1.9 | *** | 0.129 | <0.001 | 0.197 |
| July | 92.0 ± 3.6 | 85.8 ± 3.8 | 85.5 ± 3.6 | 93.1 ± 2.4 | ||||
| September | 93.3 ± 2.2 | 81.6 ± 10 | 80.1 ± 5.8 | 86.7 ± 3.6 | ||||
| November | 77.2 ± 2.6 | 77.8 ± 4.2 | 73.8 ± 8.9 | 75.7 ± 2.1 | ||||
- *,**,*** indicates significant differences at P < 0.05; 0.01 and 0.001, respectively and italic indicates marginal differences (0.05 < P < 0.1).
Changes in the abundances of AOB, nirK and nosZ
Responses of gene abundances to climate change treatments varied depending on the gene considered (Table 3). Both warming and combined climate change had a positive effect on abundance of N2O reducers (nosZ; F1,16 = 6.1, P < 0.05 and F1,16 = 6.4, P < 0.01 respectively) at all measurement dates (no significant Treatment × Date interaction). In addition, nosZ gene abundance was found to increase over time (Table 3, r² = 12.5, P < 0.05). Climate treatment also had significant effects on the abundance of AOB sequences, but treatment effects varied overtime (significant treatment × date interaction, Table 3). In general, numbers of AOB copies were significantly higher in November compared with those in May and July. Warming had a positive effect on the abundance of AOB in May and November (F1,16 = 6.3, P < 0.05 and F1,16 = 9.1, P < 0.01 respectively), whereas combined climate and elevated CO2 alone was only associated with an increase in AOB in November (F1,16 = 8.5, P < 0.05 for combined climate and F1,16 = 16.5, P < 0.001 for elevated CO2). Unlike nos Z and AOB, abundance of nirK genes showed no significant response to climate treatments over the four measurement dates.
| Climate treatments | Repeated measures anova | |||||||
|---|---|---|---|---|---|---|---|---|
| C | T | TD | TDCO2 | P | Treatments | Dates | Treatments × dates | |
| (a) Mean copy numbers of ammonia-oxidizing bacteria (106 copy per g of dry soil) | ||||||||
| May | 3.97 ± 0.15 | 5.58 ± 0.67 | 4.34 ± 0.80 | 4.13 ± 0.15 | *** | 0.083 | <0.001 | 0.021 |
| July | 4.84 ± 0.70 | 4.95 ± 0.46 | 4.77 ± 0.69 | 5.32 ± 0.76 | ||||
| September | 5.14 ± 0.39 | 4.73 ± 0.50 | 4.40 ± 0.66 | 5.01 ± 0.32 | ||||
| November | 5.12 ± 0.43 | 6.40 ± 0.59 | 7.23 ± 0.54 | 8.36 ± 0.86 | ||||
| (b) Mean copy numbers of Cu nitrite reductors nirK (107copy per g of dry soil) | ||||||||
| May | 1.70 ± 0.23 | 1.93 ± 0.12 | 1.48 ± 0.14 | 1.64 ± 0.18 | ns | 0.371 | 0.051 | 0.740 |
| July | 2.10 ± 0.38 | 2.06 ± 0.35 | 2.31 ± 0.37 | 2.22 ± 0.33 | ||||
| September | 1.62 ± 0.25 | 2.20 ± 0.33 | 1.82 ± 0.31 | 1.94 ± 0.19 | ||||
| November | 1.36 ± 0.23 | 1.76 ± 0.26 | 1.98 ± 0.24 | 2.03 ± 0.84 | ||||
| (c) Mean copy numbers of nitrous oxide reductors nosZ (107copy per g of dry soil) | ||||||||
| May | 2.24 ± 0.39 | 2.46 ± 0.38 | 2.54 ± 0.90 | 2.57 ± 0.85 | * | 0.017 | 0.048 | 0.942 |
| July | 1.49 ± 0.26 | 2.76 ± 0.28 | 3.76 ± 0.91 | 2.98 ± 0.84 | ||||
| September | 1.64 ± 0.13 | 2.78 ± 0.38 | 3.22 ± 0.91 | 3.56 ± 0.94 | ||||
| November | 2.33 ± 0.12 | 3.93 ± 0.45 | 4.13 ± 0.66 | 4.71 ± 0.97 | ||||
- C, control treatment; T, elevated temperature treatment; TD, temperature and drought treatment; TDCO2, temperature, drought, and elevated CO2.
- *,*** indicates significant differences at P < 0.05 and 0.01, respectively and italic indicates marginal differences (0.05 < P < 0.1).
Relationship between microbial activities, microbial population abundances and abiotic factors
Across treatments, field N2O fluxes showed a positive correlation with denitrification product ratio, which is consistent with its positive correlation with the denitrification enzyme activity producing N2O (N2ODEA) and a negative correlation with the reduction of N2O to N2 (N2DEA) during the study period (Table 4). This pattern was mirrored by N2O fluxes in the control treatment. In addition, N2O fluxes in the C treatment showed a significant negative correlation with NEA (Table 4). Variation in gene abundances played a relatively more important role for N2O fluxes under warmed conditions compared with the control. In the T treatment, N2O fluxes showed a significant negative correlation with both NEA and the nosZ/nirK ratio (Table 4). In the TD treatment, N2O fluxes were positively correlated with the denitrification product ratio, but negatively correlated with nosZ abundance (Table 4). Finally in the TDCO2 treatment, N2O fluxes showed a positive correlation with the denitrification product ratio, but a negative correlation with N2DEA and the nosZ/nirK ratio. No significant relationships were observed between N2O fluxes and gene abundances across treatments (Table 4).
| N2O fluxes | Microbial activities | Gene abundances | ||||||
|---|---|---|---|---|---|---|---|---|
| NEA | N2ODEA | N2DEA | Denitrification product ratio | AOB | nirK | nosZ | nosZ/nirk | |
| Pooled treatments | 0.016 | 0.335 | −0.291 | 0.411 | −0.179 | 0.117 | −0.166 | −0.216 |
| C | −0.462 | 0.477 | −0.734 | 0.768 | −0.205 | 0.281 | 0.017 | −0.211 |
| T | −0.428 | 0.221 | −0.321 | 0.398 | 0.006 | 0.359 | −0.177 | −0.426 |
| TD | 0.206 | 0.275 | −0.356 | 0.475 | −0.314 | −0.252 | −0.447 | −0.327 |
| TDCO2 | −0.101 | 0.305 | −0.393 | 0.599 | −0.346 | 0.120 | −0.264 | −0.394 |
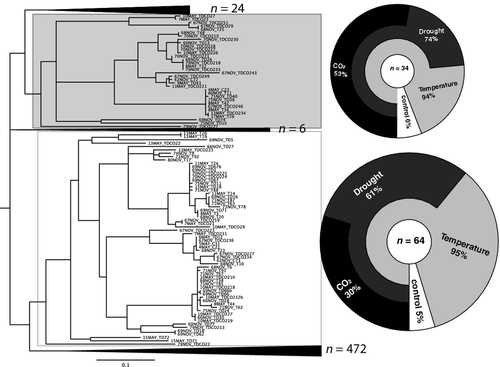
Changes in nirK community and diversity structure
On the basis of the complete maximum likelihood tree (Appendix 1), both the Unifrac significance and the P-test significance indicated that the nirK community sampled in May was significantly different in its structure from the community sampled in November (P < 0.03). In addition, the sequences from the November samples had a significant number of unique branches compared to the rest of the tree (P ≤ 0.01). When clustering the environments according to the full tree topology (Appendix 4) the nirK communities from the treatments T and TDCO2 shared the most sequences, and were closer to the control nirK community than to the TD community. All pairwise comparisons of the treatments showed significant differences (Jackknife analysis, P < 0.05). At a branch threshold of 0.05, two lineages showed significant biases toward specific treatments (Fig. 2). Lineage A showed significant dominance in the TDCO2 treatment (dominance 18 observed while 8.5 expected) and the C treatment (recession 2 observed instead of 8.5 expected). Lineage B showed significant dominance in all elevated temperature treatments compared with control (C = 3; T = 22; TD = 20; TDCO2 = 19; expected = 16). Compared to the rest of the tree, the sequences belonging to both lineages showed significant differences in high-GC% sequences (mean GC% = 62.92% for lineages A and B; GC% = 62.03% for all other sequences; Kruskal–Wallis, X2 = 32.5, P < 0.001).
Microbial drivers of N2O fluxes, a structural equation modeling
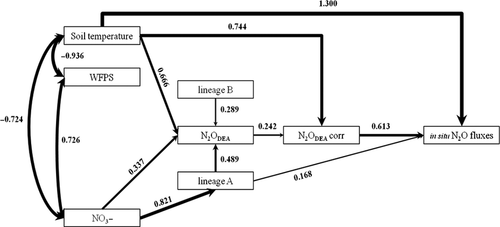
Structural equation modeling (SEM) identified potential causal relationships between variables significantly correlated with in situ N2O fluxes (χ² = 5.204, P = 0.391; Fig. 3). Non-standardized path coefficients and tests of path significance are available in Appendix 5. Almost all of the N2O flux variance (87%) was explained by denitrifier processes (N2ODEA, N2ODEA corr), relative abundance of specific nirK lineages (lineage A, B) and by abiotic factors (soil temperature). Abundances of nirK and nosZ per se or their ratio had no effect on denitrification activity and in situ N2O fluxes in the SEM (data not shown). NO3− availability impacted in situ N2O fluxes indirectly via impacts on potential denitrification and the nirK community structure (lineage A). The nirK community structure influenced N2O fluxes either directly with lineage A or indirectly via impacts on potential N2O emissions (N2ODEA with lineage A and B; Fig. 3). Soil temperature was identified as the driver of denitrification and N2O fluxes. The path coefficients indicated that changes in soil temperature were the major driver of altered in situ N2O fluxes. However, neither soil temperature nor WFPS measured on the day of sampling were related to changes in nirK community structure (Fig. 3, Appendix 5). SEM performed with nitrification (i.e., NEA and AOB gene abundances) were not significant (P < 0.05; data not shown) implying the weak effect of nitrifiers-related parameters on field N2O fluxes. Similarly, SEM performed with gene abundances of denitrifiers (nirK and nosZ) were not significant (P < 0.05; data not shown), implying uncoupled responses of gene abundances and the denitrification process.
Discussion
Global changes are known to enhance soil N2O fluxes (Cantarel et al., 2011; Carter et al., 2011; Niboyet et al., 2011). In the present study, we aimed to improve the mechanistic understanding of soil microbial functioning and the processes contributing to the emissions of N2O for grasslands subjected to sustained climate change.
Warming and all combined climate change drivers induced strong modifications in field N2O fluxes and microbial functioning
Throughout our study, we found that N2O fluxes and microbial activities responded more strongly to warming and combined climate changes (simultaneous application of warming, summer drought and elevated CO2) than to summer drought or elevated CO2 under warmed conditions. Flux data from the present study confirms the importance of warming as a key driver of climate-induced changes for N2O-N losses in grassland ecosystems (Cantarel et al., 2011). Accordingly, we found positive effects of warming on N2O fluxes recorded during the growing season, but no significant warming effects for the winter sampling date (Cantarel et al., 2011) due to an insufficient warning to compensate for the winter temperature. Such seasonal variation may reflect interactions between soil temperature and soil moisture on microbial processes (Flechard et al., 2007), as well as variation in root exudation and soil nutrient availability. Moreover, both nitrification enzyme activity (NEA) and the in situ nitrate pool increased in response to elevated temperature, in agreement with previous results observed in well-aerated soils (Barnard & Leadley, 2005). Warming was found to have a positive impact on AOB abundance in May, whereas combined warming, drought and elevated CO2 had a positive impact on AOB abundance in November. Given the relatively limited changes in gene abundances observed, and their transient nature, it is likely that the increase of AOB abundances was probably a result of indirect effects, most likely mediated by the plant community (Horz et al., 2004). AOB are believed to be inferior competitors for nutrient resources (Belser, 1979), and temporal changes in AOB community size may reflect a shifting competitive hierarchy for nutrient resources (mainly NH4+) between AOB, heterotrophic microbes and plants.
Irrespective of measurement date, combined climate change (TDCO2) was found to increase N2ODEA and N2O + N2 emissions in the laboratory measurements (N2Otot). Surprisingly, these two variables did not show a significant response to warming alone. This lack of response did not result from the confounding effects of soil moisture during the measurement campaigns, since similar soil moisture conditions were observed in the C and T treatments. Barnard & Leadley (2005) recently reported that denitrification enzyme activity (DEA) was generally less responsive to temperature in field experiments compared with laboratory studies, a phenomenon attributed to acclimation of DEA to ambient environmental conditions over time (French et al., 2009). Denitrifying bacteria harboring nosZ genes also carry nirK or nirS genes, though denitrifying bacteria may solely harbor nirK and/or nirS genes (Jones et al., 2008). Therefore, shifts in nosZ community may not always reflect nirK and/or nirS community changes. In our study, the abundances of nosZ denitrifiers increased more than those of nirK denitrifiers in response to warming and combined climate changes, suggesting a shift in nirK and/or nirS community structure. Between nirK and nirS communities, the former have been shown to respond to environmental changes (Hallin et al., 2009; Szukics et al., 2010). Herein, changes in nirK community structure were found under warming and combined climate change treatments. Indeed, we found two deeply branching lineages with significant biases for warmed treatments. This result suggests a selective process under warmed treatments; it is noteworthy that the sequences included in these two lineages harbored a higher GC-content than the other sequences on the tree, consistent with bacteria adapted to higher temperatures (Madigan & Martinko, 2006).
Low variation in soil water status modifies microbial community structure but does not affect N-related microbial activities and abundance
Combined summer drought and warming had no significant effect on microbial parameters (enzymatic activities and gene abundances) compared with warming alone. Previous study indicates that decreases in soil moisture are often associated with a decrease in DEA and an increase in NEA products (Barnard & Leadley, 2005; Bateman & Baggs, 2005). In our study, the variation in soil water status across T and TD treatments on measurement dates was weak, despite a 20% reduction of summer precipitation. Consequently, the limited effects of drought treatment on soil moisture conditions may have diminished the impact of experimental drought on soil processes. Another explanation could be that changes in nitrite community structure under warmed and drought treatment mitigated drought responses in enzymatic activities and gene abundances. Irrespective of the sampling dates (May or November), phylogenetic analysis of nirK sequences indicated a strong divergence between the nitrate reducer community in the TD treatment and those communities found in the other climate change treatments. This suggests a key selective process linked to drought under warmed conditions, which could explain why no difference was found in denitrification activities in the various treatments.
Elevated CO2 was expected to increase soil water status due to reduced plant stomatal aperture and transpiration rates (Schulze, 1986), which can have indirect consequences on denitrification by releasing the soil O2 partial pressure (Smith et al., 2003). Although we measured significantly higher soil moisture conditions in July and September in the TDCO2 treatment compared with the TD treatment (37% vs. 29% and 47% vs. 31% for July and September respectively), we found no impact of elevated CO2 on enzymatic activities and N2O fluxes. The lack of response to drought and elevated CO2 observed here mirrors the patterns of N2O fluxes recorded in 2007–08 at the same site (Cantarel et al., 2011) and suggests that N2O-related microbial processes may also be insensitive to minor variations in soil water content. Moreover, drought and elevated CO2 did not highly modify the relationship between field N2O fluxes and microbial activities and gene abundances. The maintenance of soil functioning in combined warming, drought and elevated CO2 conditions despite substantial modifications in the bacterial community structure, agrees with other studies which show high-functional redundancy of microbial communities (Wertz et al., 2007; Cabrol et al., 2011).
Relationships among microbial parameters and field N2O emissions
N2O flux variations were better explained by the denitrification product ratio (N2ODEA/[N2ODEA + N2DEA]) both across treatments and under cool, wet conditions (control site). Changes in DEA products over time (i.e., decrease of N2ODEA and increase of N2DEA) were correlated with a decrease in field N2O fluxes. This may result from continuous N losses via N2 fluxes in our grassland ecosystem even when no N2O fluxes were detected. The relative importance of microbial activities and microbial population size was modified under warmed conditions, with stronger correlations between field N2O fluxes and gene abundances in the T, TD and TDCO2 treatments. Nevertheless, field N2O fluxes showed a stronger correlation with enzymatic activities than with community abundance across climate treatments. Links between N2O fluxes and microbial abundances are known to be elusive, and may depend on soil properties or ecosystem type (Ma et al., 2008). Furthermore, the activity of a given enzyme may be uncoupled from the size of the corresponding functional gene pool due to subsequent enzyme regulation. Additional study is needed to examine the relative importance of other denitrifying and nitrifying genes on patterns of microbial activities and associated field N2O fluxes under future climate conditions.
A challenge in this study was to link in situ N2O fluxes and functioning microbial ecosystem, and particularly the nirK denitrifiers community. The SEM supported the importance of changes in abiotic conditions (i.e., soil temperature) toward in situ N2O fluxes. However, additional significant path coefficients suggested that other factors e.g., changes in denitrification activities and community structure were important in determining field N2O fluxes. Moreover, the availability of NO3− pool influenced in situ N2O fluxes indirectly by providing substrate for denitrification and impacting the nirK community structure (lineage A). The observed direct and indirect influences of nirK diversity suggest that the mechanisms driving field N2O fluxes are subtler than simple warming effects on denitrifying enzymatic activities. Taken together, our results strongly suggest that the combined effects of soil temperature, denitrifier community structure and activity, provide a much better predictor of N2O fluxes than nitrifier-related parameters. Further study coupling automated N2O measurements with more frequent soil sampling over the course of the year is required to confirm these findings, and improve our understanding of climate change impacts on annual N2O fluxes and N-related microbial functioning.
Acknowledgements
The authors would like to thank Alexandre Salcedo and Laurent Gaumy for assistance with soil sampling and chamber measurements, to Robert Falcimagne and Patrick Pichon for maintenance at the mini-FACE site. The authors acknowledge the financial support of the French Ministry of Education and Research for the doctoral fellowship to AAMC and of the EC FP6 ‘NitroEurope-IP’ project and of the French ANR VMCS ‘VALIDATE’ project. Quantitative PCR were carried out at the platform DTAMB (IFR 41, Université Lyon 1). Nitrification and denitrification measurements were performed at the Chromatography platform (UMR5557-USC1193). The authors declare that there is no conflict of interest in the present manuscript.




