Robustness to thermal variability differs along a latitudinal gradient in zooplankton communities
Abstract
Determining how thermal variability will affect the structure, stability, and function of ecological communities is becoming increasingly important as global warming is predicted to affect not only average temperatures but also increase the frequency of long runs of high temperatures. Latitudinal differences in the responses of ecological communities to changes in their thermal regimes have also been predicted based on adaptations over evolutionary time to different thermal environments. We conducted an experiment to determine whether variability in temperature leads to consistent changes in community structure, temporal dynamics, and ecosystem functioning in laboratory analogues of natural freshwater supralittoral rock pool communities inhabited by meiofauna and zooplankton collected from sub-Arctic, temperate, and tropical regions. Thermal variability of +4 °C around mean temperature led to increased extinction frequency, decreases in consumer abundance, increases in temporal variability of consumer abundance, and shifts from predominately negative interactions observed under constant temperature to positive interactions in the temperate and tropical communities but not in the sub-Arctic communities. That sub-Arctic zooplankton communities may be more robust to thermal variability than temperate or tropical communities’ supports recent studies on macrophysiological adaptations of species along latitudinal gradients and suggests that increasing thermal variability may have the greatest effects on community structure and function in tropical and temperate regions.
Introduction
Environmental temperatures and the thermal regimes ecological communities are exposed to are among the most important determinants of species’ life history traits and macroecological patterns (Clarke, 2003; Brown et al., 2004; Helmuth et al., 2005; Berg et al., 2010). Although it is widely acknowledged that increases in mean temperature may affect processes at many levels of biological organization, from the metabolism of individuals (Pörtner et al., 2006; Folguera et al., 2008, 2010; Dillon et al., 2010) to the functioning of ecosystems (Traill et al., 2010; Walther, 2010), the effects of changes in thermal regimes, defined as the amplitude and frequency of temperature changes, on the structure, dynamics, and functioning of ecological communities are only beginning to be explored (Folguera et al., 2009).
Two aspects of temperature change, other than the effects of increasing temperature per se, are particularly crucial to our understanding of how ecological communities will respond to climate change: (1) how changes in thermal variability will affect the structure, dynamics and function of ecological communities, and (2) whether or not ecological communities will respond similarly to changes in thermal regimes across latitudinal gradients.
Climate change is altering variability in thermal regimes (Easterling et al., 2000; Houghton et al., 2001; Katz et al., 2005). As warming increases, variability in temperature is expected to decrease on average with an increased frequency of long runs of extreme high temperatures [IPCC (Intergovernmental Panel on Climate Change), 2007]. This change is thought to have a wide range of effects on ecological communities. The higher frequency of long runs of unfavourable conditions may lead to decreases in population persistence, particularly for high growth rate species that track environmental changes more closely (Ripa & Lundberg, 1996; Petchey et al., 1997; Pike et al., 2004; Schwager et al., 2006; Ruokolainen & Fowler, 2008). Changes in population abundance and persistence may also differ depending on the species’ trophic roles. Berg et al. (2010) showed that thermal sensitivity of ectotherm species was higher for predators than for plants, detritivores, or microbivores, and Rall et al. (2010) suggested that the abundance of consumers decreases with increasing thermal variability as high and low temperature events can be stressful to species affecting their metabolic and growth rates as well as changing the intensity of their resource-use. The higher frequency of long runs of unfavourable conditions may also lead to increases in rates of species extinction (Hiltunen et al., 2006; Gonzalez & deFeo, 2007). When species are lost from communities the number of alternative pathways for energy flow and the potential for species complementarity is also reduced, which in turn affects the nature and strength of species interactions (Thebault & Loreau, 2005; Gonzalez & Loreau, 2009), leading to changes in temporal stability. Finally, although the effects of thermal variability on ecosystem functions have not yet been addressed, thermal regime changes will likely result in major changes to community productivity and respiration due to the temperature dependence of metabolic rate (Brown et al., 2004; Bulling et al., 2010; Traill et al., 2010; Yvon-Durocher et al., 2010).
Species have adapted over evolutionary time to different regimes of thermal variability suggesting that the effects of changes in thermal regime are likely to differ along latitudinal gradients (Deutsch et al., 2008; Dillon et al., 2010; Sunday et al., 2011). Thermal variability generally increases from the equator to the poles with high-latitude species generally exhibiting acclimation to a wider range of temperatures than low-latitude species (Chown et al., 2004; Angilletta, 2009; Sunday et al., 2011). Robustness of community and ecosystem properties to thermal variability should thus be higher in communities exposed to naturally occurring higher amplitude thermal changes such as Arctic communities (Folguera et al., 2011; Sunday et al., 2011). Tropical communities are predicted to be the most sensitive due to the low ambient variability of their natural habitats. Temperate communities, which are exposed to an intermediate level of thermal variability, are predicted to show intermediate responses (Sunday et al., 2011).
We tested two sets of predictions related to the effects of increasing thermal variability on freshwater aquatic meiofaunal and zooplankton communities collected from three sites (sub-Arctic, temperate, tropical) arrayed along a 40°N latitudinal gradient. First, we tested hypotheses related to how thermal variability affects ecological community structure, dynamics, and function focussing on responses that may be sensitive to changes in thermal variability: extinction frequency, community and trophic group abundance, temporal variability in abundance, the strength and nature of species interactions, primary productivity, bacterial abundance, and ecosystem respiration. Second, we assessed whether or not these responses differed along a latitudinal gradient, explicitly testing the hypothesis that robustness (lack of change) would be highest in sub-Arctic communities, intermediate in temperate communities and lowest in tropical communities due to latitudinal differences in the variability of thermal regimes (Chown et al., 2004; Angilletta, 2009; Sunday et al., 2011).
We conducted a 3 × 2 factorial experiment [latitude (sub-Arctic, temperate, tropical) × thermal variability (constant, 4 °C above and 4 °C below mean temperature)] using laboratory analogues of the natural meiofauna and zooplankton communities found in freshwater supralittoral rock pools. Supralittoral rock pools are a common habitat type located on rocky shores around the world. The pools form either by dissolution of limestone or in granite depressions and are above the high-tide line and thus are primarily rain-fed. Rock pool communities are dominated by a crustacean fauna composed primarily of cladocerans such as Daphnia spp., copepods, and ostracods. Rock pool communities are useful as model systems to address the generality of environmental changes along latitudinal gradients as the somewhat extreme and constrained nature of these habitats, such as frequent desiccation events, has resulted in high taxonomic and functional similarities in species composition along wide latitudinal gradients.
Our results highlight potential differences in community structure, dynamics, and function that can result from increased thermal variability along a latitudinal gradient. Thermal variability of +4 °C around mean temperature led to increased extinction frequency, decreases in consumer abundance, increases in temporal variability of consumer abundance, and shifts from predominately negative interactions observed under constant temperature to positive interactions in the temperate and tropical communities but not in the sub-Arctic communities.
Materials and methods
We collected naturally occurring freshwater aquatic meiofaunal and zooplankton species along with rock pool water containing phytoplankton, bacteria, and protists from rock pools from three locations arrayed along a 40°N latitudinal gradient. Sub-Arctic rock pools were located near the Churchill Northern Research Center (58°46′N, 94°10′W) in Churchill, Manitoba, Canada; temperate rock pools were located at Prospect Point (44°28′N, 63°47′W), Nova Scotia, Canada, and tropical rock pools were located near the Discovery Bay Marine Laboratory (18°28′N, 77°24′W) on the Northern coast of Jamaica. Species were collected in the field and brought to Dalhousie University where they were maintained under laboratory conditions in aquaria. Communities were transported in closed vials containing 40 mL of water and were generally in transport for 2–3 days prior to culturing in glass aquaria.
Rock pool species from different latitudes differ in species identity but in general the community composition of rock pools is taxonomically and functionally similar and for some taxa such as Daphniidae, the same species (e.g. Daphnia magna) can be found in both the temperate and sub-Arctic rock pools. The rock pool species in our laboratory model system consist primarily of cladocerans, ostracods, and copepods in the following functional feeding (i.e. trophic) groups: herbivores [Daphniidae (Daphnia magna, Daphnia ambigua, and Ceriodaphnia lacustris) and Ostracoda (Cypridinae eucypris sp., and Cypridopsis cf. mariae Rome)], detritivore/herbivores [Chydoridae (Chydorus sp., Alona sp., Alonella sp., Alonopsis sp.)], detritivore/omnivores [Ostracoda (Cypridinae megalocypris sp., Candona sp., Cypricercus sp., Potamocypris sp.)], and copepod predators [Cyclopoid Copepods (Microcyclops varicans, Paracyclops sp.)]. Rock pool meiofauna differ strongly in functional traits related to feeding (Romanuk et al., 2010). To determine the trophic role for each species, feeding trials were set up by placing three individuals of each species in a small (20 mL) plastic container with three individuals of another species, with ten replicates for each pair-wise trial. Containers were checked regularly until all individuals of one species were missing or dead. Direct observations of feeding behaviour were also used. Based on these pair-wise feeding trials, species were classified into trophic groups as herbivores, species that only feed on algae; detritivore/herbivores, species that feed on live as well as dead algae and other detritus; detritivore/omnivores, species that feed on live or dead consumers; or predators, species that feed on live consumers.
Experimental design
Replicates of rock pool communities from tropical, temperate, and sub-Arctic habitats were created by removing 500 mL of rock pool water from the culturing aquaria and were housed in 1.5 L plastic (polypropylene) containers marketed as aquaria/terraria from Boreal Northwest (16 × 12 × 9 cm) with perforated lids. The experimental design was a 3 × 2 factorial with three regions (tropical, temperate, and sub-Arctic) and the communities were exposed to either constant temperature (TEMPCONSTANT) or variable temperature (TEMPVAR). The mean temperature of TEMPVAR was identical to the temperature of the control (TEMPCONSTANT). The temperature of control microcosms (TEMPCONSTANT) differed regionally and was set at 15 °C for the sub-Arctic microcosms and 24 °C for the temperate and tropical microcosms. Temperature variability (TEMPVAR) was manipulated by moving microcosms between water baths of three different temperatures (mean, 4 °C above mean, 4 °C below mean) every 2 days for 8 weeks for a total of 28 moves. This short time frame allowed rock pool species to experience substantial variability within each generation. In TEMPVAR microcosms had an 80% chance of remaining at their current temperature and a 20% chance of moving to either a higher or lower temperature every 2 days. This probability of movement between temperatures resulted in reddened temperature variability, i.e. long runs of similar temperature. Two sets of six (for tropical and sub-Arctic) or five (for temperate) replicates were used in the TEMPVAR treatment to account for potential differences in the specific pattern of temperature changes that occurred (mean temperature was identical for both TEMPVAR sets and TEMPCONSTANT). In total there were n = 6 TEMPCONSTANT microcosms and n = 12 TEMPVAR microcosms for tropical, n = 5 TEMPCONSTANT and n = 12 TEMPVAR microcosms for sub-Arctic communities and n = 6 TEMPCONSTANT microcosms and n = 10 TEMPVAR microcosms for the temperate communities. No significant differences in any of community structure or dynamics response variables between sets in TEMPVAR were observed thus microcosms from both sets were analysed together. Temperate and tropical microcosms were located in heated water baths, and sub-Arctic microcosms were housed in temperature controlled fridges. Full spectrum light was provided on a 12 h cycle for all three regions with no exposure to natural light. No nutrients or food were added over the course of the experiment as the rock pool communities are self-sustaining with adequate light.
Sampling and response variables
Microcosms were sampled weekly for 8 weeks following a 2 weeks acclimation period. Species richness, number of species in each microcosm, was determined by observing the entire contents of each microcosm and counting the number of species present. Extinction frequency was calculated as [(maximum no. spp – final no. spp)/maximum no. spp] for each microcosm over 8 weeks. Total consumer community abundance was determined by counting the number of individuals of each consumer species, defined as all species that were not primary producers or bacteria, for a 50 mL sub-sample. Total functional group abundance was also determined based on the functional feeding groups identified in the feeding trials: herbivores, herbivore/detritivores, detritivore/omnivores, and predators. Temporal variability in community abundance was calculated as the coefficient of variation (standard deviation in abundance/mean abundance) across the eight sampling dates.
Ecosystem functioning
We assessed differences in productivity and respiration in the final week (Week 10) using the light/dark bottle method. Oxygen in each microcosm was measured using a YSI datasonde. A 250 mL mason jar was then filled with water from each microcosm, sealed underwater to ensure that no oxygen bubbles or air was present inside the jar, and then either covered in tin foil (respiration) or left uncovered (productivity) before being returned to the microcosm. After ~24 h, oxygen in the container was measured and the water was returned to the microcosm. Respiration and productivity rates were calculated as final minus initial oxygen/time (for productivity) or initial minus final/time (respiration). To determine final bacterial abundance, which is a proxy for function, each microcosm was sampled for bacteria using an inoculation loop which was then smeared on a plate of marine agar. Plates were incubated at room temperature for 48 h, at which point the number of colonies was counted as an estimate of bacterial density. Changes in bacterial abundance were treated as a functional rather than a structural response following Jiang (2007) and Peter et al. (2011) as bacteria drive numerous ecosystem processes such as the degradation of dissolved organic carbon which is accompanied by respiration.
Statistical analysis
anovas were used to determine significant differences between TEMPCONSTANT and TEMPVAR in mean species richness, total community abundance, functional group abundance, extinction frequency, and temporal variability in abundance across the eight sampling dates. anova was also used to determine differences in productivity, respiration, and bacterial abundances at the end of the final week of the experiment (week 10). Post-hoc Tukey tests were used to determine significant differences between regions for all univariate anovas. Data was checked for assumptions of anova including normality and homogeneity of variances, both of which were met for all variables except for summed abundance and functional group abundance which was log transformed. All factors were fixed. To detect positive and negative covariances for species abundances we computed variance ratios, VR, as the ratio of the temporal variance of total community abundance to the sum of the variances in abundance for each species for each microcosm (Schluter, 1984). For this test a ratio <1 is indicative of negative covariances and a ratio >1 of positive covariances.
Results
Species richness and extinction frequency
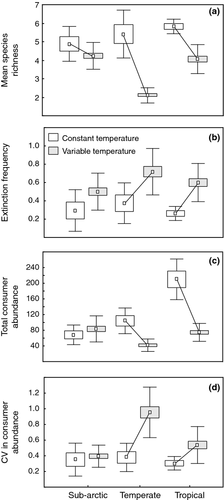
The initial latitudinal gradient in species richness, with highest species richness in the tropics (7 ± 0.49 SD), intermediate species richness in temperate regions (6.5 ± 0.51 SD), and lowest species richness in the sub-Arctic communities (5.5 ± 0.62 SD) was maintained throughout the experiment under constant temperature (Table 1; Fig. 1a). In the thermal variability treatment, species richness declined significantly in the tropical and temperate communities but not in the sub-Arctic communities, leading to a significant interaction between region and variability (F2,51 = 7.724, P < 0.001; Fig. 1a; Table 1).
| df | SS | MS | F | P | |
|---|---|---|---|---|---|
| (a) Mean species richness | |||||
| Intercept | 1 | 869.809 | 869.809 | 1664.488 | <0.001 |
| Region | 2 | 7.726 | 3.863 | 7.392 | 0.002 |
| Treatment | 1 | 53.173 | 53.173 | 101.753 | <0.001 |
| Region×Treatment | 2 | 8.073 | 4.037 | 7.724 | 0.001 |
| Error | 46 | 24.038 | 0.523 | ||
| Total | 51 | 94.038 | |||
| (b) Extinction frequency | |||||
| Intercept | 1 | 9.629 | 9.629 | 206.703 | <0.001 |
| Region | 2 | 0.249 | 0.124 | 2.667 | 0.080 |
| Treatment | 1 | 0.975 | 0.975 | 20.933 | <0.001 |
| Region×Treatment | 2 | 0.145 | 0.072 | 1.555 | 0.222 |
| Error | 46 | 2.143 | 0.047 | ||
| Total | 51 | 3.614 | |||
| (c) Total consumer abundance (log) | |||||
| Intercept | 1 | 897.002 | 897.002 | 9362.814 | <0.001 |
| Region | 2 | 4.584 | 2.292 | 23.924 | <0.001 |
| Treatment | 1 | 5.234 | 5.234 | 54.633 | <0.001 |
| Region×Treatment | 2 | 1.594 | 0.797 | 8.321 | <0.001 |
| Error | 46 | 4.407 | 0.096 | ||
| Total | 51 | 14.578 | |||
| (d) Temporal variability in consumer abundance | |||||
| Intercept | 1 | 11.091 | 11.091 | 245.913 | <0.001 |
| Region | 2 | 0.705 | 0.353 | 7.818 | 0.001 |
| Treatment | 1 | 0.955 | 0.955 | 21.168 | <0.001 |
| Region×Treatment | 2 | 0.551 | 0.275 | 6.107 | 0.004 |
| Error | 46 | 2.075 | 0.045 | ||
| Total | 51 | 4.652 | |||
| (e) Variance ratio, VR | |||||
| Intercept | 1 | 60.348 | 60.348 | 445.148 | <0.001 |
| Region | 2 | 3.636 | 1.818 | 13.411 | <0.001 |
| Treatment | 1 | 1.098 | 1.098 | 8.101 | 0.007 |
| Region×Treatment | 2 | 0.298 | 0.149 | 1.098 | 0.342 |
| Error | 46 | 6.236 | 0.136 | ||
| Total | 51 | 11.763 | |||
Extinction frequency was affected by thermal variability with 28% more extinctions on average with thermal variability than under constant temperature across all regions (F1,51 = 20.93, P < 0.001). There was no main effect of region on extinction frequency (F1,51 = 2.667, P = 0.08) and no significant interaction between region and thermal variability (F1,51 = 1.555, P = 0.222). Extinction frequency was greater under TEMPVAR than in TEMPCONSTANT in the temperate communities (P = 0.04) and tropical communities (P = 0.01).
Total consumer abundance and functional group abundance
Total consumer abundance declined in the tropical and temperate communities but not in the sub-Arctic communities, leading to a significant interaction between region and thermal variability (F2,51 = 23.134; P < 0.001; Table 1; Fig. 1c). The magnitude of the decline in consumer abundance was greatest in the tropical communities.
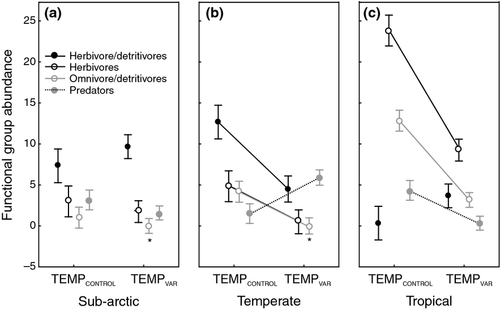
Thermal variability led to changes in the functional composition of communities in terms of relative abundance and the identity of the numerically dominant functional group. Consistent changes across latitudes in functional group abundance with TEMPVAR were only observed for herbivores and omnivore/detritivores (Table 2; Fig. 2) with consistent decreases in abundance of herbivores (F1,51 = 41.79, P < 0.001) and omnivore/detritivores (F1,51 = 95.19, P < 0.001) across all communities. Complete extinction under TEMPVAR was observed for the omnivore/detritivores in the temperate and sub-Arctic communities. No functional group went completely extinct under TEMPVAR in tropical communities, although abundance of predators declined to one individual on average in the 50 mL sub-sample.
| df | SS | MS | F | P | |
|---|---|---|---|---|---|
| (a) Herbivores (log) | |||||
| Intercept | 1 | 22.546 | 22.546 | 589.257 | <0.001 |
| Region | 2 | 5.942 | 2.971 | 77.656 | <0.001 |
| Treatment | 1 | 1.599 | 1.599 | 41.790 | <0.001 |
| Region×Treatment | 2 | 0.301 | 0.150 | 3.929 | 0.027 |
| Error | 46 | 1.760 | 0.038 | ||
| Total | 51 | 9.941 | |||
| (b) Herbivore/detritivores (log) | |||||
| Intercept | 1 | 25.017 | 25.017 | 469.048 | <0.001 |
| Region | 2 | 4.269 | 2.135 | 40.020 | <0.001 |
| Treatment | 1 | 0.031 | 0.031 | 0.576 | 0.452 |
| Region×Treatment | 2 | 1.286 | 0.643 | 12.057 | <0.001 |
| Error | 46 | 2.453 | 0.053 | ||
| Total | 51 | 7.494 | |||
| (c) Omnivore/detritivores (log) | |||||
| Intercept | 1 | 8.768 | 8.768 | 299.187 | <0.001 |
| Region | 2 | 4.558 | 2.279 | 77.771 | <0.001 |
| Treatment | 1 | 2.790 | 2.790 | 95.188 | <0.001 |
| Region×Treatment | 2 | 0.407 | 0.203 | 6.941 | 0.002 |
| Error | 46 | 1.348 | 0.029 | ||
| Total | 51 | 8.959 | |||
| (d) Predators (log) | |||||
| Intercept | 1 | 10.797 | 10.797 | 255.728 | <0.001 |
| Region | 2 | 0.325 | 0.162 | 3.844 | 0.029 |
| Treatment | 1 | 0.155 | 0.155 | 3.682 | 0.061 |
| Region×Treatment | 2 | 2.274 | 1.137 | 26.928 | <0.001 |
| Error | 46 | 1.942 | 0.042 | ||
| Total | 51 | 5.524 | |||
In contrast to the consistent cross-latitude decreases in the abundance of herbivores and omnivore/detritivores under TEMPVAR across all three communities, abundance of the herbivore/detritivore (P = 0.452) and predator (P = 0.061) functional groups did not show consistent changes in abundance across latitudes (Table 2; Fig. 2). Herbivore/detritivores in sub-Arctic (P = 0.851) were not affected by TEMPVAR although in temperate (P = 0.022) and tropical (P = 0.011) communities abundance of herbivore/detritivores decreased (P < 0.001) with TEMPVAR. Abundance of predators was not significantly affected by TEMPVAR in sub-Arctic communities (P = 0.362) whereas in tropical communities, abundance of predators decreased (P < 0.001) and in temperate communities, predator abundance increased (P < 0.001). In tropical communities, these changes in functional group abundance did not alter the relative abundance or dominance of the different functional groups in the communities. Instead, abundance declined for all four functional groups maintaining the same relative abundance profile. In the sub-Arctic communities, relative abundances were similar for TEMPCONSTANT and TEMPVAR except for the extinction of the omnivore/detritivore group. In contrast, in the temperate communities TEMPVAR led to significant changes in relative abundance of different functional groups with a shift from the dominant functional group of herbivore/detritivores in TEMPCONSTANT to predators as the dominant functional group in terms of numbers of individuals in TEMPVAR. Herbivores also declined to very low numbers and, as reported above, omnivore/detritivores went extinct in all replicates in the temperate communities.
Variance ratios: strength of interspecific interactions
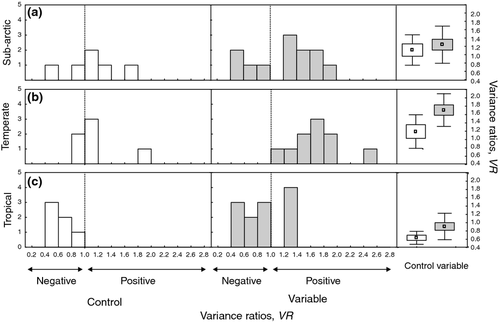
There was a significant difference in the whole-microcosms variance ratios, VR, between TEMPCONSTANT and TEMPVAR across all regions (F1,51 = 8.1, P = 0.007; Table 1; Fig. 3). There was also a significant regional effect with higher VR in temperate microcosms, followed by sub-Arctic and tropical microcosms (F1,51 = 13.41, P < 0.0001). Distributions of VR by region and treatment show that in the TEMPVAR treatment the variance ratios are right-shifted (more positive) relative to TEMPCONSTANT. There was no significant interaction effect between region and treatment for VR (P = 0.342).
Temporal variability in abundance
Thermal variability also showed a significant interaction with region for temporal variability in abundance showing that variability in temperature destabilized community dynamics in the temperate and tropical communities but not in the sub-Arctic communities (F2,51 = 6.107, P < 0.004; Table 1; Fig. 1d).
Ecosystem function
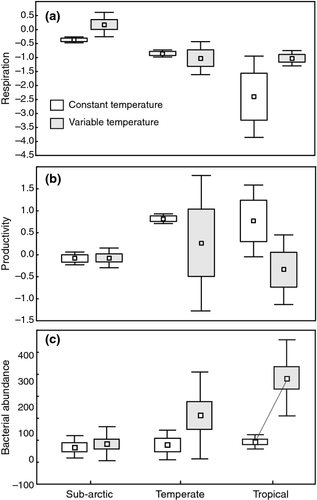
Thermal variability also had effects on ecosystem functioning, however, changes in functioning were much weaker than changes in community structure. Region × treatment effects were not observed for either respiration (P = 0.098) or productivity (P = 0.411; Table 3; Fig. 4). Across all regions, respiration (F1,22 = 4.84, P = 0.042) and bacterial abundance (F1,50 = 14.23, P = 0.0005) were significantly greater in TEMPVAR than in TEMPCONSTANT, however, post hoc tukey tests showed no significant difference between TEMPVAR and TEMPCONSTANT within regions. There was a significant region x treatment interaction effect for bacterial abundances (F2,50 = 4.65, P = 0.015; Table 3; Fig. 4c), with no differences in bacterial abundance in the sub-Arctic or temperate communities, and a significant increase in abundance in the tropical communities (P = 0.0005). Although mean productivity did not differ significantly between TEMPCONSTANT and TEMPVAR, in the temperate communities there was a large increase in across-replicate variability in productivity in TEMPVAR. In TEMPCONSTANT, productivity varied from 0.71 to 0.93 (±0.11 SD) whereas in TEMPVAR (Fig. 4b) productivity varied from −1.92 to 1.6 (±1.53 SD).
| df | SS | MS | F | P | |
|---|---|---|---|---|---|
| (a) Respiration | |||||
| Intercept | 1 | 18.051 | 18.051 | 47.282 | <0.001 |
| Region | 2 | 9.723 | 4.862 | 12.734 | <0.001 |
| Treatment | 1 | 1.848 | 1.848 | 4.841 | 0.042 |
| Region×Treatment | 2 | 2.041 | 1.021 | 2.674 | 0.098 |
| Error | 17 | 6.490 | 0.382 | ||
| Total | 22 | 20.892 | |||
| (b) Productivity | |||||
| Intercept | 1 | 1.122 | 1.122 | 1.807 | 0.196 |
| Region | 2 | 1.437 | 0.718 | 1.157 | 0.338 |
| Treatment | 1 | 1.625 | 1.625 | 2.618 | 0.124 |
| Region×Treatment | 2 | 1.163 | 0.582 | 0.937 | 0.411 |
| Error | 17 | 10.553 | 0.621 | ||
| Total | 22 | 14.526 | |||
| (c) Bacterial abundance | |||||
| Intercept | 1 | 646 037.292 | 646 037.292 | 51.903 | <0.001 |
| Region | 2 | 159 053.282 | 79 526.641 | 6.389 | 0.004 |
| Treatment | 1 | 177 111.259 | 177 111.259 | 14.229 | <0.001 |
| Region×Treatment | 2 | 115 736.105 | 57 868.053 | 4.649 | 0.015 |
| Error | 45 | 560 117.498 | 12 447.056 | ||
| Total | 50 | 1 130 396.305 | |||
Discussion
Increases in mean and maximum temperatures due to climate change will have effects at many levels of biological organization (Pörtner et al., 2006; Folguera et al., 2008, 2010; Dillon et al., 2010; Traill et al., 2010; Walther, 2010). Climate change, however, does not only lead to increases in temperature per se, but can also lead to changes in thermal regimes. Our results show strong effects of increases in thermal variability on community structure, dynamics, and ecosystem functioning in zooplankton communities as well as significant differences in how ecological communities’ respond to thermal variability along a latitudinal gradient.
Across all communities, thermal variability led to significant decreases in species richness, increases in extinction frequency, decreases in total consumer abundances, and increases in the temporal variability of consumer abundances. Community covariances also became more positive under thermal variability. The abundance of herbivores and omnivore/detritivores increased with thermal variability, whereas abundances of herbivore/detritivores and predators did not show a main effect of thermal variability. Changes in functioning were also observed with significant increases in bacterial abundance and respiration under thermal variability, but no main effect of thermal variability on productivity, Of the nine structural and dynamical responses to increased thermal variability measured, seven showed a significant effect of thermal variability as a main effect and two of the three ecosystem function responses showed a significant main effect of thermal variability. Importantly, however, significant interactions between thermal variability and latitude were also observed for seven of nine structural and dynamical responses and one functional response, bacterial abundance.
It is becoming increasingly recognized that the effects of climate change may differ along latitudinal gradients. For example, increases in temperature have been suggested to have more deleterious effects on tropical communities, as tropical species are already living closer to their thermal maxima (Deutsch et al., 2008; Tewksbury et al., 2008; Dillon et al., 2010). Metabolic adaptations to different thermal regimes over evolutionary time may also play a role (Pörtner, 2002; Pörtner et al., 2005). The consequences of differences in adaptations to different thermal regimes over evolutionary time scales may affect how robust different communities are to warming as well as lead to latitudinal differences in the structural and functional features of communities. Few studies have addressed the role of thermal variability on whole-community responses. Significant interactions between thermal variability and latitude were observed for species richness and total consumer abundance, which declined in tropical and temperate communities but not in sub-Arctic communities, and for temporal variability in community abundance, which increased in temperate and tropical communities but not in sub-Arctic communities. Abundance of the four functional groups also showed strong thermal variability × latitude interactions. Herbivore/detritivore abundance decreased in temperate communities but not tropical or sub-Arctic communities. Predator abundance decreased in tropical communities, increased in temperate communities, and was unaffected by thermal variability in sub-Arctic communities.
We observed a significant interaction between thermal variability and latitude for species richness, with declines in tropical and temperate communities but not in sub-Arctic communities, Extinction frequency, however, did not show a significant interaction effect. Extinction frequency increased with thermal variability across all latitudes, with significant increases observed under thermal variability in the tropical and temperate communities. The effect of thermal variability on extinction frequency or persistence has been well studied in the context of environmental variability (Ripa & Lundberg, 1996; Petchey et al., 1997; Pike et al., 2004; Schwager et al., 2006; Ruokolainen & Fowler, 2008). One potential explanation for this higher magnitude species loss in tropical and temperate communities relative to sub-Arctic communities is that populations with higher growth rates, such as the tropical species, were able to track, or respond, to changes in environmental conditions more strongly (Gonzalez & deFeo, 2007) than the sub-Arctic species, which were larger on average and had longer generation times. Higher mean temperature in both the tropical and temperate communities also likely played a role by increasing rates of growth and reproduction more in temperate and tropical species than in sub-Arctic species.
Changes in species richness and extinction frequency with thermal variability were also accompanied by decreases in total consumer abundance, however, the significant interaction effect showed that thermal variability had different effects depending on latitude. Consumer abundance declined in the tropical and temperate communities but not in the sub-Arctic communities. Consumer abundance has been predicted to decrease with increasing thermal variability as high and low temperature events can be stressful to species, affect rates of metabolism and growth, and change the intensity of resource-use (Rall et al., 2010). Sub-Arctic communities may have been less sensitive to changes in total consumer abundance because for species that live in sub-optimal conditions, such as sub-Arctic zooplankton, variability in temperature that includes some time periods of higher than average temperatures may provide important opportunities for growth and reproduction and thus persistence (Callaghan et al., 2004). Species that inhabit Polar Regions have also been shown to have higher intrinsic, i.e. basal, rates of metabolism than species from warmer regions, a metabolic adaptation that allows these species to take advantage of short periods of more favourable conditions (Addo-Bediako et al., 2002). It has also been suggested that species with higher thermal tolerance, such as the sub-Arctic species, should experience weaker changes in abundance with increased thermal variability than species that are less thermally tolerant (Wittman et al., 2010). The strong decreases in community abundance observed in the tropical and temperate communities may also be related to metabolic costs associated with acclimation which are associated with decreases in maximum reproduction and per capita growth rate which may decrease as thermal variability increases, particularly at high temperatures that are beyond thermal optima (Estay et al., 2011). Due to the wide variability in temperatures experienced by the sub-Arctic species in their natural habitats, a 4 °C increase may not have been sufficient to push the sub-Arctic communities beyond their thermal optima.
Thermal sensitivity has been shown to vary strongly for different functional groups of ectotherms. For example, herbivores and predators are often found to be disproportionately affected by warming (Petchey et al., 1999; Voigt et al., 2003; Berg et al., 2010). Likewise, Rose & Caron (2007) have shown that growth rates of herbivorous protists are considerably lower than the rates the bacterivorous protists for a given temperature when standardized for cell size. The only functional groups to respond similarly to thermal variability across all three communities were herbivores and omnivore/detritivores, both of which decreased in abundance with thermal variability. Declines in herbivore abundance with warming have previously been reported in protist microcosms (Petchey et al., 1999). Large declines in abundance of the omnivore/detritivore functional group, which was composed of ostracods that fed on dead and decaying consumers, was unexpected and may have been due to higher stress responses to thermal variability. Omnivore/detritivores went extinct in the sub-Arctic communities, one of the few significant changes observed with thermal variability in the sub-Arctic, and also went extinct in the temperate communities. In the tropical communities, abundance of omnivore/detritivores declined to very low abundances. Predator abundance declined with thermal variability in tropical communities, increased in temperate communities, and was unaffected in sub-Arctic communities. These results suggest that how predators, which in our microcosms were cyclopoid copepods, respond to thermal variability, is not due to unique features associated with their trophic role per se, but is instead due to either differences in thermal sensitivity or differences in food-web structures across the latitudinal gradient.
Thermal variability had the most consistent effect on functional group abundances in the tropical communities, with all functional groups showing a trend towards declines in abundance, whereas in the temperate communities there was a shift in the relative abundance of different functional groups from the dominance of herbivore/detritivores under constant temperature to dominance of predators under thermal variability. This type of change in functional group dominance, which has been called metastability, has been shown to result from environmental fluctuations that lead to a shift in species composition where previously dominant species are replaced with species that are optimally or at least better adapted to new environmental constraints (Gonzalez & deFeo, 2007).
The strength and direction of community covariances was most affected by thermal variability in the temperate communities, shifting covariances from a mix of positive and negative covariances to only positive covariances. In the tropical communities negative covariances were maintained for some microcosms. In the sub-Arctic communities a mix of positive and negative community covariances were observed under both constant temperate and thermal variability. In general, when environmental stress is high, interactions between populations in communities tend to become more positive, potentially reflecting weakening of competitive interactions (Menge & Sutherland, 1987; Bertness & Ewanchuk, 2002).
In addition to these structural changes we also observed a significant interaction effect between thermal variability and latitude on community dynamics. Temporal variability in community abundance was higher with thermal variability in all three communities, however, significant increases were only observed in the temperate and tropical communities. Increased temporal variability in the temperate and tropical communities with thermal variability likely resulted from a combination of increased extinction frequency under thermal variability and shifts in the directions of community covariances from a mixture of positive and negative under constant conditions to more positive under thermal variability. Periods of high temperature would also have increased rates of growth, reproduction, and foraging (Vasseur & McCann, 2005; Rall et al., 2010), all of which could have had an impact on population dynamics. Few empirical tests of environmental fluctuations on temporal variability have been conducted for aggregate community properties such as temporal variability in abundance. Gonzalez & Descamps-Julien (2004) showed that in algal-rotifer microcosms, temperature fluctuations induced lower community covariance thus stabilizing biomass. In contrast, our results show an increase in community covariance with thermal variability and a de-stabilizing effect. Using theoretical methods Vasseur (2007) showed that as variability moved from random to autocorrelated, variability of consumers increased although variability of resources decreased and Gonzalez & deFeo (2007) that autocorrelated environmental variability results in decreases in stability 50 times greater than the stabilizing effect of species richness.
Thermal variability also affected some aspects of ecosystem functioning, showing significant main effects of thermal variability on respiration and bacterial abundances, both of which increased with thermal variability, but not productivity. Significant interaction effects between thermal variability and latitude were also observed for bacterial abundances which increased significantly in the tropical communities but not in the temperate or sub-Arctic communities. Theory predicts that environmental variance should affect ecosystem functioning, particularly those functions that are temperature dependent (Ruel & Ayres, 1999; Brown et al., 2004; Yvon-Durocher et al., 2010; Sierra et al., 2011). It is difficult to assess the generality of our responses as similar empirical studies, using similar response variables, have not been conducted.
Overall our results using zooplankton and meiofauna communities from sub-Arctic, temperate, and tropical locations showed that thermal variability leads to significant changes in community structure, function, and dynamics and that nine of 12 structural, dynamical and ecosystem function responses differed with latitude. Tropical and temperate communities responded strongly to thermal variability with major changes in community structure and dynamics relative to constant temperature controls. In contrast, in the sub-Arctic communities the only significant effect of thermal variability was a small reduction in species richness driven by the extinction of the omnivore/detritivore functional group.
A number of potential explanations exist for the high robustness of the sub-Arctic communities to thermal variability relative to the temperate and tropical communities: polar species may have adapted over evolutionary time to high environmental variability (Pörtner et al., 2005; Verde et al., 2006); for species that live in sub-optimal conditions, such as sub-Arctic zooplankton, variability in temperature that includes some time periods of higher than average temperatures, may provide important opportunities for growth and reproduction and thus persistence (Callaghan et al., 2004). Species that inhabit Polar Regions have also been shown to have higher intrinsic rates of metabolism than species from warmer regions, a metabolic adaptation that allows these species to take advantage of short periods of more favourable conditions (Addo-Bediako et al., 2002). Evolution of reaction norms may also differ for species that have adapted to different thermal regimes (Angilletta et al., 2003). For example, species adapted to colder conditions generally have faster growth rates at lower temperatures (Angilletta et al., 2003). In addition to reaction norms evolving differently for species adapted to different thermal regimes, differences have also been observed for the relationship between growth and temperature for grazers and their resources for species at high latitudes relative to warmer latitudes, with greater temperature-related growth constraints on consumers than their algal resources, which can lead to fundamentally different food-web dynamics at high latitudes (Rose & Caron, 2007) and potentially greater stability with increasing temperature in high-latitude communities.
In contrast, the similar responses of temperate and tropical communities to thermal variability were unexpected. Environmental variability in temperature is high in temperate regions relative to that in tropical regions (Chown et al., 2004; Sunday et al., 2011) thus we initially predicted that species from the temperate region should be less sensitive to thermal variability than species from the tropical regions. Our results did not support this prediction. Temperate and tropical community responses to thermal variability were of similar magnitude and of the nine responses affected by thermal variability only one, bacterial abundance was significantly affected by thermal variability in tropical communities but not in temperate communities.
Recent studies have suggested that the effects of increases in temperature will be greater for communities in temperate and in particular tropical regions than in Polar Regions despite the greater magnitude of warming that is predicted to occur in Polar Regions. For example, Dillon et al. (2010) have shown that the consequences of increased temperature on metabolic rate will be greatest for ectotherms in tropical and temperate regions and much less severe in Polar Regions. Likewise, Deutsch et al. (2008) have suggested that extinction risk will be greatest for terrestrial ectotherms in tropical environments as organisms in these areas are already living closer to their thermal maxima than species at higher latitudes. Our results suggest that temperate and tropical communities may also be more sensitive to thermal variability.
A number of facets of our experimental design may limit the applicability of our results to other ecological communities. For example, the relatively low species richness of the laboratory microcosms with a range of 5.5–7 zooplankton and meiofaunal species on average in the sub-Arctic and tropical communities, respectively, is much lower than the species counts in natural ecosystems. Likewise, responses to thermal variability in supralittoral rock pool species may differ from other ectotherm species. However, the organisms used in the experiments are widely distributed and common inhabitants of many freshwater habitats including lakes and ponds and zooplankton are important components of the food-webs of most aquatic habitats.
It is becoming increasingly recognized that the effects of climate change may differ along latitudinal gradients (Pörtner, 2002; Pörtner et al., 2005; Deutsch et al., 2008; Tewksbury et al., 2008; Dillon et al., 2010), however, the possibility that communities will respond differently to changes in the variability of thermal regimes along latitudinal gradients has not previously been experimentally addressed. Our results highlight potential differences in community structure, dynamics, and function that can result from increased thermal variability in zooplankton and meiofauna that have adapted over evolutionary time to different environmental conditions. More broadly, our results also suggest that changes in thermal variability are as important to consider as changes in average temperature to predict how ecological systems will respond to climate change, a conclusion that has also been reached in studies of the effects of thermal variability at the organismal level (Folguera et al., 2011).
Acknowledgements
We would like to thank Vanessa Brisson and Nicholas Lacusta for assisting with the experiment. Veronik Campbell commented and improved on an earlier version of the manuscript. TNR is supported by an NSERC Discovery Grant.




