The effect of experimental warming and precipitation change on proteolytic enzyme activity: positive feedbacks to nitrogen availability are not universal
Abstract
Nitrogen regulates the Earth's climate system by constraining the terrestrial sink for atmospheric CO2. Proteolytic enzymes are a principal driver of the within-system cycle of soil nitrogen, yet there is little to no understanding of their response to climate change. Here, we use a single methodology to investigate potential proteolytic enzyme activity in soils from 16 global change experiments. We show that regardless of geographical location or experimental manipulation (i.e., temperature, precipitation, or both), all sites plotted along a single line relating the response ratio of potential proteolytic activity to soil moisture deficit, the difference between precipitation and evapotranspiration. In particular, warming and reductions in precipitation stimulated potential proteolytic activity in mesic sites – temperate and boreal forests, arctic tundra – whereas these manipulations suppressed potential activity in dry grasslands. This study provides a foundation for a simple representation of the impacts of climate change on a central component of the nitrogen cycle.
Introduction
Proteolytic enzymes depolymerize protein, a large pool of organic nitrogen (N) in soil organic matter (SOM, Schulten & Schnitzer, 1998), into amino acids. The activity of proteolytic enzymes is a principal driver of the within-system cycle of soil N and the amino acids produced by proteolytic enzymes contribute significantly to the N economy of plants and microbes (Schimel & Bennett, 2004; Finzi & Berthrong, 2005; Gallet-Budynek et al., 2009; Nasholm et al., 2009). Changes in soil temperature and moisture as result of global change have the potential to impact proteolytic enzyme activity by altering the production of enzymes and substrates (Melillo et al., 2002; Allison et al., 2010a). Given the importance of proteolytic enzymes to N cycling and primary production, it is essential to develop an empirical understanding of their response to warming and changes in precipitation so that this understanding can eventually be incorporated into current ecosystem models.
Most ecosystem models predict that N mineralization and the availability of soil N to support primary production increases with increasing temperature in nearly all biomes (TEM, Raich et al., 1991; CLM, Thornton & Rosenbloom, 2005; CENTURY, Parton et al., 1993). In these models, the rate of N mineralization is determined by the rate of C mineralization in pools with different turnover times, temperature and moisture sensitivities, and C : N ratios. This linear dependence assumes that the decomposition of N from SOM follows that of C. Recent data from arctic and temperate forest biomes show that the activity of N-degrading enzymes in the soil are less responsive to temperature than those that degrade soil C (Wallenstein et al., 2009; Brzostek & Finzi, 2011), suggesting that current generation of models may overestimate the availability of N to support primary production. In defense of models, however, there is limited data to parameterize these models, and the empirical basis for enzymatic responses to climate change is only now emerging (e.g., Schimel & Weintraub, 2003; Allison et al., 2010b; Davidson et al., 2012; Wang et al., 2012).
Results from short-term laboratory experiments suggest that among-biome differences in climate will significantly impact the response of proteolytic enzyme activity to rising temperature and changes in precipitation. In the short term, increases in temperature and soil moisture have been shown to enhance the activity of enzymes by increasing the collision frequency and solubility of enzymes and substrates (e.g., Wallenstein et al., 2009; Allison et al., 2010a), with the largest temperature effects observed in soils collected during the winter and early spring when they are cold (e.g., Fenner et al., 2005; Wallenstein et al., 2009; Brzostek & Finzi, 2012). Further, low soil moisture has been shown to limit the positive response of enzyme activity to increasing temperatures (Zak et al., 1999; Davidson et al., 2012). This evidence suggests that warming will have the greatest impact on proteolytic enzyme activity in cold, mesic biomes, whereas changes in soil moisture will control responses in warmer, xeric biomes.
Over longer time scales, the response of proteolytic enzyme activity to changes in soil temperature and moisture may differ substantially from laboratory manipulations where enzyme pool size and substrate availability are held constant. Initial increases and subsequent declines in SOM decomposition in response to warming (Melillo et al., 2002; Bradford et al., 2008) suggest that low substrate availability or reduced enzyme production can lead to declines in enzyme activity with increasing temperature (Allison et al., 2010b). Thus, even if proteolytic enzymes were highly sensitive to short-term changes in temperature and moisture, low levels of enzyme and substrate could limit enzymatic responses to global change in many ecosystems.
The aggregate effects of warming and precipitation change on microbial processes are often linked through their impact on the water balance of ecosystems (Knapp et al., 2002, 2008; Austin et al., 2004; Arnone et al., 2008). Climate change is therefore likely to result in a continuum of enzymatic responses across biomes; though to date there have been no systematic evaluations of such changes in the N cycle. In this study, we use soils from 16 existing global change experiments to understand the long-term effect of climate change on the potential activity of proteolytic enzymes (Table 1). We tested two hypotheses: (1) warming increases potential proteolysis in mesic sites and decreases potential activity in xeric sites and (2) precipitation manipulations that exacerbate large, water balance deficits decrease potential proteolysis, while those that cause reductions in water balance deficits or surpluses increase potential activity. The experiments are located in several North American biomes, ranging from dry grasslands to temperate and boreal forest to arctic tundra. We evaluated changes in potential proteolysis among biomes using a single methodology to measure changes in potential proteolysis in response to experimental manipulations of temperature and/or precipitation.
| Site | Abbrev. | Location | Biome | Manipulations | LAT | LONG | MAT (°C) | MAP (mm) |
|---|---|---|---|---|---|---|---|---|
| Toolik-Moist Acidic Tundra | T-MAT | Toolik Lake, AK | Tundra | Warming | 68.6 | −149.6 | −8.6 | 328 |
| Toolik Nonacidic Tundra | T-NAT | Toolik Lake, AK | Tundra | Warming | 68.6 | −149.6 | −8.6 | 328 |
| Toolik-Shrub | T-SHB | Toolik Lake, AK | Tundra | Warming | 68.6 | −149.6 | −8.6 | 328 |
| Toolik-Wet Sedge Grass | T-WSG | Toolik Lake, AK | Tundra | Warming | 68.6 | −149.6 | −8.6 | 328 |
| Boreal Forest Warming at a Ecotone in Danger | B4W-1 | Cloquet, MN | Boreal Forest | Warming | 46.7 | −92.5 | 4.6 | 807 |
| B4WarmED | B4W-2 | Ely, MN | Boreal Forest | Warming | 47.9 | −91.8 | 1.4 | 739 |
| Harvard Forest-Prospect Hill | HF1 | Petersham, MA | Temperate Forest | Warming | 42.5 | −72.2 | 7.8 | 1172 |
| Harvard Forest-Barre Woods | HF2 | Petersham, MA | Temperate Forest | Warming | 42.5 | −72.2 | 7.8 | 1172 |
| Harvard Forest-N × Warming | HFN | Petersham, MA | Temperate Forest | Warming | 42.5 | −72.2 | 7.8 | 1172 |
| Boston Area Climate Experiment | BACE | Waltham, MA | Temperate Old Field | Warming, ↑↓ Precipitation | 42.4 | −71.2 | 10.9 | 1104 |
| Long Leaf Pine Irrigation | LLP | Newton, GA | Coastal Pine Forest | ↑ Precipitation | 31.3 | −84.4 | 19.1 | 1356 |
| Biodiversity, CO2, and Nitrogen | BioCON | Cedar Creek, MN | Grassland | ↓ Precipitation | 45.4 | −93.3 | 6.27 | 796 |
| Prairie Heating and CO2 Experiment | PHACE | High Plains, WY | Prairie | Warming, ↑ Precipitation | 41.1 | −104.8 | 7.2 | 384 |
| Rainfall Manipulation Plot Study | RAMPS | Konza Prairie, KS | Prairie | Warming, precipitation timing | 39.1 | −96.6 | 12.7 | 872 |
| Konza Irrigation Transect | KIT | Konza Prairie, KS | Prairie | ↑ Precipitation | 39.1 | −96.6 | 12.7 | 872 |
| Warming and Rainfall Manipulation Experiment | WARM | College Station, TX | Oak-savannah | Warming, precipitation timing | 30.6 | −96.4 | 20.3 | 981 |
- LAT, latitude; LONG, longitude; MAT, mean annual temperature; MAP, mean annual precipitation.
Materials and methods
Site description and soil collection
We collected soils from 16 global change experiments from 10 different sites across the United States (Table 1). At each site, experiments manipulated soil temperature, precipitation input, or a combination of both factors. The sites were located across a broad latitudinal gradient from arctic tundra (LAT: 68.6N) to lower mid-latitude grasslands and savannah (LAT: 30.6N). Soils were sampled from replicate control and treatment plots between July and September of 2009, near the peak of the growing season at each site. The exception was the N × Warming (HFN) experiment at the Harvard Forest, MA, where soils were sampled in mid-October 2009. There were more warming studies than precipitation-change studies in the data set, allowing for meaningful horizon-specific analyses of enzymatic response to warming. Only mineral-soil samples were available from experiments manipulating precipitation.
Soils were sampled following established protocols at each site. Organic horizons when present and the top 15 cm of mineral soil were collected and analyzed separately. Immediately after coring the soils were shipped or transported on ice to Boston University for analysis. Upon arrival, each soil sample was sieved, roots and rocks removed, and a 30 g subsample frozen at −80 °C. Given the large number of soil samples (>800 cores), it was necessary to store samples at −80 °C prior to the analysis of proteolytic activity. It was not logistically possible to analyze the samples at the same rate as they were received from the different sites.
Potential proteolytic enzyme activity
We assayed potential proteolytic enzyme activity following a method modified from Watanabe & Hayano (1995) and Lipson et al. (1999). To compare rates of proteolysis among experiments, we warmed the soils for 4 h to a common laboratory temperature of 23 °C. Initial and incubated subsamples of soil (2–3 g) received 10 ml of a 0.5 mm sodium acetate buffer (pH 5.0) with a small volume of toluene (400 μl) added to inhibit microbial uptake. After these reagent additions, the initial samples were treated with 3 ml of a trichloroacetic acid solution to halt the activity of proteolytic enzymes. The remaining subsamples were incubated for 4 h and then treated with the same trichloroacetic acid solution (Finzi & Berthrong, 2005; Rothstein, 2009). The concentration of amino acids in the initial and incubated samples was quantified using the o-phthaldialdehyde and β-mercaptoethanol method (Jones et al., 2002). Concentrations of amino acid N were determined by comparing the fluorescence of the samples relative to a standard curve composed of glycine. Potential activity was calculated as the difference between amino acid concentrations in the incubated and initial samples (Brzostek & Finzi, 2011). We acknowledge that by assaying proteolytic enzyme activity at common temperature, moisture, and pH conditions, we did not specifically measure in situ rates of activity. In particular, the in situ activity in soils from very cold and very dry sites is likely much lower than the activity reported here. By holding these other factors constant, however, this assay measures a potential enzyme activity where only enzyme pool size and the availability of protein substrates varies between treatments.
Statistical analysis
We used meta-analysis to investigate the response of proteolytic enzymes to manipulations of soil temperature and precipitation inputs across the different experiments. Meta-analysis provides a quantitative statistical approach for synthesizing the results of multiple independent experiments (e.g., Rustad et al., 2001; Knorr et al., 2005; Treseder, 2008). We used meta-analysis instead of multiple independent anova analyses for each experiment because this approach would preclude a quantitative, cross-site estimate of the magnitude of the response of proteolytic enzymes to temperature and precipitation change (Rosenberg et al., 2000). Potential proteolytic activity in the organic horizon was analyzed separately from that in the mineral-soil horizon, because these horizons are functionally distinct and proteolytic activities differ by an order of magnitude or more (e.g., Rothstein, 2009; Brzostek & Finzi, 2011; Reiskind et al., 2011).
For each site, we performed a weighted meta-analysis that factors in the sample size and variability in the responses for each experimental observation (meta-win Version 2.1; Rosenberg et al., 2000). For each study, we calculated the effect size of a given treatment by calculating the natural log of the response ratio [i.e., ln(RR)], defined here as the mean of the potential proteolytic rate in the treatment divided by the mean rate in the control plot. Values of ln(RR) > 0 indicate stimulatory effects, and values <0, inhibitory effects. For each ln(RR), we used bootstrapping, a nonparametric approach, to calculate 95% confidence intervals (CI). CIs that did not overlap zero indicate a significant treatment response.
One assumption of meta-analysis is that all observations are independent (Rosenberg et al., 2000). Recently, Hungate et al. (2009) highlighted how different definitions of what constitutes an independent observation can influence the results of meta-analysis. We adopted their recommended and conservative suggestion for independent observations by calculating a single ln(RR) for experiments that had multiple treatment levels but only a single set of control plots (e.g., BACE, B4W 1 & 2, PHACE).
In addition to experiment-level responses, we used meta-analyses to examine responses across experiments. We calculated the grand mean and the bias corrected bootstrapped CIs for the ln(RR) for the response of potential proteolytic activity to the warming and precipitation manipulations (Adams et al., 1997). We used a categorical random effects model to test for differences in potential proteolytic activity among biomes and warming methods (Rustad et al., 2001).
Finally, weighted regression analysis was used to investigate the relationship between the response of potential proteolytic activity with the duration and magnitude of the experimental treatments and the climate at each site. Weighted regression analyses were performed in meta-win 2.1 using a continuous random effects model. Mean (1971–2000) growing season and annual climate information (MAT, MAP) for each site was obtained from the National Climate Data Center (Table 1 and Supporting Information Table S1). Soil moisture deficit (or surplus) was calculated as the difference between precipitation (P) and potential evapotranspiration (PET) (i.e., P-PET; Liski et al., 2003; McCarthy et al., 2010). Monthly mean temperatures and latitude were used to calculate PET during the growing season using the Thornthwaite method (Thornthwaite, 1948). We used data from the precipitation and warming manipulations to calculate treatment specific P-PET (Table 2). The details of the experimental treatments for each site are listed in Table S2. We acknowledge that differences between sites in the method of warming may have a modest impact on the accuracy of our P-PET estimate (Aronson & McNulty, 2009). The error in our PET estimates is likely lowest in those sites that use IR heaters, which accurately simulate energy balance changes and highest in those sites that use cables or chambers, which have been shown to decrease soil moisture (Kennedy, 1995; Shaver et al., 2000).
| Site | Growing-season P-PET (mm) | ||||
|---|---|---|---|---|---|
| Ambient | Warming | ↑ Precipitation | ↓ Precipitation | Warming × ↓ Precipitation | |
| Toolik Lake (4 exp.) | −144.8 | −163.6 | |||
| B4W-1 | −24.1 | −65.0 | |||
| B4W-2 | 10.4 | −25.7 | |||
| Harvard Forest (3 exp.) | −43.8 | −135.9 | |||
| BACE | −147.1 | −188.9 | −24.5 | −269.6 | |
| LLP | −82.8 | 47.8 | |||
| BioCON | −41.2 | −141.2 | |||
| PHACE | −182.4 | −213.0 | −102.4 | ||
| KIT | −135.2 | 164.8 | |||
| RaMPS | −135.2 | −199.9 | −135.2 | −199.9 | |
| WaRM | −348.5 | −383.2 | −433.2 | −552.9 | |
- P-PET, precipitation (mm) minus potential evapotranspiration (mm).
Results
Potential proteolytic enzyme activity
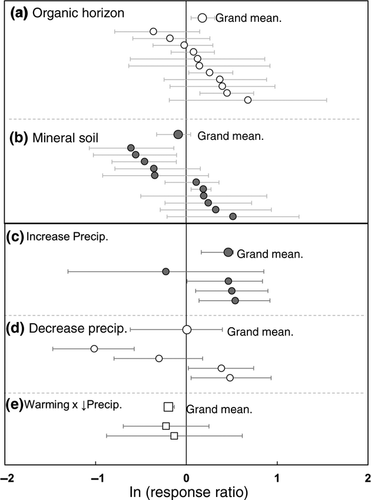
The response of potential proteolytic enzyme activity to warming in the organic and mineral-soil horizons was highly variable (Fig. 1, Table 3). Overall, experimental warming led to a significant 18% increase in potential proteolytic activity in organic horizons (Fig. 1a). Warming did not have a significant overall effect on potential proteolytic activity in the mineral soil (Fig. 1b). There were no significant differences in potential proteolytic activity between horizons, among biomes or in response to different methods of warming.
| Site | Horizon | Manipulations | Mean potential proteolytic activity (μg AA-N g soil−1 4 h−1) | Effect size | ||
|---|---|---|---|---|---|---|
| Control | Treatment | ln(RR) | Var. | |||
| T-MAT | OH | Warming | 22.83 (1.72) | 24.74 (3.61) | 0.080 | 0.027 |
| T-NAT | OH | Warming | 28.82 (7.40) | 24.13 (3.87) | −0.178 | 0.092 |
| T-SHB | OH | Warming | 19.83 (2.02) | 31.09 (5.94) | 0.450 | 0.047 |
| T-WSG | OH | Warming | 8.60 (3.38) | 6.00 (0.31) | −0.359 | 0.157 |
| B4W-1 | OH | Warming-Closed | 38.72 (4.33) | 43.91 (10.73) | 0.126 | 0.142 |
| B4W-1 | OH | Warming-Open | 10.29 (1.93) | 11.89 (2.05) | 0.145 | 0.157 |
| B4W-2 | OH | Warming-Closed | 2.23 (0.52) | 3.54 (0.79) | 0.399 | 0.087 |
| B4W-2 | OH | Warming-Open | 2.49 (0.61) | 4.93 (1.81) | 0.680 | 0.195 |
| HF1 | OH | Warming | 15.66 (2.31) | 20.17 (1.16) | 0.260 | 0.025 |
| HF2 | OH | Warming | 4.29 (0.63) | 4.19 (0.67) | −0.022 | 0.047 |
| HFN | OH | Warming | 5.95 (0.77) | 8.61 (2.81) | 0.370 | 0.123 |
| B4W-1 | MS | Warming-Closed | 1.42 (0.42) | 1.72 (0.34) | 0.194 | 0.125 |
| B4W-1 | MS | Warming-Open | 0.81 (0.17) | 1.12 (0.22) | 0.326 | 0.097 |
| B4W-2 | MS | Warming-Closed | 0.34 (0.08) | 0.57 (0.20) | 0.515 | 0.137 |
| B4W-2 | MS | Warming-Open | 0.29 (0.10) | 0.37 (0.04) | 0.244 | 0.059 |
| BACE | MS | Warming | 1.36 (0.29) | 1.52 (0.15) | 0.187 | 0.055 |
| HF1 | MS | Warming | 3.36 (0.56) | 2.36 (0.59) | −0.354 | 0.089 |
| HF2 | MS | Warming | 1.88 (0.30) | 1.19 (0.19) | −0.458 | 0.052 |
| HFN | MS | Warming | 2.10 (0.52) | 1.49 (0.53) | −0.345 | 0.189 |
| PHACE | MS | Warming | 2.75 (0.74) | 3.19 (0.93) | 0.111 | 0.325 |
| RaMPS | MS | Warming | 3.43 (0.66) | 1.87 (0.41) | −0.606 | 0.084 |
| WaRM | MS | Warming | 0.57 (0.19) | 0.33 (0.08) | −0.554 | 0.080 |
| BioCON | MS | ↓ Precipitation | 1.01 (0.16) | 1.63 (0.35) | 0.482 | 0.073 |
| BACE | MS | ↓ Precipitation | 1.08 (0.16) | 1.50 (0.21) | 0.387 | 0.093 |
| RaMPS | MS | ↓ Precipitation | 3.43 (0.66) | 2.55 (0.65) | −0.300 | 0.103 |
| WaRM | MS | ↓ Precipitation | 0.57 (0.13) | 0.21 (0.03) | −1.016 | 0.076 |
| PHACE | MS | ↑ Precipitation | 2.13 (0.98) | 1.70 (0.51) | −0.226 | 0.303 |
| BACE | MS | ↑ Precipitation | 1.08 (0.16) | 1.86 (0.27) | 0.501 | 0.041 |
| KIT | MS | ↑ Precipitation | 1.31 (0.22) | 2.46 (0.38) | 0.538 | 0.059 |
| LLP | MS | ↑ Precipitation | 0.31 (0.08) | 0.50 (0.05) | 0.463 | 0.077 |
| RaMPS | MS | Warming + ↓ Precip. | 3.43 (0.66) | 3.01 (0.99) | −0.133 | 0.145 |
| WaRM | MS | Warming + ↓ Precip. | 0.57 (0.19) | 0.40 (0.04) | −0.224 | 0.058 |
In the mineral-soil horizon, among-study variations in the response ratio of potential proteolytic activity was positively correlated with latitude (R2 = 0.55, P < 0.002, Fig. 2a) and negatively correlated with mean annual temperature (R2 = 0.47, P < 0.01, Fig. 2b). The response to warming declined significantly with the number of years of experimental treatment (R2 = 0.50, P < 0.005, Fig. 2c). There were no significant relationships between these variables and potential proteolytic activity in response to warming in the organic horizon.
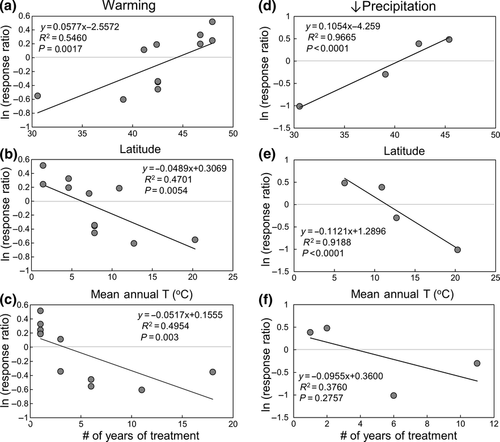
Experimental increases in precipitation significantly increased potential proteolytic activity (Fig. 1c), whereas decreases in precipitation had both positive and negative effects on potential enzyme activity (Fig. 1d). Variation in the response ratio of potential proteolytic activity among studies was negatively correlated with temperature and PET, but not precipitation (Table S3). As with warming, the response ratio of potential proteolytic activity was positively correlated with latitude (R2 = 0.97, P < 0.0001, Fig. 2d), negatively correlated with mean growing-season temperature (R2 = 0.92, P < 0.0001, Fig. 2e), and tended to decline with length of treatment (R2 = 0.38, P = 0.28, Fig. 2f).
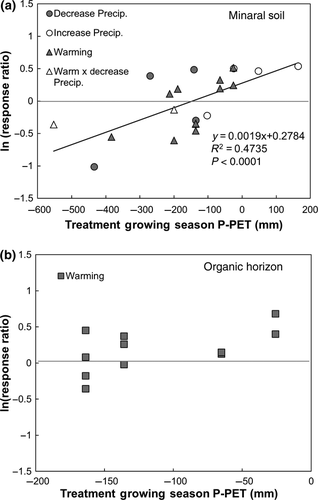
There were only two experiments that both warmed and manipulated precipitation (RAMPS and WARM; Table 1). In these studies, warming and changes in the timing of precipitation decreased potential proteolytic activity (Fig. 1e). Across all manipulations, however, treatment induced changes in the magnitude of the soil moisture deficit (i.e., growing-season P-PET; Table 2) best predicted the variability in enzymatic response (Fig. 3a). Potential proteolytic activity in the mineral soil decreased linearly with increasing soil moisture deficit (R2 = 0.47, P < 0.0001; Fig. 3a). There was no such relationship in the organic horizon (Fig. 3b). Given this strong relationship between P-PET and the response of proteolytic enzymes in the mineral soil, we used a weighted multiple regression to further examine this relationship. Ambient P-PET conditions combined with the magnitude and direction of the change in P-PET provided a good prediction of the response of potential proteolytic activity to global change (R2 = 0.43, P < 0.003, Table S3).
Discussion
Nitrogen limitation of primary production is widespread in the biosphere and N, in part, regulates the Earth's climate system by constraining terrestrial uptake of atmospheric CO2 (Vitousek & Howarth, 1991; Melillo et al., 1993; Hungate et al., 2003). Here, we show that the depolymerization of organic N, primarily in the form of protein, is highly sensitive to climate change. The experimental treatments altered the water balance of each ecosystem. The rate of potential proteolysis in the treatment relative to control plots declined linearly as the magnitude of the growing-season soil moisture deficit increased (i.e., P-PET became more negative; Fig. 3a), and all biomes plotted along the same line. This suggests the existence of a single relationship between climate and proteolytic enzymes and provides a foundation for a simple representation of climate change impacts on a key component of the terrestrial N cycle.
There was a relatively narrow range of soil moisture deficit (−200 < P-PET < −100) above which potential proteolytic rates tended to increase and below which they tended to decline (Fig. 3a). In the experimental treatments that reduced soil moisture below this threshold, it is likely that the diffusion of substrates and enzymes decreased, leading to a decline in the enzymatic return of N that limited proteolytic enzyme production and potential activity. (Zak et al., 1999; Allison et al., 2010a; Davidson et al., 2012). Above this threshold, warming and adequate soil moisture stimulated potential proteolytic activity, which in this case is likely to reflect some combination of an increase in substrate supply, and an increase in enzyme production by a larger or more active microbial biomass (Fig. 3a). Given that we did not measure extractable protein concentrations or microbial biomass, we cannot definitively argue which of these processes, or others, explain the observed response. Regardless, this analysis indicates that relatively subtle variations in climate have the potential to substantially alter the activity of proteolytic enzymes in mid- and high-latitude ecosystems.
In the mineral-soil horizon, the increase in potential proteolytic activity in response to warming was negatively correlated with MAT and positively correlated with latitude (Fig. 2a, b). The correlation with latitude was, in part, a reflection of the distribution of the global change experiments. The lower latitude sites tended to be in grasslands where warming enhanced the soil moisture deficit below the threshold of −200 mm. The higher latitude sites tended to be in cold, mesic forests where warming did not move the soil moisture deficit below this threshold. However, given the low ambient temperatures at these sites, warming had a proportionately larger effect on temperature that appeared to stimulate potential proteolytic activity (Table 1, Table S3). These considerations do not, however, compromise the major conclusion reported here. The two largest stimulations in potential proteolytic activity were measured in response to irrigation at the Konza prairie site (KIT: treatment P-PET = 165 mm) and in the lower latitude long leaf pine study (LLP: treatment P-PET = 48 mm), indicating that mid-latitude grasslands and warm-temperate forests respond to soil moisture deficit in a manner that is quantitatively similar to higher latitude forests.
The response of mineral-soil potential proteolytic activity to warming declined with the duration of experimental treatment (Fig. 2c). One interpretation of this relationship is that there is an initial increase in proteolytic activity followed by a decline in activity as readily available substrates are depleted (Melillo et al., 2002). However, three of the five experiments that anchor this relationship were from warming experiments of variable duration at the Harvard Forest. Whether collected following three (HFN), six (HF2), or 18 years (HF1) of experimental treatment, potential proteolytic activity declined to a similar degree with warming (Table 3). The similarity in response regardless of experimental duration and their plotting on the cross-biome line (Fig. 3a) suggest that the negative correlation between potential proteolytic activity and years of treatment is less important than spatial or experimental variations in climate.
The sites with organic horizons in this study were mid- and high-latitude forests and tundra (Table 1). In only 3 of the 11 studies did potential proteolytic activity in the organic horizon decline in response to warming (Table 3); overall, warming significantly increased potential proteolytic activity in this horizon (Fig. 1a). Experimental warming of the organic horizon did not result in P-PET values below the threshold seasonal soil moisture deficit described above (Table 2). Rather, it appears that any potential, negative effect of warming on soil moisture deficit was offset by the positive effect of warming on potential proteolytic activity. The consistent positive response to warming is likely driven by high protein substrate concentrations that are characteristic of organic horizons (Berthrong & Finzi, 2006; Reiskind et al., 2011).
The results of this study are consistent with an earlier meta-analysis of N mineralization responses to warming in boreal and arctic ecosystems (Rustad et al., 2001); both suggest that warming will increase the rate of N cycling in high-latitude soils. In contrast to Rustad et al. (2001), who found little relationship between potential N mineralization, MAT, or MAP, we found that variation in potential proteolytic activity was highly correlated with among-site variations in climate and the impact of climate manipulations on seasonal soil moisture deficit. Moreover, other studies have shown a soil moisture sensitivity of net N mineralization (Cassman & Munns, 1980; Emmett et al., 2004). Analysis of soil moisture deficit for the sites in Rustad et al. (2001) suggests an explanation for the apparent discrepancy between studies. The seasonal soil moisture deficit with warming in the organic-matter rich soils studied by Rustad et al. (2001) ranged from −100 to −230 mm, well within the range of positive responses observed in the organic horizons in this study (Fig. 3b).
Amino acids released from protein substrate by proteolytic enzymes contribute to terrestrial productivity and serve as substrates for mineralization and nitrification (Chapin et al., 1993; Nasholm et al., 1998; Jones & Kielland, 2002; Gallet-Budynek et al., 2009). As such, amino acid cycling is a key component of the terrestrial N cycle. The results of this study suggest that regional-scale changes in temperature and precipitation can control the magnitude and direction of the N-cycle responses to climate change. Understanding regional climate change is therefore essential to understanding the global-scale consequences of rising concentrations of radiatively active trace gasses on terrestrial productivity.
Acknowledgements
We would like to thank Colin Averill, Joy Cookingham, Verity Salmon, Poliana Lemos, Marc-Andre Giasson, Alison Greco and Winston MacDonald for laboratory assistance and Janet Chen for field assistance (PHACE). Funding for the work presented in this paper was provided by grants from the National Science Foundation (NSF, DEB-0743564, DEB-1011479) and a Northern Forest Scholar fellowship from the Northeastern States Research Cooperative, a joint program of the University of Vermont, the University of Maine and the Northern Research Station, USDA Forest Service to E.R.B. The following sources supported core operation for the global change experiments: (1) WaRM: Department of Energy's (DOE) Office of Science (BER) through the Southeastern Regional Center of the National Institute for Climatic Change Research, DOE-BER National Institute of Global Environmental Change and Texas AgriLife Research; (2) BioCON & B4Warmed: DOE-BER National Institute for Climate Change Research (2 awards), and NSF programs in Biocomplexity, Long-term Ecological Research (LTER, 2 awards), Division of Environmental Biology (DEB) and Long-term Research in Environmental Biology (LTREB); (3) PHACE: US Department of Agriculture (USDA)-Agricultural Research Service Climate Change, Soils & Emissions Program and Extension Service Soil Processes Program; (4) Arctic Sites T-MAT, -NAT, -SHB, -WSG: NSF programs in DEB, Office of Polar Programs and the Arctic LTER; (5) HFN: NSF Faculty Early Career Development Award to Serita Frey and Harvard Forest LTER; (6) HF-1, HF-2: NSF Harvard Forest LTER and DOE-BER Northeastern Regional Center of the National Institute for Climatic Change Research; (7) RaMPs: USDA, DOE-BER Northeastern Regional Center of the National Institute for Climatic Change Research and NSF LTREB; (8) KIT: NSF Konza Prarie LTER to Kansas State University; (9) BACE: NSF, Division of Environmental Biology and DOE-BER Northeastern Regional Center of the National Institute for Climatic Change Research; (10) DOE-BER National Institute for Climate Change Research. (Correction added after online publication 09/04/2012: Acknowledgements section has been updated by the authors.)




