Disturbance, rainfall and contrasting species responses mediated aboveground biomass response to 11 years of CO2 enrichment in a Florida scrub-oak ecosystem
Abstract
This study reports the aboveground biomass response of a fire-regenerated Florida scrub-oak ecosystem exposed to elevated CO2 (1996–2007), from emergence after fire through canopy closure. Eleven years exposure to elevated CO2 caused a 67% increase in aboveground shoot biomass. Growth stimulation was sustained throughout the experiment; although there was significant variability between years. The absolute stimulation of aboveground biomass generally declined over time, reflecting increasing environmental limitations to long-term growth response. Extensive defoliation caused by hurricanes in September 2004 was followed by a strong increase in shoot density in 2005 that may have resulted from reopening the canopy and relocating nitrogen from leaves to the nutrient-poor soil. Biomass response to elevated CO2 was driven primarily by stimulation of growth of the dominant species, Quercus myrtifolia, while Quercus geminata, the other co-dominant oak, displayed no significant CO2 response. Aboveground growth also displayed interannual variation, which was correlated with total annual rainfall. The rainfall × CO2 interaction was partially masked at the community level by species-specific responses: elevated CO2 had an ameliorating effect on Q. myrtifolia growth under water stress. The results of this long-term study not only show that atmospheric CO2 concentration had a consistent stimulating effect on aboveground biomass production, but also showed that available water is the primary driver of interannual variation in shoot growth and that the long-term response to elevated CO2 may have been caused by other factors such as nutrient limitation and disturbance.
Introduction
Increased carbon (C) uptake by plants grown in elevated atmospheric carbon dioxide (CO2) concentration suggests that terrestrial ecosystems will partially mitigate the anthropogenic rise in CO2 (Cramer et al., 2001; Schimel et al., 2001; IPCC, 2007). Hundreds of studies demonstrate initial stimulation of photosynthesis and plant growth to elevated CO2 (for reviews see Drake et al., 1997; Curtis & Wang, 1998; Saxe et al., 1998; Norby et al., 1999; Long et al., 2004). For example, in glasshouse and controlled growth chamber experiments, biomass increased an average of 37% in response to a doubling of ambient CO2 (Poorter, 1993). In field studies, free air CO2 enrichment (FACE) experiments at ∼1.5 times ambient CO2 reported 20% stimulation of biomass (Ainsworth & Long, 2005). The question remains whether this positive biomass response to elevated CO2 will be sustained during the entire growth cycle of perennial species. Norby et al. (2005) demonstrated that forest net primary production (NPP) response to elevated CO2 is consistent across species and ecosystems, but responses of shoot growth are inconsistent. For example, in a closed-canopy sweet gum plantation, sustained stimulation of C uptake under elevated CO2 induced faster C cycling via increased leaf and fine root production and turnover, but yielded no significant increase in standing shoot biomass (Norby et al., 2002). On the other hand, several long-term field studies report sustained biomass stimulation under elevated CO2 (Ainsworth et al., 2003; Rasse et al., 2005; Kimball et al., 2007). In a recent review, Körner (2006) suggests that biomass response to elevated CO2 will decline as resources are depleted in spatially-limited systems. Frequently cited environmental limitations to long-term CO2 stimulation of plant growth are water stress, nitrogen deficiency, and access to light in closing canopies.
Water availability almost universally limits plant growth. Water stress is alleviated by elevated CO2, by reducing stomatal conductance, thereby improving water-use efficiency (Drake et al., 1997; Long et al., 2004). For example, in sweet gum, reductions in water use under elevated CO2 increased annual biomass increment by 4.1% (Wullschleger & Norby, 2001; Wullschleger et al., 2002). Baker et al. (1997) reported a 5–10% reduction in water use in rice (Oryza sativa L.) under CO2 enrichment, which allowed 1–2 days more growth during a 20-day drought cycle. Finally, aboveground biomass stimulation by elevated CO2 was higher during years with more rainfall, while stimulation of root biomass was reduced in a Chesapeake Bay tidal wetland (Erickson et al., 2007).
Nutrient deficiency, specifically nitrogen (N), commonly limits plant growth. Elevated CO2 typically reduces N concentration in plant tissues (McGuire et al., 1995), but growth stimulation can persist through increased nitrogen-use efficiency (Drake et al., 1997). However, in natural ecosystems, plant responses to elevated CO2 may decline over time due to progressive nitrogen limitation. With time, an increasing proportion of N becomes bound in organic form in plant tissues, litter, and soil organic matter (Luo et al., 2004; Johnson, 2006). Nitrogen availability is closely linked to sink capacity, the ability of a plant to utilize photosynthate (Stitt & Krapp, 1999; Paul & Foyer, 2001). Rasse & Tocquin (2006) demonstrated that sink limitation leads to increased leaf respiration, effectively removing excess assimilates. A limited sink capacity and excess carbohydrates trigger photosynthetic down-regulation by reducing the carboxylation rate of Rubisco (Vcmax) and the regeneration rate of ribulose 1,5-bisphosphate (Jmax) (Ainsworth & Rogers, 2007).
While the canopy is relatively open, elevated CO2 stimulates biomass production through its direct effect on photosynthesis, and indirectly through stimulating light interception through increased leaf area. This results in the so-called compound interest effect whereby CO2 stimulates growth through a self-perpetuating cycle of increased leaf area production and carbon uptake (Ceulemans & Mousseau, 1994; Norby et al., 1996). After canopy closure, further leaf area expansion is restricted. In this way, long-term canopy development will cause a reduction of the community growth response to elevated CO2 over time, while for individual species, growth response may become limited due to reduced access to light.
Here we report on the response of the aboveground biomass to 11 years (1996–2007) of CO2 enrichment in a Florida scrub-oak ecosystem. The Florida scrub-oak ecosystem is an ideal system for investigating long-term biomass responses to elevated CO2 for three main reasons. First, because of a short fire-return period (10–15 years), we were able to analyze the effects of elevated CO2 concentration on a natural scrub-oak community through an entire growth cycle from emergence through canopy closure. Canopy closure was reached in 2000 (Ainsworth et al., 2002; Li et al., 2007b), 4 years since fire, indicating a transition from leaf area expansion to a spatially-limited system (Körner, 2006). Root closure, whereby root production and turnover reach a dynamic equilibrium, coincided with canopy closure (Day et al., 2006). These observations suggest that the oak species became increasingly light limited and potentially sink limited as well. Second, the soils are N poor, therefore any long-term nutrient limitation can be easily detected. For example, after initial CO2 stimulation of the biomass of a leguminous vine Galactia elliottii, Hungate et al. (2004) observed long-term decline of this important nitrogen fixer. Elevated CO2 initially increased plant N uptake (Hungate et al., 1999), but after 4 years, Hungate et al. (2006) observed decreased soil N availability, suggesting progressive N limitation. Third, this system is subject to water stress (Powell et al., 2006), thereby evaluate the importance of rainfall on the biomass responses. We previously reported reductions in stomatal conductance and transpiration under elevated CO2 (Lodge et al., 2001; Hymus et al., 2002; Li et al., 2003) and increased relative photosynthetic stimulation under drought conditions (Li et al., 2007a).
The objective of the present research is to test whether a nutrient- and water-limited natural ecosystem can maintain a long-term biomass response to elevated CO2 concentration after canopy closure. A second objective is to quantify the potential links between growth responses to elevated CO2 on the one hand, and water stress and canopy-closure, on the other.
Materials and methods
Ecosystem description
The study site was located on the John F. Kennedy Space Center within Merritt Island National Wildlife Refuge, coastal central Florida (28 °38′N, 80 °42′W), USA. Climate is subtropical, characterized by mild, dry winters and hot, humid summers. Annual precipitation displays high variability around a 100-year mean of 131 cm yr−1. Typically, a dry period occurs from April through early June, followed by the wet season from late June to October. Mean daily maximum and minimum temperatures are 22.3 and 9.6 °C for January and 33.3 and 21.9 °C for July. Soils are Pomello Sands (Arenic Haplahumod) which are acidic, well-drained, and nutrient-poor. A Bh horizon was found at different locations at approximately 1 m depth. The water table typically fluctuates between 1.5 and 2.5 m, but rising close to the surface following extreme rain events. Vegetation is typical of a fire-regenerated coastal Florida scrub-oak saw palmetto community (Schmalzer & Hinkle, 1992a). This ecosystem is dominated by three species of evergreen scrub-oaks which rapidly resprout after fire: Quercus myrtifolia Willd, Quercus geminata Small, and Quercus chapmanii Sargenti, together typically comprising 85–90% of aboveground biomass (Schmalzer & Hinkle, 1996). Less abundant species include: saw palmetto (Serenoa repens), rusty Lyonia (Lyonia ferruginea), tarflower (Befaria racemosa), shiny blueberry (Vaccinium myrsinites), wax myrtle (Myrica cerifera), and a leguminous vine (G. elliottii). At our site, Q. myrtifolia and Q. geminata are co-dominant. Over time, Q. myrtifolia remains low and bushy and Q. geminata continues to grow vertically, exceeding maximum Q. myrtifolia height by as much as 5 m (Guerin, 1993). Both oaks utilize groundwater at the study site, but xylem deuterium measurements show that Q. geminata uses the water table to a greater extent than does Q. myrtifolia (Hungate et al., 2002).
Experimental design
CO2 was manipulated using open-top chambers (OTCs) consisting of 4 in PVC frames wrapped in a clear polyester film (‘Mylar’; Melinex 071; Courtaulds Performance Films, Martinsville, VA, USA). Chambers were octagonal, 2.5 m tall, 3.45 m diameter at parallel sides, 3.66 m diameter diagonally, and enclosing 9.42 m2 ground area. To minimize effects of wind intrusion on CO2 treatment, a frustum was constructed atop each chamber, reducing the opening to 5.9 m2, or about 60% of the chamber footprint. Before starting the experiment, 19 plots were selected for representative species composition. Within these plots, aboveground standing biomass was estimated, followed by a controlled burn of the entire site in August 1995 and January 1996. Immediately following the second burn, OTCs were constructed on 16 of the 19 plots. Treatments were randomly assigned to plots using a random block design. Blocks were selected based on preburn aboveground oak species composition. Eight additional plots were established postburn as unchambered controls (C). Fumigation with CO2 commenced in May 1996 after all vegetation that had resprouted was cut back to ground level. CO2 concentrations in the chambers were maintained at ambient (A) (∼350 ppm in 1996 to ∼380 ppm in 2007) and elevated (E) (ambient+350 ppm) levels throughout the experiment except for periods between 13 September and 11 October 1999 and 13 August and 18 October 2004 during repairs following extreme wind events. See Dijkstra et al. (2002) for further details of experimental setup, chamber design and operation, and plot selection.
Biomass and shoots
Each year, between late December and early February, a comprehensive, nondestructive census of oaks was conducted within the plots. At each census, shoots of the individual species were counted and stem basal diameters measured at 2–5 cm above soil surface. A destructive harvest was conducted in June 2007 to determine final aboveground biomass in each plot. To estimate aboveground biomass from census data, allometric relationships were developed between aboveground biomass per shoot and shoot basal diameter for each oak species (Table 1). To compute allometric relationships, data from three destructive harvests were combined: (1) a pilot study conducted at an adjacent site between April 1992 and July 1995 (Day et al., 1996; Dijkstra et al., 2002), (2) an adjacent, unmanipulated site in 1998, and (3) final harvest in 2007. At final harvest, no significant effects of CO2 concentration on allometric relationships were observed (GLM procedure with treatment as a categorical variable; P=0.44, 0.50, 0.58 for Q. myrtifolia, Q. geminata, and Q. chapmanii, respectively). Hence, allometric relationships were calculated by species, but not separately by treatment, in contrast to Dijkstra et al. (2002). Because of the presence of only a few individuals of Q. chapmanii in the pilot study, the relationship between stem diameter and biomass for this species was calculated from the 1998 and final harvest data. Allometry was then applied to all census data from the start of the experiment. To estimate annual accumulation, biomass increment was calculated from census biomass estimates of the current year minus the previous year; for example, biomass increment for 2002 represents growth that occurred between December 2001 and December 2002. For analysis, CO2 stimulation was calculated as an effect relative to ambient treatment [Rel. stim. (%)=[(E−A)/A] × 100]. Absolute stimulation is calculated as elevated minus ambient (Abs. stim.=E−A).
| Species | N | Regression equation | r 2 | P |
|---|---|---|---|---|
| Quercus myrtifolia | 375 | ln(mass)=−1.8420+ln(diam) 2.8307 | 0.945 | <0.001 |
| Quercus geminata | 265 | ln(mass)=−1.4289+ln(diam) 2.6133 | 0.956 | <0.001 |
| Quercus chapmanii | 71 | ln(mass)=−1.4387+ln(diam) 2.5742 | 0.967 | <0.001 |
Statistical analyses
To test for treatment differences in aboveground biomass and shoot density, repeated measures analysis of variance (anova) was used to test between E and A (CO2 effect) and between A and C (chamber effect) using the mixed model procedure of the sas statistical system (Littell et al., 1998). An unstructured covariance model was used for evenly distributed observations in time with year treated as a repeated categorical variable. Degrees of freedom were determined using the Satterthwaite approximation. Differences in final harvest data were tested using one-way anova between subject treatments. Linear regressions were conducted using sigmaplot v8.0 (SPSS Inc., Chicago, IL, USA). Where applicable, means are displayed with calculated standard error (SE) values.
Results
Biomass
At harvest in 2007, aboveground biomass (including standing dead biomass) in the elevated CO2 treatment (E) significantly exceeded biomass in the ambient treatment (A) (P<0.01) (Table 2). Oak aboveground biomass increased substantially throughout the experiment, displaying markedly linear growth (Fig. 1a). Significant differences were observed between E and A within the first year of fumigation. The relative effects of CO2 concentration on community biomass increased steeply during the first 3 years, reaching 67.3% in 1999, and were stable for the remainder of the experiment. Repeated measures analysis showed significant differences between ambient and elevated chambers (P=0.01) and treatment × time interaction (P=0.01). At final harvest, the CO2 effect on biomass was 67.5%. No significant difference was observed between A and C total biomass (P=0.65), although a significant treatment × time interaction was observed (Table 3).
| Species | Biomass (g m−2) | P-values | |||
|---|---|---|---|---|---|
| Ambient (A) | Elevated (E) | Control (C) | E vs. A | A vs. C | |
| Quercus myrtifolia | 519.7 ± 155.7 | 1186.2 ± 263.9 | 607.9 ± 116.0 | <0.05 | 0.66 |
| Quercus geminata | 613.7 ± 96.1 | 651.6 ± 171.6 | 173.4 ± 45.7 | 0.85 | <0.01 |
| Quercus chapmanii | 40.4 ± 10.0 | 128.1 ± 43.7 | 109.1 ± 30.2 | 0.07 | <0.05 |
| Other species | 83.2 ± 23.6 | 136.6 ± 22.2 | 437.9 ± 91.8 | 0.12 | <0.01 |
| Standing dead | 74.7 ± 14.5 | 83.7 ± 29.2 | 70.0 ± 16.7 | 0.81 | 0.85 |
| Total | 1313.1 ± 110.7 | 2186.2 ± 161.0 | 1398.4 ± 143.7 | <0.01 | 0.65 |
- P-values from one-way anova tests for significant biomass differences in elevated vs. ambient chambers (E vs. A) and ambient chambers vs. unchambered control plots (A vs. C). Bold values indicate P≤0.05.
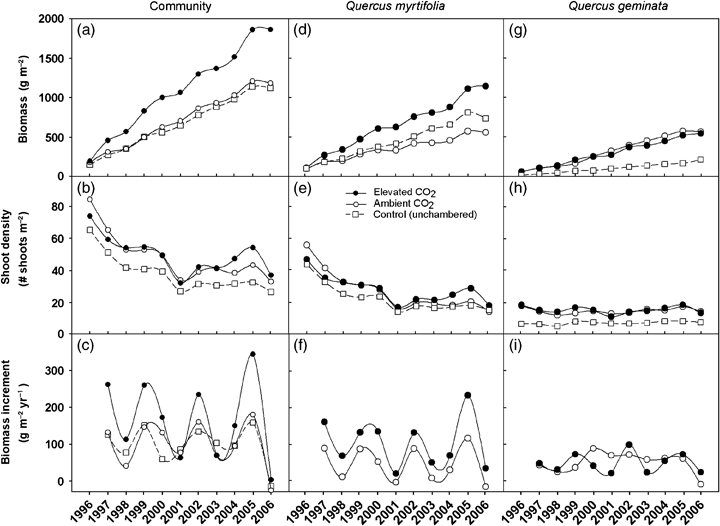
Aboveground biomass, shoot density, and aboveground biomass increment by open-top chamber (OTC) treatments (ambient and elevated CO2) and unchambered control for a scrub-oak community (a–c) and separately for two co-dominant scrub-oak species, Quercus myrtifolia (d–f) and Quercus geminata (g–i) over time. Data were collected at annual census conducted between late December and early February each year. Biomass values were estimated using allometric relationships (Table 1).
| Species | Test | Effect | P-values | ||
|---|---|---|---|---|---|
| Abovegroundbiomass | Biomassincrement | Shootdensity | |||
| All oaks | E vs. A | Treatment | 0.01 | 0.03 | 0.47 |
| Treatment × Time | <0.01 | 0.09 | <0.01 | ||
| A vs. C | Treatment | 0.66 | 0.32 | 0.06 | |
| Treatment × Time | <0.01 | 0.33 | <0.01 | ||
| Quercus myrtifolia | E vs. A | Treatment | 0.05 | 0.09 | 0.85 |
| Treatment × Time | <0.01 | 0.82 | <0.01 | ||
| A vs. C | Treatment | 0.36 | 0.37 | 0.25 | |
| Treatment × Time | 0.03 | <0.01 | 0.03 | ||
| Quercus geminata | E vs. A | Treatment | 0.83 | 0.90 | 0.89 |
| Treatment × Time | 0.03 | 0.02 | 0.11 | ||
| A vs. C | Treatment | <0.01 | <0.01 | <0.01 | |
| Treatment × Time | 0.11 | 0.27 | 0.05 | ||
- Bold values indicate P≤0.05.
Biomass responses were species specific. Q. myrtifolia displayed a strong CO2 response throughout the study (Fig. 1d), resulting in a 128% greater biomass at final harvest in 2007 (Table 2). In contrast to Q. myrtifolia, Q. geminata displayed no significant effect of CO2 concentration (Fig. 1g, Table 3). Therefore, we conclude that the community biomass response was driven primarily by Q. myrtifolia (Fig. 1d, Table 3). However, Q. geminata displayed a significant CO2× time interaction, associated with a shift in species composition under ambient CO2, while species composition remained constant under elevated CO2 concentrations. (Table 4). At harvest, Q. chapmanii revealed a marginally significant CO2 effect (P=0.07). However, this species comprised only 3% and 6% of aboveground biomass in A and E, respectively, and thus did not influence the overall community response to CO2. The three oak species combined comprised 89% and 90% of total A and E aboveground biomass, respectively, but only 64% of biomass in C plots in 2007. The low proportion of oak biomass in C plots was already present at the start of the experiment. No significant effects of CO2 were detected for nonoak species biomass (P=0.12) (Table 2).
| Species | Year | Treatment (%) | ||
|---|---|---|---|---|
| Ambient | Elevated | Control | ||
| Quercus myrtifolia | 1996 | 54 | 57 | 68 |
| 2007 | 44 | 60 | 68 | |
| Quercus geminata | 1996 | 33 | 30 | 10 |
| 2007 | 52 | 33 | 20 | |
Shoot density
Total shoot density displayed an exponential decay trend characteristic of self-thinning (Westoby, 1984) (Fig. 1b). An anomalous drop observed in 2001 coincided with the second year in a 2-year drought period (Li et al., 2007a), while the sudden increase in 2005 was associated with recovery after hurricane disturbance (Li et al., 2007b) (Fig. 1b). Repeated measures analysis showed no significant effects of CO2 concentration on shoot density (Table 3). Thus, observed biomass stimulation reflected increased mass of individual shoots. Trends in shoot density were dominated by Q. myrtifolia (Fig. 1e), while Q. geminata displayed stable shoot densities throughout the experiment (Fig. 1h). Significant treatment differences between A and C shoot densities were observed at the time of the first census (Dijkstra et al., 2002) and persisted over time (Fig. 1b) associated with lower number of Q. geminata shoots (Table 3) and greater abundance of nonoak species in the control plots (Table 2).
Biomass increment and correlation with rainfall
Biomass increment showed large interannual variability in all treatments (Fig. 1c) and was positively correlated with annual rainfall across all treatments (Fig. 2). Repeated measures analysis showed significantly higher biomass increments in E and a marginally significant treatment × time interaction, but no significant differences between A and C (Table 3). Stimulation of biomass increment (g m−2 yr−1) generally declined over the course of the study (Fig. 3). When the biomass increment for 2005 was excluded from analysis, this decline over time was statistically significant. This suggests that ecosystem recovery after hurricane disturbance was able to make use of the combined effects of increased light and nitrogen made available by the decay of leaves dropped in the storm (Fig. 3).
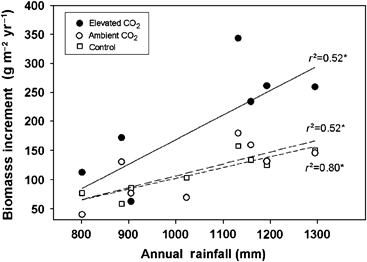
Relationship between total oak biomass increment (g m−2 yr−1) and annual rainfall (mm) for elevated, ambient and control treatments; * indicates significance at P<0.05. Outliers were excluded from the regression: data from 2004 based on atypical rainfall associated with hurricane events, and 2006 due to negative growth values.
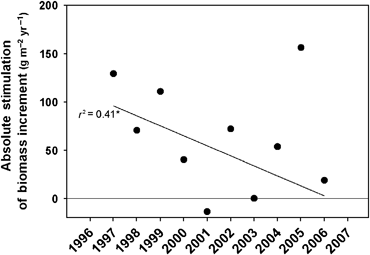
Absolute stimulation (Abs. stim.=E−A) of biomass increment over time for all oaks combined. The linear regression excludes 2005 growth, an anomalous year, due to recovery from hurricane damage; * indicates P<0.05.
Q. myrtifolia displayed a consistent and significant CO2 response in biomass increment (Fig. 1f; Table 3). Biomass increment for Q. geminata did not show a significant CO2 effect, although a significant treatment × time interaction was observed (Fig. 1i; Table 3). The relative effect of CO2 on annual biomass increment exhibited a negative correlation with annual rainfall for Q. myrtifolia, but not for Q. geminata (Fig. 4), demonstrating that CO2 provided a relative advantage for Q. myrtifolia under dry conditions; a trend consistent with observed leaf level gas exchange data under drought conditions (Li et al., 2007a). Q. geminata biomass increment displayed negligible interannual variability under ambient CO2 concentrations between 2000 and 2005 and was higher than under elevated CO2 concentration during several of the driest years (2000, 2001, and 2003) (1, 4).
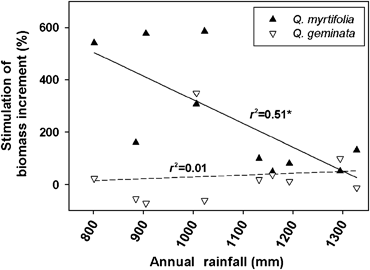
Relationship between relative CO2 stimulation of Quercus geminata and Quercus myrtifolia biomass increment and total annual rainfall; * indicates P<0.05.
Discussion
Long-term aboveground biomass response
CO2 enrichment significantly increased aboveground biomass of the scrub-oak community. This response was driven primarily by the significant response to elevated CO2 by the dominant oak species, Q. myrtifolia. Several other long-term field studies have reported sustained biomass stimulation (Rasse et al., 2005; Wittig et al., 2005; Erickson et al., 2007; Kimball et al., 2007). Our results are unique in which stimulation was sustained after the community reached canopy closure (Ainsworth et al., 2002; Li et al., 2007b), root closure (Day et al., 2006), and displayed nitrogen limitation (Hungate et al., 2006): three critical constraints predicted to limit CO2 response (Norby et al., 1999; Körner, 2006). Canopy closure was reached in 2000, after which leaf area index (LAI) reached stable values, except during recovery from hurricane damage sustained in 2004 (Li et al., 2007b). Studies on forests have typically reported an absence of biomass stimulation for closed canopies (Norby et al., 2004; Körner et al., 2005). However, Oren et al. (2001) showed biomass stimulation after canopy closure to be sustainable in the absence of nutrient limitation (i.e. nitrogen). At our site, plant nitrogen uptake initially increased under elevated CO2, reaching maximum uptake in 1999. Thereafter, N uptake declined and indications of progressive nitrogen limitation became apparent (Hungate et al., 2006). Fine root response paralleled N uptake trends. Initial CO2 stimulation hastened fine root closure by several months, after which the CO2 effect on fine roots diminished, suggesting that the community reached dynamic equilibrium in terms of fine root production and mortality (Day et al., 2006). Aboveground growth appeared linked to these trends, as cumulative biomass stimulation increased through 1999, but then stabilized for the remainder of the experiment. Similarly, NPP estimated from leaf litter production rates showed an initial increase (Hungate et al., 2006). However, canopy damage incurred from two hurricanes in September 2004 temporally renewed the effect of CO2 concentration on aboveground biomass production. At the site, 113 km h−1 winds caused extensive defoliation, producing a sharp decrease in LAI, but only minor structural damage (Li et al., 2007b). Defoliation effectively opened the canopy, as evidenced by a surge of new shoots observed in 2005, a common trend among hurricane-disturbed ecosystems (Brokaw & Walker, 1991; Fernandez & Fetcher, 1991). This hurricane effect is clearly demonstrated as a spike in stimulation of incremental growth in 2005 (Fig. 3). Excluding 2005 data, the effects of CO2 concentration on absolute annual biomass increment declined throughout the study (Fig. 3). Additionally, previous studies demonstrate that plant growth increases following hurricanes due to the pulse of nutrients from decomposing canopy leaves relocated to the forest floor (Lodge et al., 1991; Herbert et al., 1999). At our site, nitrogen from dropped leaves represented a substantial increase over typical annual rates of N uptake (Hungate et al., 2006; Li et al., 2007b). This extra nitrogen would not be immediately available, but would be gradually released through decomposition, resulting in a delayed effect on plant growth (Dilustro et al., 2001). Thus, the reopening of the canopy and additional pulse of available nitrogen may have effectively removed previous light and nutrient limitations and contributed to a renewed biomass response to elevated CO2. LAI and ecosystem CO2 uptake, determined via eddy covariance measurements at an adjacent site, declined immediately after the hurricanes and did not return to stable, prehurricane levels until 2006 (Powell et al., 2006; Li et al., 2007b). Despite a 22% reduction in ecosystem C uptake following the hurricanes (Powell et al., 2006), biomass increment values were higher across all treatments in 2005 than in any other year of the experiment (Fig. 1). The discrepancy between reduced assimilation and increased growth suggests that storage carbohydrates may have been remobilized during recovery from hurricane damage. This high regrowth after the hurricane may also have been the result of high N availability (Fig. 3). This response to hurricane damage may be similar to ecosystem recovery after fire, when scrub-oaks remobilize belowground reserves for growth following fire disturbance (Schmalzer & Hinkle, 1992b; Langley et al., 2002). This comparison underscores the potential effects of disturbance on plant C allocation patterns which may alter the ecosystem carbon budget. In the absence of hurricane disturbance, biomass response may have continued to decline.
Species-specific response
Q. myrtifolia showed a strong biomass response to elevated CO2 throughout the experiment, in contrast to Q. geminata. Variation in the growth responses to CO2 enrichment is commonly observed among species (Poorter, 1993; Ainsworth & Long, 2005), and is typically attributed to differences in the response of photosynthesis (Nowak et al., 2004). Over the experiment, response of photosynthesis in the two oaks mirrored the effects of CO2 on biomass increments (Li et al., 1999, 2007a, unpublished data; Hymus et al., 2001, 2002; Ainsworth et al., 2002). Q. geminata generally displayed photosynthetic acclimation to elevated CO2, as reductions in Vcmax and Jmax (Table 5). Q. myrtifolia initially displayed reduction in Vcmax (Li et al., 1999), but never displayed reduction in Jmax (Table 5). Under growth conditions, both oak species consistently showed increased rates of leaf net photosynthesis (Li et al., 1999, 2007a, unpublished data; Hymus et al., 2001, 2002; Ainsworth et al., 2002). However, long-term mean stimulation of Q. myrtifolia net photosynthesis was approximately twice that of Q. geminata (63% and 35%, respectively). Although the mechanisms are not fully understood, it is believed that sink limitations trigger acclimation of the photosynthetic system (Ainsworth & Rogers, 2007). Contrasting photosynthetic responses observed between these species may thus have resulted from fundamental differences in how each species controls sink activity. By examining responses in Arabidopsis thaliana mutants unable to synthesize starch, Rasse & Tocquin (2006) demonstrated that starch production exerts strong control over photosynthesis and growth. Early in this study, Li et al. (1999) reported significant increases in leaf starch content in Q. myrtifolia under elevated CO2, but not in Q. geminata, corresponding to the brief time period in which Q. myrtifolia displayed photosynthetic acclimation and Q. geminata did not (Table 5).
| Species | Measure | Was significant reduction observed under elevated CO2? | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aug 1996 | Jul 1997 | Apr 1998 | Aug 1998 | Dec 1998 | Mar 1999 | Jun 1999 | Jan 2000 | Jul 2000 | Aug 2000 | Aug 2002 | Oct 2003 | ||
| Quercus geminata | V cmax | No | * | * | * | No | Yes | No | Yes | Yes | Yes | Yes | Yes |
| J max | No | * | * | * | Yes | Yes | No | No | Yes | Yes | Yes | Yes | |
| Quercus myrtifolia | V cmax | Yes | Yes | No | Yes | No | No | No | No | No | No | Yes | No |
| J max | No | No | No | No | No | No | No | No | No | No | No | No | |
| Jul 2004 | Dec 2004 | Jun 2005 |
|---|---|---|
| Yes | Yes | Yes |
| No | No | No |
| No | No | No |
| No | No | No |
- Values indicate results of one-way anova to test significant reductions (P<0.05) in leaf maximum carboxylation capacity (Vcmax) and maximum rate of RubP regeneration (Jmax) under elevated and ambient CO2 treatment (Li et al., 1999, 2007a, unpublished data; Hymus et al., 2001, 2002; Ainsworth et al., 2002). See references for measurement details.
- * No data available.
Although acclimation in Q. geminata reduced photosynthetic capacity in E (Table 5), net photosynthesis was consistently and significantly stimulated over the course of the study. Similarly, stimulation of Q. myrtifolia photosynthesis exceeded stimulation of aboveground biomass, reiterating a common observation that there is no 1 : 1 relationship between leaf photosynthesis and plant growth. This study reports only aboveground response but there is now evidence that additional carbon assimilated as a result of CO2 stimulation of photosynthesis was allocated belowground. Plants may increase assimilate allocation to belowground structures under nutrient limitation (Ericsson, 1995) and water stress (Schenk & Jackson, 2002). At this site, from measurements using ground penetrating radar, Stover et al. (2007) showed that belowground biomass of coarse roots and storage structures in E exceeded A by approximately 2400 g m−2 (37%) by December 2005, an increase of about 284 g m−2 yr−1 or almost three times the average increment of biomass of shoots. Further investigation into the fate of assimilated carbon is needed to quantify the relationships between C uptake, allocation, and growth response to elevated CO2.
Species-specific responses also contributed to a shift in species composition. Natural ecosystems often demonstrate a species shift in aboveground biomass under elevated CO2. This is attributed to changes in competitive relationships (Niklaus et al., 2001; Ramseier et al., 2005), derived from species-specific responses to CO2 and environmental factors such as nitrogen (Berendse et al., 2001; Joel et al., 2001) and water availability (Owensby et al., 1999; Belote et al., 2004; Morgan et al., 2004). In this study, a species composition shift was observed, but only in the chambers exposed to ambient CO2 (Table 4). In terms of percent biomass composition, over the course of the study, Q. geminata steadily increased (+19%) and Q. myrtifolia steadily decreased (−10%) from 1996 values in A, while composition remained stable in E and C (Table 4). This composition shift may have been partially responsible for the disconnect between stimulation of Q. geminata net photosynthesis and the apparent lack of biomass response to elevated CO2. This trend may also indicate that chamber effects on species composition were ameliorated by elevated CO2. OTCs commonly alter microclimate, particularly temperature, light, and relative humidity (Drake et al., 1989; Van Oijen et al., 1999), and minor effects on plant growth may compound over time (Leadley & Drake, 1993). At our site, OTCs increased daytime air temperature by 4 ± 0.2 °C and vapor pressure deficit (VPD) by 0.7 ± 0.05 kPa compared with unchambered control sites (Dore et al., 2003). Additionally, frustums reduced rainfall penetration into OTCs by 40%. These unintentional effects created a warmer, drier growth environment, consistent with predicted future climate scenarios (Weltzin et al., 2003; IPCC, 2007). Our study was not explicitly designed to quantify OTC microclimate effects on biomass; however, the observed shift in A biomass composition, not apparent in E, qualitatively demonstrates that the combination of warmer, drier conditions under ambient CO2 favors Q. geminata growth relative to Q. myrtifolia and that elevated CO2 may counteract this effect. The observed interactions between growth and water availability also suggest this interpretation, as discussed below.
Interactions with rainfall
Water availability limits ecosystem productivity (Huxman et al., 2004) and the scrub-oak ecosystem is no exception (Powell et al., 2006). Although these oaks are phreatophytic, hence use both soil- and groundwater sources, annual rainfall showed significant correlations with community growth response to elevated CO2 (Fig. 2). Because of sandy, well-drained soils, groundwater provides a stable water source for scrub-oaks compared with soil moisture which is only a transient resource immediately following rain events (Hungate et al., 2002). In fact, Schmalzer & Hinkle (1992a) demonstrated that water table depth mediates scrub-oak ecosystem composition, suggesting that annual rainfall captured the general long-term trends of both soil water content and water table depth. This study observed significant positive correlations between annual rainfall and community biomass increment across treatments, with plants exposed to elevated CO2 showing a steeper response to rainfall than those exposed to ambient CO2 (Fig. 2). This trend is consistent with observations from our site of a positive correlation between CO2 stimulation of NEE and water availability (Hymus et al., 2003). Similar results were reported for a Chesapeake Bay wetland (Rasse & Tocquin, 2006) and for Mojave Desert shrubs (Naumberg et al., 2003), where larger photosynthetic responses to CO2 enrichment were observed during years with more rainfall. Our results also indicate that relative CO2 stimulation of Q. myrtifolia biomass increment significantly decreased as rainfall increased, while no significant interaction was observed for Q. geminata (Fig. 4). This reflects a relative ameliorating effect of elevated CO2 on Q. myrtifolia growth under water stress (Fig. 4), consistent with theoretical predictions based on reduced stomatal conductance (Drake et al., 1997) and consistent with a previous study from our site showing increased stimulation of net photosynthesis in Q. myrtifolia under drought conditions (Li et al., 2007a). The ability of Q. geminata to use groundwater may explain its competitive advantage over Q. myrtifolia under dry conditions at current CO2 levels, resulting in the aboveground composition shift observed in A. Xylem deuterium measurements from our site show that Q. geminata uses groundwater more readily than Q. myrtifolia (Hungate et al., 2002). Under drier soil conditions in the OTCs, these competitive traits manifest as a species shift in A, without significant impact on total biomass production relative to C. The ameliorative effects of elevated CO2 on Q. myrtifolia growth under water stress may have decoupled the ecosystem relationship with groundwater, thus preventing a similar shift from occurring in E. Over time and compounded by natural fire cycles, these competitive interactions could potentially yield significant changes in ecosystem composition and C cycling.
Conclusions
Our results suggest that future research should focus on determining the interactive processes and mechanisms linking photosynthesis, assimilate allocation, and sink development, as these relationships may be the key to understanding the species-specific responses observed in this study, as well as the progressive limitations on ecosystem growth response to rising CO2.
Acknowledgements
This work was supported by a grant from the Department of Energy to the Smithsonian Institution (DE-FG02-95ER61993). We are grateful to everyone who dedicated hard work and enthusiasm in support of the site over the years. We thank John Erickson and Jessica Hines for their helpful comments on an earlier version of this manuscript. We acknowledge the support and encouragement of NASA Kennedy Space Center, Dynamac Corp., and US Fish and Wildlife Service, Merritt Island National Wildlife Refuge.




