Comparing carbon sequestration in temperate freshwater wetland communities
Abstract
High productivity and waterlogged conditions make many freshwater wetlands significant carbon sinks. Most wetland carbon studies focus on boreal peatlands, however, with less attention paid to other climates and to the effects of hydrogeomorphic settings and the importance of wetland vegetation communities on carbon sequestration. This study compares six temperate wetland communities in Ohio that belong to two distinct hydrogeomorphic types: an isolated depressional wetland site connected to the groundwater table, and a riverine flow-through wetland site that receives water from an agricultural watershed. Three cores were extracted in each community and analyzed for total carbon content to determine the soil carbon pool. Sequestration rates were determined by radiometric dating with 137Cs and 210Pb on a set of composite cores extracted in each of the six communities. Cores were also extracted in uplands adjacent to the wetlands at each site. Wetland communities had accretion rates ranging from 3.0 to 6.2 mm yr−1. The depressional wetland sites had higher (P < 0.001) organic content (146 ± 4.2 gC kg−1) and lower (P < 0.001) bulk density (0.55 ± 0.01 Mg m−3) than the riverine ones (50.1 ± 6.9 gC kg−1 and 0.74 ± 0.06 Mg m−3). The soil carbon was 98–99% organic in the isolated depressional wetland communities and 85–98% organic in the riverine ones. The depressional wetland communities sequestered 317 ± 93 gC m−2 yr−1, more (P < 0.01) than the riverine communities that sequestered 140 ± 16 gC m−2 yr−1. The highest sequestration rate was found in the Quercus palustris forested wetland community (473 gC m−2 yr−1), while the wetland community dominated by water lotus (Nelumbo lutea) was the most efficient of the riverine communities, sequestering 160 gC m−2 yr−1. These differences in sequestration suggest the importance of addressing wetland types and communities in more detail when assessing the role of wetlands as carbon sequestering systems in global carbon budgets.
Introduction
Wetlands have an important role in global carbon cycles. They are highly productive ecosystems that accumulate large amounts of organic matter in the soil, functioning as significant carbon sinks (Odum et al., 1995; Chmura et al., 2003; Mitra et al., 2005). Despite covering 6–8% of the freshwater surface, wetlands are estimated to account for one-third of the world's organic soil carbon pool (Mitra et al., 2005; Lal, 2007; Mitsch & Gosselink, 2007). Part of the carbon that the wetland produces is released to the atmosphere as methane (about 3% of the net wetland production; Schlesinger, 1997; Jokic et al., 2003), a powerful greenhouse gas. Wetlands are estimated to be responsible for 25% of the current yearly methane emissions to the atmosphere, representing 60% of the naturally originated methane emitted each year (Bartlett & Harris, 1993; Whalen, 2005; IPCC, 2007).
The amount of carbon that a wetland stores and emits every year depends greatly on the hydrogeochemical characteristics of the ecosystem, which, in turn, determine the wetland vegetation communities. Therefore, to estimate with precision a wetland's carbon pool and carbon sequestration capacity, it would be more accurate to differentiate between wetland types, especially if wetlands are to be used as a carbon–sequestering systems to reduce net greenhouse gas emissions (Stern, 2007). Despite the known importance of wetlands in global carbon budgets, the lack of systematic studies and adequate models, and the limited information on their carbon turnover rates and temporal dynamics, has probably led to an underestimation of their relevance to global and regional levels, to the point that they are typically omitted from large-scale assessments (Trenttin & Jurgensen, 2003).
Wetlands are ecosystems defined by the presence of standing water and/or saturated soil during at least part of the year, a condition that is subsequently responsible for the development of specialized vegetation (hydrophytes) and hydric soil (Mitsch & Gosselink, 2007). These factors (hydrology, vegetation, and soil) and their interaction create the signature characteristics of wetland ecosystems and communities, and can be used to differentiate and classify wetland types. The U.S. Fish and Wildlife Service recognizes five wetland systems (marine, estuarine, riverine, lacustrine, and palustrine; Cowardin et al., 1979) while the U.S. Army Corps of Engineers uses a wetland classification system developed by Brinson (1993) based on wetland hydrogeomorphology (HGM classification), that recognizes four geomorphic settings (depressional, riverine, fringe, and extensive peatlands), three water sources (precipitation, groundwater discharge, and surface inflow), and three hydrodynamics (vertical fluctuation, unidirectional flow, and bidirectional flow). Much of the rest of the world uses the 32 Ramsar wetland classes identified mainly for wildlife and biodiversity (http://www.ramsar.org). Wetland plant communities (plant associations generally described by the dominant plant species) adapt to the conditions of particular wetland zones and often reflect an environmental gradient as they change through the wetland from deep water to the upland (Boutin & Keddy, 1993; Mitsch & Gosselink, 2007). Recognition of a wetland community can be used to identify not only the plant species, but also hydrogeomorphic features that define the wetland site.
In this study, we compare the ability of wetlands to accumulate carbon in two hydrogeomorphic conditions and several vegetation communities by estimating their soil carbon pool and sediment accretion rate. Given the depositional nature of wetlands, a frequently used technique to estimate accretion rates is radiometric dating with 137Cs and 210Pb, two independent atmospherically deposited radionuclides of similar half-life that bind strongly to the sediments and accumulate in wetlands, functioning as a reference to date the soil (Appleby & Oldfield, 1978; Craft & Richardson, 1993; Graham et al., 2005; Stark et al., 2006). We determine carbon pools and sequestration rates of six different wetland communities in two different hydrogeomorphic settings of temperate humid Ohio – one flow-through riverine, inland deltaic wetland with surface inflow and directional flow (Ramsar classes L and M) and one isolated palustrine and depressional wetland, with vertical fluctuation of the water table mainly fed by groundwater and precipitation (Ramsar classes Ts and Xf). The communities associated with the riverine system would be likely to have high total carbon accumulation because of additional external organic inputs and nutrients in the inflow water, while wetland communities on depressional and isolated sites are usually less productive than flow-through or slow-flow ones (Mitsch & Ewel, 1979; Mitsch et al., 1991; Conner & Day, 1992; Watt & Golladay, 1999; Cronk & Fennessy, 2001; Wilson et al., 2005). On the other hand, we might expect to find high carbon accumulation in a forested wetland community that is intermittently flooded because of the recalcitrant nature of the organic matter produced there (high in lignin and cellulose, two organic compounds that are harder to degrade by microbes due to their complexity; Dalva & Moore, 1991; Schlesinger, 1997), and because of the protection from direct wind and sun exposure that the tree canopy provides, potentially retarding plant litter decomposition rates (Kirschbaum, 1995; Fierer et al., 2005; Bernal & Mitsch, 2008).
Materials and methods
Site descriptions
Our depressional forested wetland communities are located in a suburban area in central Ohio (40°0′ N, 82°50′ W). These woodland pools are mainly fed by groundwater and precipitation (Korfel et al., 2010), and their surface and groundwater water tables fluctuate with precipitation events. Gamble & Mitsch (2009) and Korfel et al. (2010) described the seasonality of these swamp pools as depressional bodies of water that remain saturated all year-round, with permanent standing water in some of the deeper pools that fluctuates vertically, remaining frozen from December to March and drying down for the most part between June and July. The deepest pools reach up to 40 cm of water depth and saturation under driest conditions is found at 5 cm deep in the soil (Korfel et al., 2010). All the pools are isolated and their water remains stagnant rather than flowing. Hydric soils (Pewamo and Carlisle muck) dominate these wetland areas (Natural Resources Conservation Service, U.S. Department of Agriculture, National Cooperative Soil Survey, 2010).
The wetland communities studied for soil carbon accumulation at this site (Table 1) include a permanent pool where cattail grows (Typha spp.), a semipermanently flooded forest site with pin oak (Quercus palustris), and an edge site of buttonbush (Cephalanthus occidentalis). Upland soil samples were also collected in a beech–maple (Fagus spp.–Acer spp.) forest adjacent to the wetland area.
| Wetland community | Dominant vegetation | Hydrogeomorphic category | Hydrologic features | Soil typea | Location |
|---|---|---|---|---|---|
| Shrub | Cephalanthus occidentalis | Depressional, isolated | Intermittently flooded, shallow edge | Pw | Central Ohio |
| Forested | Quercus palustris | Depressional, isolated | Intermittently flooded, shallow | Pw/Cc | Central Ohio |
| Marsh | Typha spp. | Depressional, isolated | Permanently flooded, deep water | Cc | Central Ohio |
| Marsh | Phragmites australis, Scirpus fluviatilis | Riverine, flow-through | Intermittently flooded, shallow edge | FnA | Northern Ohio |
| Mudflat | Leersia oryzoides | Riverine, flow-through | Intermittently flooded, shallow | FnA/Aa | Northern Ohio |
| Floating bed | Nelumbo lutea | Riverine, flow-through | Permanently flooded, deep water | FnA | Northern Ohio |
- a Pw (Pewamo), Cc (Carlisle muck), FnA (Fluvaquents), Aa (Adrian muck).
Our flow-through wetland communities are located in the Old Woman Creek State Natural Preserve (41°22′ N, 82°31′ W), in the southwestern shores of Lake Erie. The site is a 230-ha park that connects a 69-km2 agricultural watershed (75% cropland) with the lake through a 56-ha wetland that comprises the lower 3 km of the Old Woman Creek (Mitsch & Reeder, 1991; Francko & Whyte, 1999; Herdendorf et al., 2006). They receive water from the watershed (unidirectional flow) and from occasional wind-driven seiches when the outlet mouth is not blocked by the formation of a small barrier beach (bidirectional flow). The barrier is present almost half of the time due to lake wave action, and is usually broken by storm flows from the watershed, giving these wetland communities a pulsing hydrology and an ability to exchange nutrients and sediments with the watershed and the lake. Water depths range between 0.3 and 1.6 m at the mouth, with most of the wetland basin at about 0.5 ± 0.1–0.2 m deep daily (Herdendorf, 1990; Herdendorf et al., 2006). The soils in the wetland are hydric (Fluvaquents and Adrian muck; Natural Resources Conservation Service, U.S. Department of Agriculture, National Cooperative Soil Survey, 2010). In the deepest areas of the wetland, floating beds of Nelumbo lutea (American water lotus) dominate the deep water zones that are guarded from the wind (Klarer & Millie, 1992; Whyte et al., 2003; Herdendorf et al., 2006). Emergent plants such as Phragmites australis (common reed) and Typha angustifolia (narrow-leaved cattail), and to a lesser extent Schoenoplectus tabernamontani (softstem bulrush) and Scirpus fluviatilis (river bulrush) grow in saturated or shallowly submerged soils, up to 50 cm of standing water (Klarer & Millie, 1992; Whyte et al., 2003; Herdendorf et al., 2006). The mudflats on the shallow part of the wetland (<10 cm of standing water) are characterized by S. fluviatilis (river bulrush) and dense stands of Leersia oryzoides (rice cutgrass), as described by Whyte et al. (2003) and Herdendorf et al. (2006). The upland areas surrounding the wetland are a mixed hardwood forest dominated by red oak (Quercus rubra), pin oak (Q. palustris), white ash (Fraxinus americana), and buttonbush (C. occidentalis).
Samples were collected in the three wetland communities at this riverine site: the deep water floating beds of N. lutea, the emergent vegetation marshes dominated by P. australis and S. fluviatilis, and the mudflats dominated by L. oryzoides (Table 1). The upland forest adjacent to the wetland and creek bank of the stream that connects the wetland to the watershed were also sampled.
Soil sampling and samples preparation
Two sets of samples were collected in each of the six wetland communities (units of stratified sampling), one for radiometric analysis and the other for bulk density and carbon content determination. For radiometric analysis, a composite sample consisting of three 7-cm diameter sediment cores, up to 36 cm deep. The three cores for a composite sample were spaced within 40 cm to include variation of deposition in the sample area (Isaksson et al., 2001; Stark et al., 2006) and, divided in situ into 2-cm-thick increments. Corresponding layers were pooled together into one sample per layer and packed in sealed containers (Bernal & Mitsch, 2008). Triplicated soil samples (7 cm in diameter, 35 cm long) were extracted for the determination of bulk density and carbon content in each wetland community, divided in the field into 5 cm increments, and packed in sealed containers. One set of triplicated samples was also collected in the upland area adjacent to these wetlands. These upland samples were 10 cm diameter, 35 cm long, and were collected by the core method described by Grossman & Reinsch (2002). Upland samples were collected for carbon pool determination as a reference site for each wetland. Another extra set was collected in the creek bank feeding the wetland of Old Woman Creek, right in the inflow of the wetland basin. This set of creek cores included samples for radiometric and carbon analysis, following the procedure described above. Every soil sample taken was stored under 4 °C until analysis to minimize losses from volatilization and microbial activity, ground and passed through a 2 mm sieve after been oven-dried, and homogenized (Bernal & Mitsch, 2008). Samples collected from radiometric dating were dried at 105 °C until constant weight was reached (Craft & Richardson, 1993; Grossman & Reinsch, 2002), while the samples for carbon analysis were dried at 60 °C until constant weight to avoid potential oxidation of carbon in very rich organic soils (Grossman & Reinsch, 2002).
Estimation of sediment accretion rates
Accretion rates in the soil were determined non-destructively with 137Cs and 210Pb activity (pCi, 10−12 Ci) in each 2 cm increment soil sample by γ spectrometry for 24 h (Craft & Richardson, 1993; Smith et al., 2000; Graham et al., 2005) at 661.7 and 46.5 keV respectively, using a high efficiency germanium detector (Canberra, GL 2820R; Canberra Industries, Inc., Meriden, CT, USA). Radiocesium (137Cs) is a man-made fallout radionuclide (30.1 years half-life) worldwide distributed as consequence of deposition from atmospheric nuclear weapon tests (Smith et al., 2000; Ilus & Saxén, 2005). According to its depositional pattern, 1964 had the highest 137Cs deposition on the globe. Once in the soil, 137Cs binds strongly to the sediment and moves with it, remaining unaltered and making it a radionuclide widely used as tracer in dating studies, especially successful in depositional environments such as wetlands and floodplains (Yeager & Santschi, 2003; Ilus & Saxén, 2005; Stark et al., 2006). The identification of the layer in the soil profile with the peak in the activity is assumed to correspond to the year 1964. Thus, the sediment accumulated in the wetland since that year can be estimated, and the accretion rate calculated assuming constant sedimentation rate, unless evidence in the profile of the opposite (Craft & Richardson, 1993; Craft & Casey, 2000; Graham et al., 2005; Stark et al., 2006).
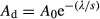 (1)
(1)Determination of soil carbon content and carbon sequestration rate
 (2)
(2)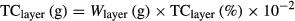 (3)
(3)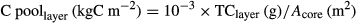 (4)
(4)The carbon accumulation since 1964 is calculated by estimating the total soil carbon pool from the soil surface to the soil layer that, according to the radiometric profile, corresponds to the year 1964.
Statistical analysis
Statistical analyses were performed with spss version 19.0 (SPSS Inc., Chicago, IL, USA) for Macintosh. Student's t-test for independent samples at 95% significant level was used to find differences between carbon concentrations and carbon pools of the six wetland communities, between the hydrogeomorphic wetland types, and between the wetland areas and their respective non-wetland sites (upland in Gahanna Woods, upland and creek bank in Old Woman Creek). Carbon sequestration rates of each wetland were also compared using a t-test, at a significance level of 95% and 90%. Carbon sequestration was compared in every wetland community individually, and within each wetland hydrogeomorphic type, at these same significance levels. Significant differences indicate P ≤ 0.05, ≤0.01 for 95% and 99% confidence, respectively (Fowler et al., 2003).
Results
Soil carbon content
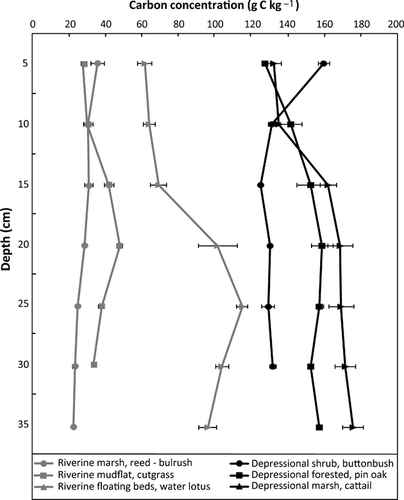
The total carbon concentration in the depressional isolated wetland sites was, on average, about three times the average concentrations of the riverine flow-through sites (146 ± 4.2 and 50.1 ± 6.9 gC kg−1, respectively; Table 2, P < 0.001). However, the concentrations of total carbon of the depressional sites were only 1.5–2 times greater than the floating bed community (water lotus). In every site, soil carbon was predominantly organic (98–99% in the isolated depressional wetland communities, 85–98% in the riverine ones). The carbon content increased with depth of soil in the riverine deep-water community dominated by floating beds of N. lutea and slightly in the mudflat, as well as in the forested community and the cattail marsh of the depressional wetland (Fig. 1). Total carbon concentration in the upland site adjacent to the depressional wetlands was 16.5 ± 6.3 gC kg−1, about 10% of the carbon content in the wetland sites, while the upland at the riverine sites was 7.1 ± 2.9 gC kg−1 (14% of what the wetland area contains; Table 2). A wetland's carbon content was significantly higher than its adjacent uplands in both cases (P < 0.001). The creek feeding the riverine wetland had low carbon content as well (9.1 ± 1.1 gC kg−1) compared to the wetland communities, but it was 30% higher than the carbon at the upland of the riverine site. The carbon content of the creek bank was significantly different to the wetland content (P < 0.001) but not to the upland.
| Wetland community | Hydrogeomorphic category | Total carbon (gC kg−1 soil) | Inorganic carbon (gC kg−1 soil) | Organic carbon (gC kg−1 soil) |
|---|---|---|---|---|
| Shrub | Depressional, isolated | 134.4 ± 5.2 (6) | 1.7 ± 0.1 (6) | 132.7 ± 5.1 (6) |
| Forested | Depressional, isolated | 143.6 ± 6.4 (7) | 2.7 ± 0.6 (7) | 141.0 ± 6.9 (7) |
| Marsh | Depressional, isolated | 159.4 ± 6.8 (7) | 2.7 ± 0.8 (7) | 156.7 ± 7.5 (7) |
| All depressional wetland sites | 146.4 ± 4.2 (20) | 2.4 ± 0.4 (20) | 144.0 ± 4.3 (20) | |
| Upland of depressional wetland | 16.5 ± 6.3 (7) | 1.0 ± 0.1 (7) | 15.5 ± 6.3 (7) | |
| Floating bed | Riverine, flow-through | 86.6 ± 8.4 (7) | 5.4 ± 1.6 (7) | 81.3 ± 9.7 (7) |
| Marsh | Riverine, flow-through | 26.6 ± 1.8 (7) | 4.3 ± 0.9 (7) | 22.3 ± 1.1 (7) |
| Mudflat | Riverine, flow-through | 35.1 ± 3.2 (6) | 0.7 ± 0.3 (6) | 34.9 ± 3.4 (6) |
| All riverine wetland sites | 50.1 ± 6.9 (20) | 3.6 ± 0.8 (20) | 46.6 ± 6.9 (20) | |
| Creek bank | 9.1 ± 1.1 (7) | 0.3 ± 0.0 (7) | 8.9 ± 1.1 (7) | |
| Upland of riverine wetland | 7.1 ± 2.9 (7) | 1.1 ± 0.0 (7) | 5.9 ± 2.9 (7) | |
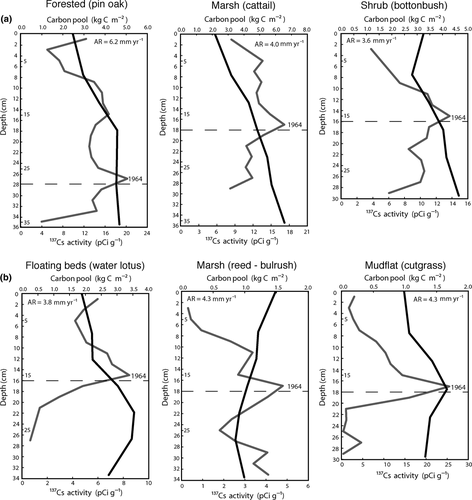
The bulk densities (Table 3) of the depressional wetland communities were very similar, and on average (0.57 ± 0.01 Mg m−3) compared to the density of the riverine ones (0.77 ± 0.06 Mg m−3). Soil bulk density was consistently lower in the wetland sites compared to their adjacent uplands (upland bulk densities ranged between 1.60 and 1.68 Mg m−3). The creek, despite being a non-wetland site, had a bulk density similar to the wetlands (0.84 ± 0.01 Mg m−3), but much lower carbon pool (0.38 ± 0.05 kgC m−2). Differences in carbon pools between wetland and non-wetland sites are evident in these sites, being the non-wetland ones one-fourth to one-third lower than in their corresponding wetland sites (1.01 ± 0.32 kgC m−2 vs. 4.18 ± 0.25 kgC m−2 in the depressional sites, and 0.64 ± 0.22 kgC m−2 vs. 1.50 ± 0.19 kgC m−2 in the riverine ones, Table 3). The average total carbon pool (up to 35 cm, Table 3) of the depressional wetland category was significantly different (P < 0.001) to the average pool of the riverine ones. In all the wetland communities studied, except of the Phragmites – Scirpus marsh, soil carbon increases with depth (Fig. 2).
| Wetland community | Hydrogeomorphic category | Carbon pool (kgC m−2) | Bulk density (Mg m−3) |
|---|---|---|---|
| Shrub | Depressional, isolated | 3.72 ± 0.27 (6) | 0.56 ± 0.05 (6) |
| Forested | Depressional, isolated | 3.93 ± 0.34 (7) | 0.54 ± 0.03 (7) |
| Marsh | Depressional, isolated | 4.62 ± 0.57 (7) | 0.57 ± 0.05 (7) |
| All depressional wetland sites | 4.18 ± 0.25 (20) | 0.57 ± 0.01 (20) | |
| Upland of depressional wetland | 1.01 ± 0.32 (7) | 1.60 ± 0.01 (7) | |
| Marsh | Riverine, flow-through | 1.10 ± 0.08 (7) | 0.82 ± 0.02 (7) |
| Mudflat | Riverine, flow-through | 1.31 ± 0.10 (6) | 0.75 ± 0.02 (6) |
| Floating bed | Riverine, flow-through | 2.73 ± 0.24 (7) | 0.63 ± 0.02 (7) |
| All riverine wetland sites | 1.50 ± 0.19 (20) | 0.77 ± 0.06 (20) | |
| Creek bank | 0.38 ± 0.05 (7) | 0.84 ± 0.01 (7) | |
| Upland of riverine wetland | 0.64 ± 0.22 (7) | 1.68 ± 0. 03 (7) |
Sediment accretion rates
Every wetland core analyzed for 137Cs showed peaks of this radionuclide's activity (Fig. 2), corresponding to 1964. On the contrary, the creek bank had no 137Cs activity detected. Old Woman Creek sites had peaks on 137Cs at very similar depths (18 cm deep in both shallow reed – bulrush sites and intermittently flooded mudflat, and 16 cm deep in the water lotus floating beds), resulting in accretion estimates of 4.3 mm yr−1 for the former, and 3.8 mm yr−1 in the latter (Table 4). Based on these accretion rates, we estimated the weighted average annual sediment accumulation of the entire riverine wetland area of Old Woman Creek to be 28 tons ha−1 yr−1. A previous study by Wilson et al. (2005) in this same wetland area reported a peak on 137Cs activity at 17.5 cm deep (with an accumulation rate of 4.3 mm yr−1), and estimated a sediment sink capacity for the entire Old Woman Creek wetland area of 47%.
| Wetland community | Hydrogeomorphic category | Annual sediment accretion (mm yr−1) | Annual sediment accumulation (tons ha−1 yr−1) | Carbon sequestration rate (gC m−2 yr−1) |
|---|---|---|---|---|
| Shrub | Depressional, isolated | 3.0 | 14 | 202 |
| Forested | Depressional, isolated | 6.2 | 34 | 473 |
| Marsh | Depressional, isolated | 3.4 | 15 | 210 |
| All depressional wetland sites | 4.5 | 23 | 317 | |
| Floating bed | Riverine, flow-through | 3.8 | 25 | 160 |
| Marsh | Riverine, flow-through | 4.3 | 35 | 105 |
| Mudflat | Riverine, flow-through | 4.3 | 31 | 112 |
| All riverine wetland sites | 4.1 | 28 | 140 | |
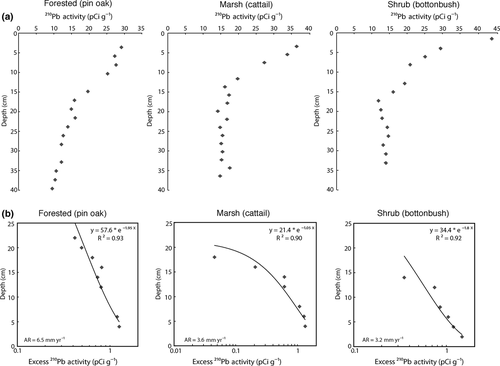
In the depressional wetland sites, the 137Cs activity peaked at 16 cm deep in the buttonbush community and at 18 cm deep in the Typha marsh, both very similar to the depths obtained in the riverine wetland sites (Fig. 2). The forested community dominated by pin oaks, however, had a much deeper peak at 28 cm. Due to the lack of consistency between these three wetland communities and because of the absence of accretion rates from the wetlands in Gahanna Woods reported in literature, we used 210Pb to estimate accretion rates (Fig. 3) and support the values obtained with 137Cs. The resulting accumulation of 14 cm since 1964 in the buttonbush community, 16 cm in the Typha marsh, and 29 cm in the forested site, are similar to the depths obtained by the 137Cs method. Thus, these sites are accumulating 3.2, 3.6, and 6.5 mm yr−1 of soil respectively, and that this entire depressional wetland area is accumulating 23 tons ha−1 yr−1 of sediment (Table 4).
Carbon sequestration rates
On average, the depressional wetland sites accumulated almost 2.5 times more carbon per year than did the riverine sites (317 ± 93 vs. 140 ± 16 gC m−2 yr−1, respectively; Table 4). Sequestrations are weighed averages relative to the surface area of each wetland community. The two general hydrogeomorphic types of wetlands (depressional vs. riverine) are significantly different (P = 0.010). The riverine floating beds of water lotus were the most effective community sequestering carbon in the riverine type of wetland (160 gC m−2 yr−1, about 45% higher than the other two riverine sites). The forested depressional site, however, had the highest carbon sequestration rate of all the communities studied (473 gC m−2 yr−1), more than twice the rates estimated for the other two depressional communities (202 gC m−2 yr−1 in the shrub community and 210 gC m−2 yr−1 in the cattail marsh). The high sequestration rate of the forested community raises the average rate of the depressional sites, but in any case all of the depressional communities sequester carbon at a faster pace than did any of the riverine communities.
Discussion
Carbon profiles in wetland soils
Most of the soil carbon measured was in the organic form (85–99%). Inorganic percentages were slightly higher in the riverine sites (particularly in the marshes), which could be due to external inputs from the river or Lake Erie. In every site, high organic carbon content could be indicating that these wetlands are receiving important organic inputs despite their different placement in the landscape and their different hydrogeomorphic classification. This is usually the case for any type of wetland – these ecosystems are so productive that they have the ability to generate large amounts of organic matter (autochtonous organic source) and store it in a semidecomposed state in the soil due to the anaerobic conditions that water saturation creates (Gorham, 1998; Collins & Kuehl, 2001; Mitsch & Gosselink, 2007). In the cases where the wetlands receive external (allochtonous) organic inputs, their soil carbon content was even higher. For this reason, the carbon content of the wetland is much higher compared to its adjacent upland. The creek bank did not accumulate much carbon because of its slope and the constant fluvial processes of the creek and the occasional seiches, but it was higher than the concentration in the adjacent upland for two possible reasons: (1) being under water for most of the time could be retarding the decomposition of the organic matter contained in its soil, and (2) the water coming back and forth to the creek bank can be a source of stress (erosive agent) but also a constant subsidy of nutrients and organic matter (Odum, 1988; Odum et al., 1995; Mitsch & Gosselink, 2007). All these organic rich sediments that are being eroded from the creek bed are entering the wetland, where they are deposited and temporarily stored in its soil, in a way similar to runoff from the uplands surrounding the wetlands bringing nutrient-rich sediments and organic materials into the wetland.
Soil carbon concentration increased with depth in most of our sites. Chin et al. (1998) measured total dissolved carbon in the pore water of the riverine wetland at Old Woman Creek and also found that the concentration increased with depth, up to a point where it was significantly greater than the concentration in the standing water. This increase is particularly evident in the floating beds of N. lutea, a community that has increased in area considerably in Old Woman Creek in the last two decades (Francko & Whyte, 1999; Whyte et al., 2003; Herdendorf et al., 2006; Cornell & Klarer, 2008), augmenting the net primary productivity of the entire wetland area and thus the organic matter inputs into the wetland soil. The increase of carbon content with depth is frequent in sites where there is significant organic matter accumulation and the decomposition rate (carbon output) is slower than the organic matter deposition (carbon input), as described by Becker-Heidmann & Scharpenseel (1992), Gorham (1998), Schlesinger (1997), and Wolf & Wagner (2005). In the wetlands of this study, that could be due to the constant or semiconstant presence of water slowing down microbial activity (in the cattail marsh, the water lotus marsh, the reed – bulrush marsh, and the mudflat), and because of the recalcitrant nature of the organic matter entering the system (particularly in the depressional wetland sites).
The depressional isolated wetlands of Gahanna Woods are surrounded by a forested wetland community dominated by Q. palustris, which is able to survive because of seasonal flooding (Mitsch et al., 2009). When the site is not flooded the soil is exposed and organic matter accumulated can oxidize. But overall, this site seems to be accumulating enough organic matter to compensate carbon for the loses under aerobic conditions, which could be consequence of the recalcitrant character of the organic matter coming into the soil from the trees. Trees litter reaches the wetland directly or through runoff or wind. Plant litter from woody species is rich in lignin and complex polysaccharides, difficult to degrade by microorganisms (Schlesinger, 1997; Wolf & Wagner, 2005; Berg & McClaugherty, 2008) and thus, remaining longer in the soil and accumulating deeper in the profile than rapidly degradable labile compounds (Schlesinger, 1997; Trumbore, 1997; Wolf & Wagner, 2005). Also, water from precipitation falling through the tree canopy onto the wetland is likely to contain more organic compounds than water from direct precipitation onto the wetland. Dalva & Moore (1991) studied this phenomenon and found that water richer in dissolved organic carbon after passing through the forest canopy. The profiles of these depressional isolated wetland sites are more similar among themselves than are the ones in at the riverine sites, probably because the depressional communities were closer to each other and likely received similar organic inputs from the surrounding forest. The more recalcitrant woody plant input could be a reason why the carbon soil content in the depressional communities is about one order of magnitude higher than in the riverine sites.
Despite the similar or higher carbon accretion rates on the depressional sites compared to the riverine ones, total accumulation of sediment is lower because its soil has lower density. The high annual sediment accretion in the forested community can be interpreted as evidence of the large amount of organic matter accumulating in this site from the trees litter, probably due to its high productivity and recalcitrant character.
Carbon sequestration in freshwater temperate wetlands
The studied forested isolated wetland sites had greater carbon sequestration rates (317 ± 93 gC m−2 yr−1) than did the riverine sites (140 ± 16 gC m−2 yr−1), but also greater variability. The high productivity of this forested site raises the average rate of all of our depressional sites. The carbon sequestration rates of the marsh and shrub depressional communities studied, however, are also higher compared to any of the riverine ones. Such a significant difference between our two wetland types is likely due to the high productivity of the forest in which they are located, cooler temperatures provided by the shade that retards litter decomposition, and the recalcitrance of the organic matter being introduced in the wetland (Fierer et al., 2005). Within the riverine sites, the sites with highest carbon sequestration rate were the floating beds of water lotus (160 gC m−2 yr−1), probably because of the combined effect of the permanent anaerobic conditions in this deepwater area and the reported high productivity of this community. It is therefore important to take into account these differences when comparing the efficiency of wetlands as carbon sequestering systems. The flooding duration and the type of vegetation growing in the wetland are factors that control soil carbon accumulation, and therefore can be managed to enhance the natural ability of a wetland to accumulate carbon, while maintaining other valuable wetland functions and ecosystem services.
To put the carbon sequestration rates obtained in this study in perspective, we compare them with other carbon sequestration rates estimated for freshwater temperate wetlands reported in literature (Table 5). Most of these wetland studies used radiometric dating with 137Cs and/or 210Pb. The range of carbon sequestration in temperate zone inland wetlands range over an order of magnitude from 56 to 504 gC m−2 yr−1 (Table 5). The mean of all these temperate zones estimates is 174 gC m−2 yr−1 and the median is 131 gC m−2 yr−1. Eliminating outliers, most of the numbers range from 100 to 280 gC m−2 yr−1. Our carbon sequestration estimates (317 ± 93 and 140 ± 16 gC m−2 yr−1; Table 4) fall within the range of previously published rates. The average values for isolated, depressional, and/or forested wetlands reported in literature (188 ± 33 gC m−2 yr−1), however, are more similar to those of riverine, flow-through wetlands (164 ± 37 gC m−2 yr−1) than to our forested wetland measurements. All these temperate zone mineral soil wetland estimates are, in general, considerably higher than the average rates of carbon sequestration estimated for boreal peatlands (10–61 gC m−2 yr−1; Mitsch & Gosselink, 2007), a much less productive type of wetlands where most of the wetland carbon studies are focused. Many wetland carbon budgets are based on peatland carbon pools and sequestration rates, which may lead to a general underestimation of the role of wetland ecosystems in global carbon budgets and to the misconception that every wetland has a similar low accretion rate.
| Wetland site | Carbon sequestration rate (g m−2 yr−1) | References |
|---|---|---|
| Isolated, depressional, and/or forested wetlands | ||
| Dismal swamp, Virginia | 105 | Craft et al. (2008) |
| Cypress swamp, Florida | 122 | Craft et al. (2008) |
| Pocosin, North Carolina | 127 | Bridgham & Richardson (1993) |
| Okefernokee peat swamp, Georgia | 25–82 | Schlesinger (1978)*, Craft et al. (2008) |
| Peat meadow, Netherlands | 280 | Hendriks et al. (2007)* |
| Danube floodplain, Austria | 180 | Zehetner et al. (2009) |
| Cypress-gum floodplain, Georgia | 107 | Craft & Casey (2000) |
| Upper St. Johns floodplain, Florida | 117–244 | Brenner et al. (2001) |
| Pin oak swamp, Ohio | 473 | This study |
| Buttonbush swamp, Ohio | 202 | This study |
| Cattail marsh, Ohio | 210 | This study |
| Riverine and/or flow-through wetlands | ||
| Cattail marsh, nutrient enriched, Florida | 264 | Reddy et al. (1993) |
| Cattail-sawgrass marsh, Florida | 104–167 | Reddy et al. (1993), Craft & Richardson (1993) |
| Sawgrass marsh, Florida | 124 | Reddy et al. (1993) |
| Grass-sedge marsh, Georgia | 56 | Craft & Casey (2000) |
| Reed-bulrush marsh, Oregon | 116 | Graham et al. (2005) |
| Reed marsh, Denmark | 504 | Brix et al. (2001)* |
| Arum arrow marsh, Virginia | 97 | Whiting & Chanton (2001)* |
| Anderson tule marsh, California | 106–155 | Kim (2003) |
| Everglades, Florida | 99–190 | Craft & Richardson (1993) |
| Reed-bulrush marsh, Ohio | 105 | This study |
| Cutgrass mudflat, Ohio | 112 | This study |
| Water lotus marsh, Ohio | 160 | This study |
Implications of this study
Wetlands are important sinks of carbon, as evidenced in the significant differences between wetland and non-wetland sites in this study. However, we have shown that not all wetlands are equal in their ability to sequester carbon. In our study the six temperate wetland communities differed in their hydrogeomorphic type and placement in the landscape, thus allowing different vegetation communities to dominate. These differences resulted in significantly different carbon content in the soil (greater in all the depressional wetland sites than in the riverine ones, the greatest in the forested community, and greater in the riverine deeper areas than in the shallower ones). The hydrogeomorphic types of these wetland communities also had significantly different carbon sequestration rates (on average, 2.5 times higher in the depressional wetland areas than in the riverine sites). Not every wetland is equally effective in sequestering carbon; it is important to address differences in wetland types and vegetation communities when assessing the role of wetlands as carbon sinks in global carbon budgets.
Acknowledgements
Support for this project came from the U.S. Environmental Protection Agency (Agreements EM83329801-0 from Cincinnati OH and MX95413108-0 from Gulf of Mexico Program), National Science Foundation (CBET-1033451 and CBET-0829026), the School of Environment and Natural Resources, and the Olentangy River Wetland Research Park. We also appreciate a Sigma Xi (Ohio State Chapter) Grant-In-Aid of Research award. We appreciate site access provided by Gahanna Woods State Nature Preserve and Old Woman Creek State Nature Preserve (Frank Lopez and David Klarer). We thank all the colleagues and friends who advised on and helped with the research. Olentangy River Wetland Research Park Publication 12-03).




