Parapatric species and the implications for climate change studies: a case study on hares in Europe
Abstract
Parapatry is a biogeographical term used to refer to organisms whose ranges do not overlap, but are immediately adjacent to each other; they only co-occur – if at all – in a narrow contact zone. Often there are no environmental barriers in the contact zones, hence competitive interaction is usually advocated as the factor that modulates species distribution ranges. Even though the effects of climate change on species distribution have been widely studied, few studies have explored these effects on the biogeographical relationships between closely related, parapatric, species. We modelled environmental favourability for three parapatric hare species in Europe – Lepus granatensis, L. europaeus and L. timidus – using ecogeographical variables and projected the models into the future according to the IPCC A2 emissions scenario. Favourabilities for present and future scenarios were combined using fuzzy logic with the following aims: (i) to determine the biogeographical relationships between hare species in parapatry, that is L. granatensis/L. europaeus and L. europaeus/L. timidus and (ii) to assess the effects of climate change on each species as well as on their interspecific interactions. In their contact area L. granatensis achieved higher favourability values than L. europaeus, suggesting that if both species have a similar population status, the former species may have some advantages over the latter if competitive relationships are established. Climate change had the most striking effect on the distribution of L. timidus, especially when interspecific interactions with L. europaeus were taken into account, which may compromise the co-existence of L. timidus. The results of this study are relevant not only for understanding the distribution patterns of the hares studied and the effects of climate change on these patterns, but also for improving the general application of species distribution models to the prediction of the effects of climate change on biodiversity.
Introduction
Parapatry is a biogeographical pattern in which two species have separate, but contiguous distributions without physical barriers between them, and they only co-occur – if at all – in a narrow contact zone. This is a common distribution pattern in closely related species and in species with a weak phylogenetic relationship, but with a high level of ecological similarity (Bull, 1991). As parapatry is considered as an opposite state to coexistence, most explanations of parapatric distributions assume negative interactions as the cause of interspecific exclusion along geographical gradients; as a result, the species with the highest persistence potential displaces the other (Bull & Possingham, 1995 and references therein).
A key step in species distribution modelling (SDM) (Guisan & Thuiller, 2005) involves taking into account the biotic factors (i.e. interactions with other species that modify the ability for a given species to maintain populations) that contribute to delimiting species' ranges. The inclusion of biotic interactions improves SDM performance for both positively related species, such as specialist species requiring a specific biotic resource (e.g. Araújo & Luoto, 2007; Kissling et al., 2010), and for other systems in which competitive forces modulate species distribution ranges (e.g. Meier et al., 2011). Biotic interactions are especially relevant in the context of studies predicting distributional shifts under climate change scenarios, as the distribution of a species may change not only because of altered climatic conditions, but also because interactive species move in response to climate change (Meier et al., 2011). Thus, understanding the biogeographical relationships between parapatric species and how they vary in response to climate changes is needed to improve the predictions on shifts in species distributions.
Even though biotic interactions are highly relevant in SDM, studies investigating procedures to account for them are still scarce. The simple approach of including the distribution of other species as predictors in a predictive model of a given species (e.g. Araújo & Luoto, 2007) may not in fact reflect a biotic interaction, but rather the absence of important environmental predictors in the model (Guisan & Thuiller, 2005). In addition, the inclusion of these predictors in the model may only provide information on potential interaction between species, but not on the possible role of each species in the interaction. Biotic interactions, such as those producing parapatry, are usually asymmetric relationships in which the distribution of one species is strongly mediated by another, but not always vice versa (Bull & Possingham, 1995). Thus, the inclusion of other species' ranges as predictors ideally requires prior knowledge to choose the correct biotic predictors from among many alternatives; nevertheless, this is not always possible when, for example, competitive exclusion between species has not been previously documented.
Fuzzier approaches have been applied to assess interspecific relationships in a biogeographical context. For example, Chefaoui et al. (2005) explored variation in the suitability scores for two species along an environmental gradient and showed the usefulness of this approach to describe potential sympatry between two species (see also Acevedo et al., 2007a,2007b). Sattler et al. (2007) examined biogeographical relationships between cryptic species by combining ecological niche factor analysis (Hirzel et al., 2002) and discriminant analysis. The discriminant factor correlates with the variables that best segregate the species. These authors used the discriminant factor as an integrative variable to compare the ‘niches’ of the species and estimate their degree of overlap. Based on the conceptual framework applied in these studies and using the favourability function (see Real et al., 2006), Acevedo et al. (2010) developed an approach to explore the biogeographical relationships between related species. Even though this approach was proposed in a context of native vs. introduced species, it can be used to study other types of biotic relationships. This approach can be used to map species interaction and to create directional hypothesis about the role of each species in the interaction, although species interactions cannot be conclusively demonstrated using these kinds of approaches (Anderson et al., 2002; Jiménez-Valverde et al., 2007).
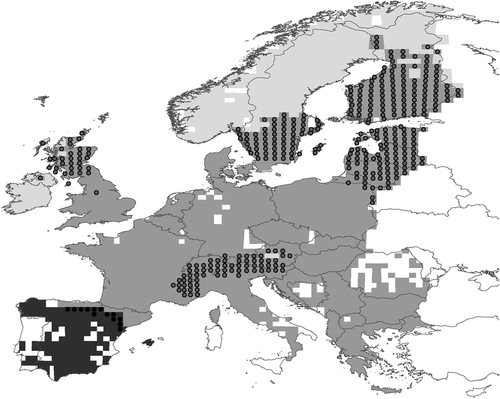
European hares provide a suitable model by which to study the biogeographical relationships between parapatric species. At present, taxonomic experts accept five species of the genus Lepus occurring naturally in Europe: L. europaeus, L. timidus, L. granatensis, L. castroviejoi and L. corsicanus (Mitchell-Jones et al., 1999; Alves & Hackländer, 2008). The latter two species, although being genetically similar (Alves et al., 2008), have restricted allopatric ranges – L. castroviejoi in the Cantabrian Mountains of the Iberian Peninsula and L. corsicanus in the Apennines and Sicily – and the other three species have wider distributions (see Fig. 1). This complex distribution pattern certainly reflects specific ecological adaptations, and enhances different putative contact zones. However, the relationship between each pair of parapatric species is not expected to be symmetrical as usually one species prevails over the other in the contact zones, even when competitive exclusion relationships have not been firmly evidenced. On the one hand, it is known that populations of L. granatensis are increasing, but those of L. europaeus are decreasing in their contact areas in the Iberian Peninsula (Gortázar et al., 2007). On the other hand, other studies have identified the expansion of L. europaeus as one of the causes of the generalized decline of L. timidus (see Thulin, 2003; Jannson & Pehrson, 2007; Patton et al., 2010; Reid, 2011).
Using European hares as a study model and the analytical procedure described in Acevedo et al. (2010), this study has the following aims: (i) to determine the biogeographical relationships between hare species with wider distributions, namely L. granatensis/L. europaeus and L. europaeus/L. timidus and (ii) to assess the effects of climate change on each species and their interspecific interactions. The results may assist in improving the general application of species distribution models for assessing the effects of climate change on biodiversity.
Material and methods
Species data
The European distribution of Lepus spp. was extracted from The Atlas of European Mammals (Mitchell-Jones et al., 1999; Fig. 1). Information refers to UTM 50 × 50 km squares as they were the territorial units used for modelling purposes. Due to the fact that the sampling effort used to create the Atlas was not spatially homogeneous, we calculated the number of mammal species in each square as a proxy of sampling effort; for modelling purposes, we only considered the UTM squares in which at least one species has been reported (n = 2557). This filter excluded most of Eastern Europe which coincided with the most incomplete area identified by the Atlas authors (A.J. Mitchell-Jones, personal communication). In the study area, the most frequent hare species was L. europaeus (n = 1119 presences), followed by L. timidus (n = 532 presences) and finally the Iberian endemism L. granatensis (n = 118 presences).
Environmental data
The occurrence of the three hare species in each UTM square was modelled using 35 potential explanatory variables related to the following factors: spatial location (2 variables), topography (1 variable), climatology (15 variables) and land use (17 variables; see Table 1). These variables were chosen on the basis of availability at this scale and potential predictive power, and were assumed to be correlated with more explanatory factors.
| Code | Description |
|---|---|
| LONG | Longitude (decimal degrees) |
| LAT | Latitude (decimal degrees) |
| ALT | Mean altitude (masl) |
| BIO1 | Annual mean temperature |
| BIO2 | Mean diurnal range (mean of monthly (max temp - min temp)) |
| BIO3 | Isothermality (BIO2/BIO7) (×100) |
| BIO4 | Temperature seasonality (SD × 100) |
| BIO5 | Max temperature of warmest month |
| BIO6 | Min temperature of coldest month |
| BIO7 | Temperature annual range (BIO5-BIO6) |
| BIO10 | Mean temperature of warmest quarter |
| BIO11 | Mean temperature of coldest quarter |
| BIO12 | Annual precipitation |
| BIO13 | Precipitation of wettest month |
| BIO14 | Precipitation of driest month |
| BIO15 | Precipitation seasonality (coefficient of variation) |
| BIO16 | Precipitation of wettest quarter |
| BIO17 | Precipitation of driest quarter |
| T11 | Post-flooding or irrigated croplands |
| T14 | Rainfed croplands |
| T20 | Mosaic Cropland (50–70%)/Vegetation (grassland, shrubland, forest) (20–50%) |
| T30 | Mosaic Vegetation (grassland, shrubland, forest) (50–70%)/Cropland (20–50%) |
| T50 | Closed (>40%) broadleaved deciduous forest (>5m) |
| T70 | Closed (>40%) needleleaved evergreen forest (>5m) |
| T90 | Open (15–40%) needleleaved deciduous or evergreen forest (>5m) |
| T100 | Closed to open (>15%) mixed broadleaved and needleleaved forest (>5m) |
| T110 | Mosaic Forest/Shrubland (50–70%)/Grassland (20–50%) |
| T120 | Mosaic Grassland (50–70%)/Forest/Shrubland (20–50%) |
| T130 | Closed to open (>15%) shrubland (<5m) |
| T140 | Closed to open (>15%) grassland |
| T150 | Sparse (>15%) vegetation (woody vegetation, shrubs, grassland) |
| T180 | Closed to open (>15%) vegetation (grassland, shrubland, woody vegetation) on regularly flooded or waterlogged soil - Fresh, brackish or saline water |
| T190 | Artificial surfaces and associated areas (urban areas > 50%) |
| T200 | Bare areas |
| T210 | Water bodies |
Land use data came from Global Land Cover 2005, which is freely available at http://www.esa.int/esaEO/SEMXB7TTGOF_index_0.html. The map (~300 m spatial resolution) covers the entire planet, and its accuracy has been successfully validated (see Bicheron et al., 2008). Bioclimatic variables (for present and future times) and altitude (~1000 m spatial resolution) were obtained from the Worldclim project database (see Hijmans et al., 2005 for details). The models calibrated for the present period were projected into the future by replacing the current bioclimatic variables in the models with those expected according to the climate change scenario for the future period up to 2080 using the A2 emissions scenario (Nakicenovic et al., 2000). This scenario is defined as a world of strengthening regional cultural identities, with an emphasis on family values and local traditions, high population growth and less concern for rapid economic development. We used only one scenario because our main interest was to assess the changes in interspecific relationships between parapatric species due to climate, rather than to assess the effect of different global circulation models or emissions scenarios (see Real et al., 2010).
Modelling
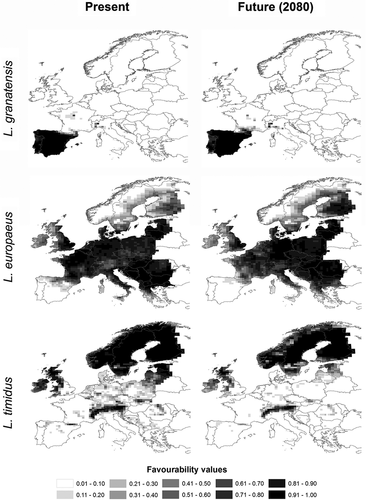
We used an inductive approach to estimate the macroecological requirements of the species from the locations in which they occurred (Corsi et al., 2000). We modelled the occurrence of each species assuming that, after correcting for the sampling effort previously described, if a species was not observed within a UTM square, this was equivalent to the absence of the species. For each species, the model was calibrated using a 70% random sample of the data and evaluated against the remaining 30%. Firstly, to control for the increase in type I errors as the number of independent variables increased, we evaluated the false discovery rate (FDR; García, 2003) using the procedure proposed by Benjamini & Hochberg (1995), and only accepted variables that were significantly (P < 0.05) related to the species distribution under an FDR of q < 0.05. The selected variables were then used in a multiple logistic regression procedure (Hosmer & Lemeshow, 1989), and the final models were selected following a forwards–backwards stepwise procedure. Finally, to establish direct comparisons between models (species), the logistic probabilities were used to obtain favourability values using the function described by Real et al. (2006). The favourability function is a valuable tool to study biogeographical relationships between models whatever the proportion of presence records (sample prevalence) in the calibration datasets (e.g. see Real et al., 2009; Acevedo et al., 2010). This is due to the fact that a favourability value of 0.5 always corresponds to the same environmental threshold, thus the independence of these values in relation to species prevalence enables direct comparisons between models (species) built with different prevalences.
Sensitivity – the percentage of correctly predicted presences to the total number of presences –, specificity – the percentage of correctly predicted absences to the total number of absences –, and the area under the ROC curve (AUC) were estimated on the validation datasets to assess the discriminative capacity of the models (Fielding & Bell, 1997; but see Lobo et al., 2008). To calculate sensitivity and specificity a threshold of 0.5 was used as a cut-off for favourability values in all the models according to the favourability concept (Real et al., 2006). All statistical analyses were performed using spss 18 (spss Inc., Chicago, IL, USA) statistical software.
Assessing relationships between parapatric species
An inherent quality of favourability values is that they can be regarded as the degree of membership in the fuzzy set of sites whose environmental conditions are favourable to the species (Robertson et al., 2004; Real et al., 2006). Thus, fuzzy logic operations can be used to compare different models. This is an advantage of the favourability function over other SDM techniques when the aim of the study is to combine models for different species, scenarios, etc. (see Estrada et al., 2008; Acevedo et al., 2010, 2011). The biogeographical relationships between two species can be assessed using the fuzzy overlap index (FOvI; see Acevedo et al., 2010), i.e. the ratio between the degree to which the study area is favourable to the two studied species simultaneously and the degree to which it is favourable for either species (Dubois & Prade, 1980; Kunchenva, 2001). This index varies from 0 (no overlap in favourability) to 1 (complete overlap in favourability). The FOvI can be decomposed into absolute local overlap values (FOvI-L) that represent the contribution of each locality (UTM square) to the FOvI. Thus, the FOvI-L shows the spatial location of the areas where spatial overlap between species is expected to occur (Acevedo et al., 2010).
Trends on species favourability were assessed across the range of FOvI-L values for each pair of parapatric species (L. granatensis/L. europaeus and L. europaeus/L. timidus) using the procedure described by Acevedo et al. (2010). Briefly, FOvI-L values were divided into 10 intervals (0.1 width), and mean favourability values at each interval were calculated for each pair of species. Throughout the gradient defined by FOvI-L, and consistent with the favourableness-severity hypothesis (Richerson & Lum, 1980), it can be assumed that competition between species increases and competitive exclusion decreases as FOvI-L increases. Subsequently, we divided the curve into fixed intervals: FOvI-L < 0.2 (areas that were unfavourable for at least one species) and FOvI-L > 0.8 (areas simultaneously highly favourable to the two species). According to the favourableness-severity hypothesis, the area with 0.2 < FOvI-L < 0.8 is where biotic interactions could limit species occurrence (Acevedo et al., 2010).
Assessing changes in distribution patterns between climatic scenarios
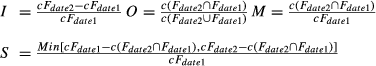
Results
Logistic regression procedure selected variables related to spatial situation, climate, topography and land uses to explain the European distribution of L. granatensis, L. europaeus and L. timidus at 50 × 50 km spatial resolution (Table 2). By applying the favourability function, maps were obtained for the studied species (Fig. 2) which determine the localities with ecogeographical characteristics that favour or constrain the presence of the species (F > 0.5 or F < 0.5 respectively). The models showed a high discrimination capacity (Se, Sp and AUC were 1, 0.950 and 0.987 for L. granatensis; 0.821, 0.787 and 0.877 for L. europaeus; 0.908, 0.935 and 0.970, for L. timidus).
| Variables | Lepus granatensis | L. europaeus | L. timidus |
|---|---|---|---|
| LONG | −0.609/45.529/*** | 0.156/120.858/*** | −0.056/6.237/* |
| LAT | −0.736/17.019/*** | 0.167/25.16/*** | |
| BIO1 | −0.098/87.23/*** | ||
| BIO3 | −0.486/18.955/*** | FDR | |
| BIO4 | −0.001/21.676/*** | ||
| BIO5 | 0.041/23.355/*** | ||
| BIO6 | FDR | ||
| BIO7 | FDR | ||
| BIO11 | FDR | ||
| BIO12 | FDR | ||
| BIO15 | −0.205/45.482/*** | −0.088/185.019/*** | |
| BIO17 | −0.042/13.173/*** | 0.011/24.01/*** | |
| T11 | 0.144/7.02/*** | FDR | |
| T14 | FDR | 0.023/17.361/*** | −0.107/59.053/*** |
| T30 | 0.076/3.99/* | ||
| T50 | 0.045/7.025/*** | −0.026/5.458/* | |
| T70 | FDR | −0.05/9.457/** | |
| T90 | −0.019/12.244/*** | ||
| T100 | FDR | ||
| T110 | FDR | ||
| T120 | −0.163/11.238/*** | −0.1/20.997/*** | |
| T140 | FDR | FDR | |
| T150 | −0.085/119.854/*** | −0.104/107.186/*** | |
| T180 | −0.065/22.62/*** | ||
| T190 | FDR | FDR | |
| T200 | FDR | ||
| T210 | −0.041/21.278/*** | −0.053/17.13/*** | |
| Intercept | 63.519/39.387/*** | 3.399/50.549/*** | −8.854/9.167/** |
When the favourability functions were projected into the future (2080), different situations for each species were observed (Fig. 2). Our results suggest that whereas the L. granatensis and L. europaeus ranges will slightly shift to the north/northeast, the L. timidus distribution range will notably decrease. Based on the maps, these interpretations are also supported by the fuzzy logic indices summarized in Table 3.
| Model | Without biotic interaction | With biotic interaction | ||||||
|---|---|---|---|---|---|---|---|---|
| I | O | M | S | I | O | M | S | |
| L. granatensis | 0.065 | 0.866 | 0.959 | 0.041 | −0.064 | 0.911 | 0.889 | 0.047 |
| L. europaeus | −0.052 | 0.835 | 0.886 | 0.062 | −0.281 | 0.842 | 0.688 | 0.031 |
| L. timidus | −0.283 | 0.717 | 0.717 | 0.000 | −0.696 | 0.646 | 0.304 | 0.000 |
| L. granatensis/L. europaeus | −0.227 | 0.418 | 0.523 | 0.250 | ||||
| L. europaeus/L. timidus | −0.228 | 0.593 | 0.660 | 0.112 | ||||
The relationships between parapatric species in terms of favourability and their trends over the gradient defined by FOvI-L are displayed in Fig. 3 (see also Table 3). Localities that are simultaneously highly favourable to both L. granatensis and L. europaeus (FOvI-L > 0.8), i.e. with ecogeographical conditions that actually favour the presence of both species, do not exist at present and are not expected to exist in future scenarios. Both species overlapped with FOvI-L > 0.2 in only 3.2% of the study area (82 squares); this means that 96.8% of the study area is highly unfavourable (F < 0.2) to, at least, one of the species, i.e. they are territories with ecogeographical conditions that constraint the presence of, at least, one of the species. In the intervals with intermediate values of FOvI-L, L. granatensis attained higher favourability values than L. europaeus, suggesting that given equal population status (e.g. balanced densities) for both species, if competitive relationships were established in these localities the former species may have some advantages over the latter. This situation is maintained in the future climatic scenario, although favourability for L. granatensis is expected to decrease slightly, with a subsequent reduction in overlap between species (Table 3). Regarding the biogeographical relationship between L. europaeus and L. timidus, the current favourability maps for these species overlapped by more than double compared with those for the previous pair of species. Lepus timidus attained higher favourabilities than L. europaeus in the intervals with intermediate values of FOvI-L. When models for these species were projected into the future climatic scenario, the situation was similar to that obtained for the present period, except for the areas with FOvI-L > 0.8 that generally shifted northwards.
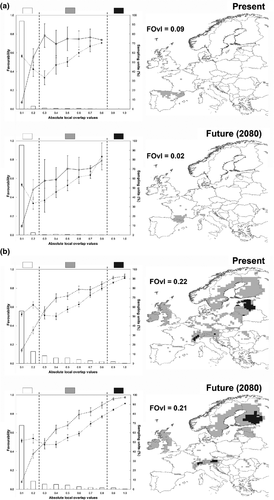
If the results of the interspecific interactions are considered, an uncertain area could be delineated where biotic interactions could limit species occurrence; this is the area with 0.2 < FOvI-L < 0.8 (see Fig. 3). This assumes 3.2% of the study area for L. granatensis/L. europaeus and 29.5% for L. europaeus/L. timidus in the models for the present period, and 2.2% and 21.5%, respectively, for models projected into the future. Two extreme values can be estimated when assessing the sensitivity of the species to climate change. The first is defined by the rates of change for each single species assuming non-negative relationships between parapatric species, that is, those rates exclusively modulated by abiotic factors (see Table 3). When biotic interactions are considered, the other extreme value can be obtained by assuming that each species could be competitively excluded from the uncertainty area (see Table 3).
Discussion
Our results focus on two issues: the methodology used and the conservation of the studied species in Europe. On the one hand, the analytical approach followed in this study is useful for a deeper assessment of the biogeographical relationship between parapatric species and its expected shifts under new scenarios according to global change. Even though this approach is based on the favourableness-severity hypothesis (Richerson & Lum, 1980) and it was used in the context of competing species, under other theoretical frameworks it can be useful for exploring the biogeographical relationships of species, for example, when species are positively related (Callaway et al., 2002). On the other hand, the effects of climate change on the distribution of L. timidus predicted for 2080, especially when combined with potential exclusion by L. europaeus, should drive managers to consider global climate change as one of the factors involved in L. timidus decline in Europe, as already appears to be occurring in some contact areas (see Thulin, 2003).
The methodological approach
A key step in SDM involves taking into account biotic interaction; thus, changes in climate may not only directly alter the distribution of a species, but also indirectly alter it through affecting the distribution of other interactive species (Meier et al., 2011). Our study offers a new perspective on the role of interspecific interactions on shaping future distribution ranges in response to climate change. The methodological approach, previously described by Acevedo et al. (2010), allowed us: (i) to determine the areas where the probability of competition between species is higher; and assuming equal population status for the species involved in the interaction; (ii) to infer a directional hypothesis on the role of each species; and (iii) to explore the spatial shifts in species interactions pattern under different scenarios, that is, to assess interspecific interactions and how they would evolve under climate change scenarios (see Klanderud & Totland, 2005). It is an improvement over other approaches previously used in the context of SDM and climate change assessment which are mainly based on including the distribution data of the interacting species as predictors during the modelling processes.
Clearly, the critical issue is not to determine whether biotic interactions have effects at biogeographical scales, but to quantify their magnitude (e.g. Leathwick & Austin, 2001). This key question is difficult to answer mainly because there is no validation data available by which to assess the predictions of the models under climate change conditions (Araújo et al., 2005). It is also often very difficult to distinguish spatial patterns generated by interspecific interactions from those caused by abiotic causes, historical factors or dispersal barriers (Wiens, 1989). With our approach two extreme situations for each species can be determined, one in which no effects of interspecific interaction were considered when assessing shifts in the species distribution area due to climate changes, and another in which it was assumed that the species was totally excluded from potentially competitive exclusion areas (see also Araújo & Luoto, 2007). In other words, it presents a range between no effects and the full effects of interspecific interactions. The real situation is likely to be between these extremes, although the exact outcome cannot be determined. The approach used allows the identification and mapping of the most probable areas for competitive exclusion, and so would be of use when designing subsequent studies on biotic interactions at local scales (Anderson et al., 2002; Jiménez-Valverde et al., 2007). Even when the real distribution of the species in the future remains unknown, with this approach a more detailed assessment can be made of the expected species distribution in response to change, based on the directional hypothesis about the role of each species and, when available, on previous knowledge of species' relationships obtained from local studies. This is the case for hare species in Europe (see below), although this may not be possible for every species.
On the hare species in Europe
Our results on the effects of climate change on L. granatensis and L. europaeus distributions indicate slight northward shifts in their ranges by 2080 according to the A2 emissions scenario. However, this situation does not apply to L. timidus, as the predictions suggest that its range will undergo notable reductions because of climate changes (Fig. 2). First, these results are consistent with studies which have documented differences in sensitivity to climate change in relation with the ecoregion inhabited by the species; i.e. species from the Boreo-Alpine region were more sensitive to climate change than those inhabiting more temperate regions (Thuiller et al., 2005). Thus, species occurring in colder regions should be affected by climate change because of a loss of suitable habitat. Given this context, and consistent with previous studies, the future range of L. timidus in Europe is highly dependent on climate (Jannson & Pehrson, 2007; Anderson et al., 2009). When interspecific interactions were considered the expected effects of climate change on these species were even more pronounced (Table 3); the real situation probably lies between these extremes, but unfortunately this cannot be determined at present as no validation data exist regarding the future.
We can interpret the predicted interactions based on previous knowledge about the ecology of the studied species. On the one hand, L. granatensis is expected to have some advantage over L. europaeus in their contact area as it is more favourable to the former (Fig. 3A). In addition, data obtained from population monitoring has described an increasing population trend for L. granatensis, whereas L. europaeus is declining in their contact area (Gortázar et al., 2007), thus enhancing the potential advantage of L. granatensis over L. europaeus. In this context, the effects of biotic interactions on L. granatensis could be considered negligible when the models are projected onto future scenarios. Therefore, the future distribution of L. europaeus is predicted to be negatively affected by L. graatensis, although it should be noted that L. europaeus has been suggested as a competitor able to exclude L. timidus in the borders of distribution (Thulin, 2003; and references therein). Thus, it is expected that L. europaeus would display an intermediate pattern between the reported extremes (Table 3), as even if the viability of the southern European populations from the Iberian Peninsula may be compromised, it is not expected that those of the north will be constrained by competition. Nevertheless, the last interpretation is not directly supported by our analysis; when the population status of both species was expected to be similar, then L. timidus seemed to be favoured over L. europaeus in their contact area (Fig. 3B). In our opinion, a plausible explanation would be related to unbalanced densities between these species when they co-occur; L. timidus is usually found at lower densities than L. europaeus (Jannson & Pehrson, 2007). Even if a territory is more favourable to L. timidus, differences in densities could drive a situation in which this species is disadvantaged compared with L. europaeus when resources become limited (Thulin, 2003). However, in addition to resources, exclusion mediated by hybridization (see Rhymer & Simberloff, 1996) can occur when species differ in density, especially in highly unbalanced situations. This was also suggested as a potential factor mediating the L. europaeus/L. timidus interactions (Thulin, 2003; but see Jansson et al., 2007), and even explaining the ancestral local extinction of L. timidus in the Iberian Peninsula due to displacement by L. granatensis (e.g. Melo-Ferreira et al., 2007). These range replacements with hybridization have also important implications on the genetic composition of the involved species, as gene introgression should predominantly occur from the resident into the invading species (Currat et al., 2008). Finally, future predictions for the distribution of L. timidus cannot be viewed with optimism; it is expected that by 2080 the distribution of this species will be reduced to 30% of its current range (72% under the most optimistic predictions) due to climate changes. According to our results, the future of this species will be compromised by climate change especially when biotic interactions with L. europaeus are taken into account. Thus, we suggest that climate change should be included among the factors to be monitored when addressing the conservation of L. timidus (Smith & Johnston, 2008).
Acknowledgments
We thank the Societas Europaea Mammalogica and Tony Mitchell-Jones for providing the distribution data used to prepare the Atlas of European mammals. We are grateful to A.M. Barbosa for her ever-useful advice. P. A. and A. J.-V. were supported by the Juan de la Cierva research program awarded by the Ministerio de Ciencia e Innovación–Fondo Social Europeo, and partially by the project CGL2009-11316/BOS from the Spanish Government and FEDER. P. A. is a current holder of the Jose Castillejo fellowship (2010–2011) in Portugal awarded by the Ministerio de Ciencia e Innovación. J. M.-F. has a post-doctoral grant funded by FCT and the European Social Fund (SFRH/BPD/43264/2008). This work was partially funded by the research projects PTDC/BIA-EVF/111931/2009 and PTDC/BIA-EVF/115069/2009 funded by FCT and FEDER.




