Carbon exchange of a mature, naturally regenerated pine forest in north Florida
Abstract
We used eddy covariance and biomass measurements to quantify the carbon (C) dynamics of a naturally regenerated longleaf pine/slash pine flatwoods ecosystem in north Florida for 4 years, July 2000 to June 2002 and 2004 to 2005, to quantify how forest type, silvicultural intensity and environment influence stand-level C balance. Precipitation over the study periods ranged from extreme drought (July 2000–June 2002) to above-average precipitation (2004 and 2005). After photosynthetic photon flux density (PPFD), vapor pressure deficit (VPD) >1.5 kPa and air temperature <10 °C were important constraints on daytime half-hourly net CO2 exchange (NEEday) and reduced the magnitude of midday CO2 exchange by >5 μmol CO2 m−2 s−1. Analysis of water use efficiency indicated that stomatal closure at VPD>1.5 kPa moderated transpiration similarly in both drought and wet years. Night-time exchange (NEEnight) was an exponential function of air temperature, with rates further modulated by soil moisture. Estimated annual net ecosystem production (NEP) was remarkably consistent among the four measurement years (range: 158–192 g C m−2 yr−1). In comparison, annual ecosystem C assimilation estimates from biomass measurements between 2000 and 2002 ranged from 77 to 136 g C m−2 yr−1. Understory fluxes accounted for approximately 25–35% of above-canopy NEE over 24-h periods, and 85% and 27% of whole-ecosystem fluxes during night and midday (11:00–15:00 hours) periods, respectively. Concurrent measurements of a nearby intensively managed slash pine plantation showed that annual NEP was three to four times greater than that of the Austin Cary Memorial Forest, highlighting the importance of silviculture and management in regulating stand-level C budgets.
Introduction
Forests of the southeastern US have been identified as an important carbon (C) sink largely resulting from a climate-driven easement of constraints on productivity (Nemani et al., 2003) and reversion of agricultural land to forest (Turner et al., 1995). Pine forests are one of the most widespread ecosystem types in this region, covering approximately 257 000 km2 and comprising 33% of the total timberland (Conner & Hartsell, 2002). Given the importance of pine forests for the region's C balance, a key element of adapting forest management to the goal of mitigating climate change is understanding how various silvicultural options available to land managers impact stand- and landscape-level C budgets.
In Florida, approximately 50% of terrestrial ecosystems are pine flatwoods, which are composed of a mixture of about two-thirds pine uplands and one-third deciduous cypress (Taxodium spp.) wetlands (Myers & Ewel, 1990). Historic pine flatwoods were characterized by open-canopy, mixed stands of longleaf pine (Pinus palustris Mill.) and slash pine (Pinus elliottii var. elliottii Engelm.), with a dense understory thought to be maintained by frequent (ca. every 3–7 years) low-intensity fires (Abrahamson & Hartnett, 1990). Following European settlement, species composition, stand structure, and regeneration were largely dictated by silviculture, first through selective logging, and more recently by even-aged management. Management intensity has generally increased over time, and pine flatwoods are now the most intensively managed forest ecosystem in Florida (Abrahamson & Hartnett, 1990). Current plantations are typically clearcut every 15–25 years, mechanically and/or chemically site prepared, regenerated using genetically improved tree seedlings, and treated one to three times per rotation with chemical fertilizers and/or herbicides.
Evaluating the effects of even-aged management on the C dynamics of pine flatwoods and cypress wetlands has been a major focus of recent research (e.g. Gholz & Fisher, 1982; Cropper & Gholz, 1993; Clark et al., 1999, 2004). Still largely unexamined are the 18% of Florida's 5.76 million ha of timberlands which are naturally regenerated, nonplantation stands. These stands are classified by the USDA Forest Service as ‘natural pine’ forests (Conner & Hartsell, 2002), and this distinction implies a contrast to short-rotation, even-aged plantations. Natural and uneven-aged management is increasingly being considered as an alternative silvicultural model for nonindustrial forest lands (Owen, 2002). Therefore, an evaluation of the C dynamics of natural flatwoods ecosystems is necessary to understand differences between these contrasting land uses, both today and into the future.
Understory C dynamics may differ significantly between ‘natural stands’ and intensively managed plantations because of the relatively open canopies of older, natural flatwoods forests and the lack of intensive silvicultural practices. Gross ecosystem production of forest understories is controlled to a great extent by understory leaf area index (LAI) and the amount of light transmitted to the understory (Misson et al., 2007). Following canopy closure in unburned plantations, understory biomass approaches a steady state (Gholz & Fisher, 1982), which suggests that long-term net CO2 exchange is insignificant. In contrast, the tree canopies of natural pine flatwoods typically have a relatively low LAI, which allows large amounts of radiation to penetrate the understory (Gholz et al., 1991), and implies that the net CO2 exchange capacity of the understory is much greater.
The objective of this study was to characterize the C dynamics of a mature, naturally regenerated Florida pine flatwoods ecosystem and compare its C budget with a contrasting, intensively managed slash pine plantation. To meet this objective, we commenced ecological measurements over a natural pine flatwoods community in north Florida to compare with simultaneous measurements and previously reported results from adjacent pine plantations. We framed our research in terms of the following questions: (1) how much C does a mature, naturally managed pine flatwoods sequester on an annual basis; (2) how much of the interannual variation in net ecosystem C exchange is explained by precipitation and water stress; (3) how much of the total ecosystem C flux is accounted for by the understory; and (4) how does long-term net ecosystem C production of a mature, less intensively managed pine flatwoods compare with that of a younger, more intensively managed pine plantation?
Materials and methods
Study sites
The study was conducted from July 2000 to June 2002 and January 2004 to December 2005 in a 41 ha pine flatwoods ecosystem in the Austin Cary Memorial Forest (ACMF). The ACMF is managed by the School of Forest Resources and Conservation of the University of Florida and located 15 km northeast of Gainesville, Alachua County, Florida, USA (29°44′17″N, 82°13′8″W; elevation 50 m). Prior to state purchase in 1936, the site had been selectively harvested for timber and used for low-intensity cattle grazing. In 1936, the area was allowed to regenerate naturally from remnant seed trees. The resulting stand was thinned in 1991 by removing 27% of the basal area to open the canopy. The current management objective is to restore an uneven-aged, mixed slash and longleaf pine stand by encouraging natural regeneration through prescribed burns every 3–5 years.
A 30-m walkup scaffolding tower was erected in the forest for this study. The fetch from the tower was greater than 1 km in the north and south directions, and approximately 0.5 km in the east and west directions. The fetch encompassed two similar administrative compartments; before this study, the understory of one was burned in the winter of 1997 and the other in the winter of 1998. The overstory was composed of longleaf pine and slash pine (72% and 28% of tree basal area, respectively), with tree ages ranging from 20 to 80 years in 2001. The top of the tree canopy averaged 22 m. The tree canopy was vertically separated by 15 m over a 1.5-m-tall, dense understory. In 2000, stand basal area was 18.6 m2 ha−1, and stem density was 363 trees ha−1. The understory consisted of native species dominated by saw palmetto [Serenoa repens (Bartr.) Small], gallberry [Ilex glabra (L.) Gray], wax myrtle (Myrica cerifera, L.), and wiregrass (Aristrida stricta Michx). The soils were poorly drained ultic alaquods (sandy, siliceous, thermic) with a discontinuous spodic horizon 30–60 cm deep and argillic horizon approximately 1.25 m deep.
Measurements covering the same periods were also made at the Donaldson Tract (DT) pine plantation located 5.75 km to the northeast of the ACMF. Slash pine trees were planted to harvest density (∼2000 trees ha−1) following a clearcut in 1990. Dominant canopy height ranged from 11.4 to 14.9 m during the study. The sonic anemometer height was raised from 15 to 21 m over the course of the study. The fetch was >800 m in all directions from the tower. Soil properties at this site were similar to the ACMF, but the water table was generally deeper. Further details about this site, experimental methods and previous flux measurements are reported in Gholz & Clark (2002) and Clark et al. (2004).
Meteorological and soil measurements
Photosynthetically active photon flux density (LI-190, LI-COR Inc., Lincoln, NE, USA), net radiation (Q7, Radiation and Energy Balance Systems Inc., Seattle, WA, USA), wind speed and direction (No. 3001-5, R. M. Young Company, Traverse City, MI, USA), temperature and relative humidity (HMP 23 UT, Vaisala Inc., Helsinki, Finland), and precipitation (tipping bucket, Sierra Misco Inc., Berkeley, CA, USA) were measured continuously from the top of each site's tower. At each site, three soil heat flux plates (HFT-3.1, Radiation and Energy Balance Systems Inc.) were buried 10 cm below the soil surface in separate locations within 8 m of the tower. All sensors were measured every minute and 30-min averages were collected on an EasyLogger EL824-GP data logger (Omnidata International, Ogden, UT, USA). Water table depth was measured adjacent to each site's tower using a Stevens water depth gauge (F-68, Leupold and Stevens, Beaverton, OR, USA).
Ecological measurements
The following description of the biomass sampling methods applies to the ACMF. DT biomass was sampled using similar procedures that are described in Gholz & Clark (2002) and Clark et al. (2004). Four inventory plots (50 m × 50 m) were established in random directions and distances between 50 and 200 m around the tower (directions constrained to place one plot in each compass quadrant). Tree density, height, and stem diameter at breast height (DBH; 1.37 m height) were measured in each plot in February 2000, 2001, and 2002. Allometric equations were used to determine standing wood, bark, and foliage biomass, and annual increment for each tissue (Taras & Clark, 1977; Taras & Phillips, 1978). Coarse root biomass was estimated as 13% of aboveground wood (plus bark) biomass (Gholz & Fisher, 1982). Litterfall was collected monthly from 10 1 m2 traps located within the inventory plots, and then dried for 72 h at 70 °C and separated into needles or other material. Needle accretion in the canopy for each year was estimated by applying needlefall from the following year to a normalized logistic accretion curve, assuming 18 months for needle turnover (Gholz et al., 1991; Dougherty et al., 1995; Jokela & Martin, 2000; Martin & Jokela, 2004). LAI was then calculated from needlefall and needle accretion estimates.
Ten randomly distributed subplots situated in each of the four inventory plots were established, and then measurements of saw palmetto and gallberry – the two dominant understory species – were applied to allometric equations to determine their biomass (Gholz et al., 1999; Powell et al., 2005). Biomass of grass and herbs was estimated from nine 1 m2 clip plots located around the tower at the time of peak biomass in 2000 and 2002. Forest floor mass was sampled in the winter of 2002 from five 100 cm2 subplots randomly located within each inventory plot. Samples were dried for 72 h at 70 °C and then weighed.
Needle litter decomposition was estimated using a simulation model for slash pine forest floor dynamics (Gholz et al., 1985), adjusted to reflect dynamics in longleaf pine forest. The model consisted of three coupled ordinary differential equations representing fresh needle litter, a labile litter fraction, and a lignin and cutin (refractory) fraction. The measured rate of fresh needle decomposition (0.15 year−1) in slash pine plantations does not accurately represent decomposition in longleaf pine-dominated ecosystems; Hendricks et al. (2002) reported a decomposition rate for longleaf pine needles on the forest floor surface which was 64% of the slash pine plantation estimate. Therefore, the parameters for fresh litter decomposition and transition rate from fresh to labile compartments were reduced to 0.096 and 0.319 year−1 to reflect this difference. To estimate the relative sizes of the three litter pools, the model was simulated from a starting point of no litter mass (postfire conditions). The spatial heterogeneity of the standing litter mass required separate simulation of each plot. Measured litterfall was used as annual inputs for the model.
Net CO2 exchange
Net ecosystem fluxes of CO2 (NEE, μmol CO2 m−2 s−1), sensible heat (H, W m−2), and latent heat (λE, W m−2) were measured with a closed-path eddy covariance system (Clark et al., 1999; Baldocchi, 2003; Lee et al., 2004; Powell et al., 2005). Both the ACMF and DT systems consisted of a three-dimensional sonic anemometer (Windmaster Pro, Gill Instruments Ltd, Lymington, UK) and an infrared gas analyzer (LI-6262, LI-COR Inc.) situated in a shed at the base of each tower. Raw data were logged at 10 Hz. The anemometer was situated at 32 m (10 m above mean canopy height) and 15–21 m (4 m above mean canopy height) at the ACMF and DT, respectively. Gas was sampled 10 cm from the center of the anemometer head at both sites and drawn through an IRGA at a flow rate of 6.0 L min−1. The sampling tubes (4 mm ID, Teflon coated) were 35 m long at both sites. The LI-6262s were calibrated twice per week using a traceable (± 1%) standard for CO2 and a dew point generator (LI-COR 610, LI-COR Inc.) for water vapor.
A 3 m mobile tower with an identical eddy covariance system was erected to measure understory fluxes between November 2000 and May 2002 in ACMF. We assumed that net ecosystem fluxes transferred across a horizontal plane existing between the bottom of the canopy and the understory (Baldocchi & Vogel, 1996; Powell et al., 2005). The understory tower was moved between three fixed locations within the footprint of the ACMF canopy tower to dampen localized effects of understory heterogeneity. No systematic patterns of variation among the three locations were observed, and thus all data were pooled.
Flux calculation software was used to rotate the horizontal wind velocities to obtain turbulence statistics perpendicular to the local streamline using Reynolds detrending (200 s constant). Net fluxes were calculated in half-hour intervals, and then collectively corrected for attenuation of the gas concentrations in the sampling tube, the nonideal frequency response of the LI-6262, and sensor separation loss using transfer functions (Moncrieff et al., 1997). Hourly barometric pressure measured at the Gainesville Regional Airport, 10 km southwest of the tower, was used to correct fluxes to ambient atmospheric pressure. The rate of change of CO2 stored within the air column below the eddy covariance system was used to estimate the CO2 storage flux (Hollinger et al., 1994; Clark et al., 2004). We used the meteorological convention that positive NEE indicated net movement of CO2 to the atmosphere from the ecosystem.
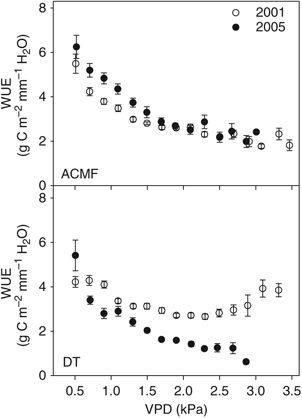
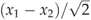 where x1 and x2 are the paired measurements meeting the conditions described above. As has been reported for other sites (Hollinger & Richardson, 2005; Richardson et al., 2006), we found that the distribution of δ was better described by a double-exponential distribution than a Gaussian distribution. Accordingly, we used the unbiased estimator of the standard deviation of δ for the double-exponential distribution:
where x1 and x2 are the paired measurements meeting the conditions described above. As has been reported for other sites (Hollinger & Richardson, 2005; Richardson et al., 2006), we found that the distribution of δ was better described by a double-exponential distribution than a Gaussian distribution. Accordingly, we used the unbiased estimator of the standard deviation of δ for the double-exponential distribution:
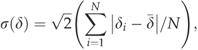 (1)
(1)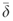 is the median δ. Statistics for the distributions of δ were calculated for various stratifications of the datasets (i.e. all data, night-time, daytime, PPFD≥1000, growing season, and non-growing season). It should be noted that we estimated δ of NEE rather than δ of FCO2, in contrast to Hollinger & Richardson (2005) and Richardson et al. (2006), and so our inferred random error includes the calculation of storage flux in addition to the measurement and calculation errors associated with the eddy covariance flux term.
is the median δ. Statistics for the distributions of δ were calculated for various stratifications of the datasets (i.e. all data, night-time, daytime, PPFD≥1000, growing season, and non-growing season). It should be noted that we estimated δ of NEE rather than δ of FCO2, in contrast to Hollinger & Richardson (2005) and Richardson et al. (2006), and so our inferred random error includes the calculation of storage flux in addition to the measurement and calculation errors associated with the eddy covariance flux term.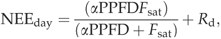 (2)
(2)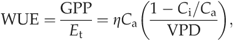 (3)
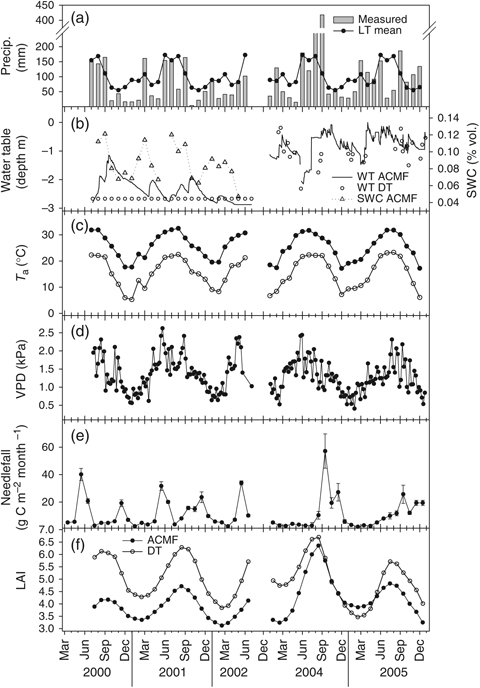
(3)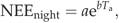 (4)
(4)To estimate net ecosystem production of C (NEP) over time steps greater than 30 min, missing values of NEEday and NEEnight were modeled using half-hourly measurements of either PPFD or Ta and monthly parameters fitted to Eqns (2) or (4), respectively (Falge et al., 2001). For gap-filled ecosystem C production, we used the convention NEP=(−NEE) and GEP=NEP−Re, where GEP is the gross ecosystem production and Re is the ecosystem respiration.
To quantify the extent to which gaps in the datasets introduced bias or error in our annual sums, we created 50 datasets with 10% of the data removed in artificial gaps for ACMF for 2005. The gaps were created as in Moffat et al. (2007), with 10 scenarios each consisting of very short gaps (isolated half-hours), short gaps (eight consecutive half-hours), medium gaps (64 consecutive half-hours), long gaps (12 consecutive days), and mixed scenarios containing a combination of the preceding scenarios. We then calculated NEP for each dataset using the procedures outlined above, and compared the distribution of annual NEP sums for the datasets with artificial gaps with the NEP calculated on the original dataset.
Results
Meteorological data
Environmental variables measured at the ACMF during the study period are shown in Fig. 1. The sum of precipitation for each measurement year is also reported in Table 5. Long-term (1971–2000) mean annual precipitation in the vicinity was 1228 mm, and mean maximum and minimum temperatures for the months of January and July were 19.0 and 5.8 °C, and 32.7 and 21.6 °C, respectively (Fig. 1a; NCDC, 2002). During the study, the general climatic pattern was dry mild winters (November–March), warm dry springs, (April and May), and warm humid summers (June–October). However, there was a great deal of variation within this pattern. Moreover, three major environmental anomalies occurred during this study. The first was a severe ‘100-year drought’ that was in progress at the commencement of the study and was not alleviated until the summer of 2002. A water table depth below 2 m at both the ACMF and DT was common from June 2000 to July 2002 and reflected the severity of the drought (Fig. 1b). The water table was generally near or at the surface for both ecosystems during 2004 and 2005 when precipitation was higher. The second anomaly was the winter between 2000 and 2001, when 32 days had minimum temperatures below 0 °C – 16 more freeze days than the long-term average (NCDC, 2002). The third anomaly was that three tropical storms hit the sites in August and September 2004. Heavy rain from these storms left both sites inundated in September and October, and high winds impacted tree canopy leaf area.
Environmental and foliage variables measured at the Austin Cary Memorial Forest (ACMF). (a) Monthly precipitation (bars) and long-term mean monthly precipitation from 1971 to 2000 (line; NCDC, 2002). (b) Monthly mean soil water content (SWC) at 10 cm depth and depth to the water table (WT). Donaldson Tract (DT) data are also shown. SWC was not available in 2004 and 2005. Flat line indicates that WT exceeded maximum sensor depth. (c) Monthly mean maximum (closed circles) and monthly mean minimum (open circles) air temperature (Ta). (d) Weekly mean afternoon (12:00–14:00 hours) vapor pressure deficit (VPD). (e) Monthly estimates of needlefall (mean ± SE). (f) Monthly estimates of all-sided, canopy, leaf area index (LAI).
| 12-monthperiod | Annual precipitation(mm) | NEP | GEP | R e | R e : GEP | ||||
|---|---|---|---|---|---|---|---|---|---|
| ACMF | DT | ACMF | DT | ACMF | DT | ACMF | DT | ||
| 2000–2001 | 956 | 192 | 616 | 1794 | 2423 | −1602 | −1807 | −0.89 | −0.75 |
| 2001–2002 | 812 | 159 | 660 | 1871 | 2687 | −1715 | −2026 | −0.92 | −0.75 |
| 2004 | 1373 | 185 | 729 | 1568 | 2348 | −1383 | −1619 | −0.88 | −0.69 |
| 2005 | 1185 | 154 | 490 | 1785 | 2248 | −1631 | −1758 | −0.91 | −0.78 |
- ACMF: Austin Cary Memorial Forest, mature, naturally regenerated pine forest.
- DT: Donaldson Tract, intensively managed, 10–15-year-old pine plantation.
- Each year either represents a calendar year or the 12-month period of July to June the following year. Values are reported as g C m−2 yr−1.
Ecological measurements
In January 2001, aboveground standing biomass was estimated as 5911 g C m−2 and belowground biomass as 721 g C m−2 (Table 1). In December 2002, total forest floor biomass was estimated as 1452 g C m−2. Annual increment for trees, understory, litterfall, and forest floor was 136 and 77 g C m−2 yr−1 for 2000 and 2001, respectively (Table 1).
| C pool | Biomass | Biomass increment | |
|---|---|---|---|
| Trees | 2001 | 2000 | 2001 |
| Stem and branch (includes bark) | 5543 ± 273 | 110 ± 25 | 59 ± 7 |
| Foliage | 244 ± 16 | 4 ± 1 | 2 ± <0 |
| Coarse roots* | 721 | 14 ± 3 | 8 ± 1 |
| Understory | 18 ± 15 | 18 ± 15 | |
| Serenoa repens | 66 ± 12 | ||
| Ilex glabra | 48 ± 7 | ||
| Grasses and herbs | 10 ± 6 | ||
| Forest floor† | 1452 ± 106 | −10 ± 12 | −10 ± 12 |
| Total | 8084 | 136 | 77 |
- * Coarse roots were estimated as 13% of stem and branches (Gholz & Fisher, 1982).
- † Estimated from 2002 forest floor census. Litterfall was incorporated into the forest floor calculation. Litterfall for year 1=161 ± 10 g C m−2 and for year 2=175 ± 13 g C m−2.
- Units are in g C m−2± SE.
During the 2 years with average precipitation (i.e. 2004 and 2005), one large pulse of needlefall occurred in the fall (Fig. 1e). In contrast, the drought caused the canopy to adjust by prematurely dropping 50% of annual needlefall in May and June of 2000 to 2002 (Fig. 1e). The unusually high pulse of needlefall in September 2004 was caused by high winds from the tropical storms. LAI was seasonal and the effects of the premature needle fall and high winds are reflected in the maximum summertime values in 2000, 2001, and 2005 (Fig. 1f). In comparison, DT LAI was approximately 30% greater than ACMF LAI during the drought years and very similar in 2004 before the wind damage (Fig. 1f). The drought appeared to have less of an effect on summertime LAI in the DT, while the tropical force winds also reduced LAI.
Measurement error
Similar to other estimates of eddy covariance C flux measurement error (Richardson et al., 2006, 2008), the distribution of NEE random measurement error (δ) in this study was better described by a double-exponential distribution than by a normal distribution (Fig. 2). The distributions of δ were similar between ACMF and DT, with approximately equivalent medians, standard deviations, and skewness between the sites (Table 2). Kurtosis for DT was higher than for ACMF, however, meaning DT δ clustered nearer the median and had slightly lower density in the tails than did ACMF (Fig. 2). Random error tended to be lower at night than during the day, especially at DT, and error was highest at higher radiation levels at both sites (Table 2). Growing season (March–October) error was slightly higher than during the winter (November–February) for both sites.
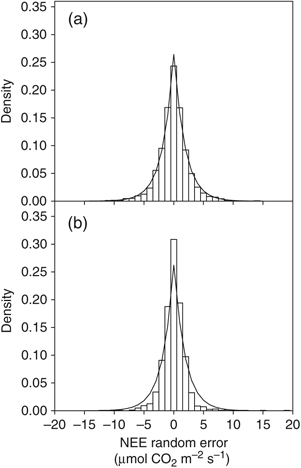
Histograms showing the distribution of the random net ecosystem exchange (NEE) measurement error δ and fitted PDF double-exponential functions for (a) Austin Cary Memorial Forest and (b) Donaldson Tract.
| Conditions | Austin Cary Memorial Forest | Donaldson Tract | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | SD | Skewness | Kurtosis | n | Median | SD | Skewness | Kurtosis | |
| All data | 8059 | 0.02 | 2.6 | −0.3 | 7.3 | 3650 | 0.03 | 2.6 | −0.8 | 14.3 |
| Day | 2254 | 0.08 | 2.9 | −0.5 | 7.5 | 1987 | 0.06 | 3.3 | −0.9 | 10.2 |
| Night | 5805 | 0.00 | 2.5 | −0.2 | 6.8 | 1663 | 0.02 | 1.8 | 0.8 | 11.9 |
| PPFD≥1000 | 610 | 0.02 | 3.2 | −0.2 | 7.1 | 715 | 0.33 | 3.7 | −0.3 | 5.4 |
| Growing season (March–October) | 5658 | 0.03 | 2.7 | −0.5 | 7.4 | 2501 | 0.08 | 2.8 | −0.7 | 12.3 |
| Winter (November–January) | 2401 | −0.03 | 2.5 | 0.2 | 6.3 | 1149 | −0.01 | 2.3 | −1.1 | 21.3 |
-
Error calculated with the daily differencing approach as
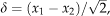 , where x1 and x2 are flux measurements taken under equivalent environmental conditions 24 h apart in time.
, where x1 and x2 are flux measurements taken under equivalent environmental conditions 24 h apart in time.
Net CO2 exchange
The ACMF was photosynthetically active during all parts of the year (Fig. 3). Light response curve parameters for selected months of 2001 and 2005, two hydrologically contrasting years, are given in Table 3. The drought and the seasonality of LAI and soil moisture cause little change in the magnitude of maximum NEEday (i.e. the dotted lines that mark the lower boundary of points in Fig. 3) during the growing season (March–October). For example, maximum NEEday reached approximately −16 to −18 μmol CO2 m−2 s−1 from May to October when soil moisture and LAI were highly variable in both 2001 and 2005.

Light response curves for seasonally representative months in 2001 (dry year) and 2005 (wet year). January: cool and dry, May: warm and dry, July and October: warm and humid. The dotted lines are a visual aid for comparative purposes to mark the bottom boundary of daytime net ecosystem CO2 exchange (NEEday). Regression parameters and statistics for each month are given in Table 3.
| Month | Model parameters | Statistics | |||
|---|---|---|---|---|---|
| F sat | α | R d | R 2 | n | |
| January 2001 | −17.6 ± 1.7 | −0.030 ± 0.010 | 4.7 ± 1.0 | 0.43 | 380 |
| May 2001 | −14.4 ± 0.9 | −0.037 ± 0.012 | 4.4 ± 1.1 | 0.32 | 594 |
| July 2001 | −18.5 ± 1.1 | −0.040 ± 0.011 | 6.0 ± 1.1 | 0.47 | 354 |
| October 2001 | −21.5 ± 0.6 | −0.047 ± 0.004 | 5.1 ± 0.3 | 0.78 | 636 |
| January 2005 | −7.2 ± 0.7 | −0.021 ± 0.007 | 1.7 ± 0.5 | 0.29 | 375 |
| May 2005 | −25.0 ± 2.0 | −0.022 ± 0.003 | 4.7 ± 0.5 | 0.67 | 465 |
| July 2005 | −23.6 ± 1.5 | −0.031 ± 0.010 | 4.9 ± 0.7 | 0.74 | 243 |
| October 2005 | −21.1 ± 1.5 | −0.037 ± 0.008 | 3.4 ± 0.7 | 0.63 | 269 |
- January: cool and dry, May: warm and dry, July and October: warm and humid.
The wide scatter of data points within the light response curves indicated that there were important secondary controls regulating NEEday , particularly during more droughty months, such as May and July 2001 – two months with relatively low R2 values (Table 3). Therefore, the midday (11:00–15:00 hours) residuals of each monthly light response regression (NEEres) were plotted against VPD and Ta to elucidate when these two variables became important constraints on NEEday (Fig. 4). These relationships were not affected by differences in soil moisture or season (data not shown) and therefore the data were pooled over each year. The ACMF reached optimum C uptake near VPD=1.0 kPa, after which C uptake declined linearly as VPD increased (Fig. 4a). Interestingly, the function of this relationship was not altered by drought. Air temperature below 10 °C severely reduced net C uptake (Fig. 4b), whereas the temperature range of 10–30 °C had a little effect on NEEday and above 30 °C caused net C uptake to decline somewhat.
(a) Residuals of daytime net ecosystem CO2 exchange (NEEday) at photosynthetic photon flux density (PPFD) (NEEres, μmol CO2 m−2 s−1) as a function of vapor pressure deficit (VPD, kPa) for 2001 (dry year) and 2005 (wet year). (b) NEEres as a function of above-canopy air temperature (Ta, °C) for July 2000–June 2001, a period that contains 32 days with below-freezing temperatures. NEEres are for the hours between 11:00 and 15:00 hours; mean ± 1 SE.
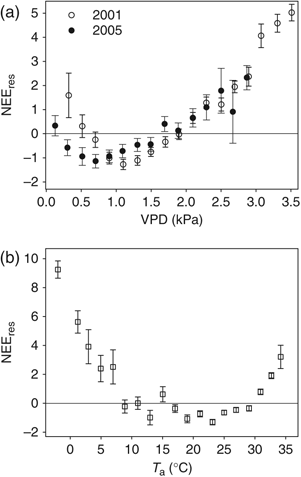
At ACMF, WUE declined linearly with increasing VPD, and was approximately constant at VPD>1.5 kPa; this relationship was similar in both droughty 2001 and wet 2005 (Fig. 5a). In contrast, the relationship between WUE and VPD at DT varied between wet and dry years (Fig. 5b). In 2005, the relationship at DT was similar to that at ACMF, but in 2001, the relationship was more complex, showing an increase in WUE at VPD above 2.5 kPa.
Water use efficiency (WUE) as a function of vapor pressure deficit (VPD, kPa) for (a) Austin Cary Memorial Forest (ACMF) and (b) Donaldson Tract (DT) during 2001 (dry year) and 2005 (wet year). WUE values are from 11:00 to 15:00 hours and photosynthetic photon flux density >500 μmol m−2 s−1; mean ± 1 SE.
Mean nightly (22:00–4:00 hours) C exchange, NEEnight, was a significant function of mean night-time Ta during well-coupled (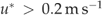 ) conditions (Table 4). Although SWC was highly variable over the year in this ecosystem, the addition of a SWC function to Eqn (4) was not statistically significant. However, separating the data into two different SWC groups (<5.5% and >5.5%) produced two significant functions, the former showing a 33% reduction in respiration rates at 25 °C (Table 4, Fig. 6). It should be noted that values for b (Table 4), which indicate how sensitive respiration was to changes in temperature, were significantly different (P=0.001), while a values were not significantly different (P=0.557) for these two conditions (determined statistically with an indicator variable; Powell et al., 2005). When SWC was >5.5%, mean NEEnight was 6.8 μmol CO2 m−2 s−1 at 25 °C, while under dry conditions, (SWC<5.5%) it was 4.6 μmol CO2 m−2 s−1 at 25 °C. Q10 for moist soil conditions was 2.0; while under dry conditions, Q10 was reduced to 1.4.
) conditions (Table 4). Although SWC was highly variable over the year in this ecosystem, the addition of a SWC function to Eqn (4) was not statistically significant. However, separating the data into two different SWC groups (<5.5% and >5.5%) produced two significant functions, the former showing a 33% reduction in respiration rates at 25 °C (Table 4, Fig. 6). It should be noted that values for b (Table 4), which indicate how sensitive respiration was to changes in temperature, were significantly different (P=0.001), while a values were not significantly different (P=0.557) for these two conditions (determined statistically with an indicator variable; Powell et al., 2005). When SWC was >5.5%, mean NEEnight was 6.8 μmol CO2 m−2 s−1 at 25 °C, while under dry conditions, (SWC<5.5%) it was 4.6 μmol CO2 m−2 s−1 at 25 °C. Q10 for moist soil conditions was 2.0; while under dry conditions, Q10 was reduced to 1.4.
| Model parameters* | Statistics | |||||
|---|---|---|---|---|---|---|
| a | b | R 2 | n | P-value | Q 10 | |
| Wet soil SWC>5.5% | 1.10 ± 0.09 | 0.070 ± 0.004 | 0.50 | 394 | < 0.001 | 2.0 |
| Dry soil SWC<5.5% | 1.85 ± 0.40 | 0.036 ± 0.011 | 0.22 | 49 | 0.001 | 1.4 |
- * In a dummy analysis, a parameters were not significantly different (P=0.557), while the b parameters were significantly different (P=0.001).
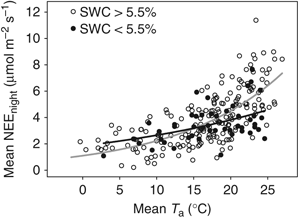
Mean nightly (22:00–4:00 hours) exchange of carbon dioxide above the slash pine canopy as a function of mean nightly air temperature (Ta) under well-coupled conditions (u>0.2). Gray regression line represents nights when soil water content (SWC)>5.5% and black regression line represents nights when SWC<5.5%. Regression parameters and statistics are given in Table 4.
An ordinary least squares regression analysis showed that over all times of the day, understory net ecosystem C exchange, NEEu, was linearly related to above-canopy NEE and accounted for approximately 37% of total ecosystem C fluxes (Fig. 7). This relationship was insensitive to changes in above-canopy VPD and soil moisture. It should be noted that random error associated with measurements of the independent variable, NEE, violates error assumptions of an ordinary least squares regression. Therefore, we also calculated the half-hourly ratio of NEEu to NEE and then examined the distribution of this ratio for 24-h, midday, and night-time periods. Median values of this ratio were 0.28 for 24-h period, 0.27 for midday, and 0.85 for night. Season did not have any apparent effect on these ratios. The frequency distribution of NEEu : NEE during both the night and midday was not normally distributed (Fig. 7b and c; Shapiro–Wilke, P<0.05). The night-time frequency distribution was broadly centered around the median value with 66% falling within the range of 0.65–1.08 (skewness of 0.66 and kurtosis of 2.0). In contrast, the midday frequency distribution was strongly centered around the median value with 66% falling within the range of 0.22–0.31 (skewness of 0.43 and kurtosis of 28.8).

Proportion of total net ecosystem exchange (NEE) accounted for by understory net ecosystem exchange (NEEu). (a) Linear regression of all half-hourly data pooled over 24 h. Histograms of NEEu : NEE for (b) night-time (22:00–4:00 hours), median=0.85 and (c) midday (11:00–15:00 hours), median=0.27.
ACMF NEP was remarkably similar (range: 154–192 g C m−2 yr−1) among the four measurement years of this study regardless of the considerable differences in precipitation (Table 5). The seasonality of C assimilation in the ACMF was also similar among the 4 years, with the greatest monthly C sequestration occurring in the early spring and then being nearly C neutral from late summer through the winter (Fig. 8a). April almost universally experienced the highest NEP each year (Fig. 8b). In comparison, NEP for DT was three to four times greater than that of the ACMF for each simultaneous measurement year (Table 5). DT seasonal NEP patterns also contrasted ACMF in that DT generally experienced high rates of C uptake during all parts of the year (Fig. 8a).
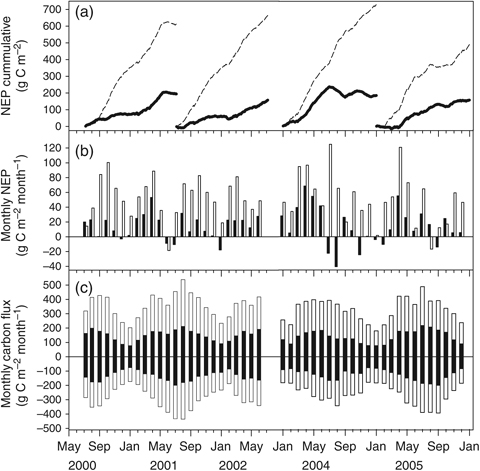
(a) Cumulative net ecosystem production (NEP) over the study period for the Austin Cary Memorial Forest (ACMF; solid) and Donaldson Tract (DT; dashed). (b) Monthly NEP for the ACMF (black) and DT (white). (c) Seasonality of total monthly gross ecosystem production (GEP; positive values) and ecosystem respiration (Re, negative values) for the ACMF (black) and DT (white).
The gap-filling analysis indicated that there was no major error or bias introduced by the distribution of gaps in our dataset. The annual NEP sum for the original, complete 2005 dataset was 154 g C m−2 yr−1. By comparison, NEP sums for the 50 gap-filling scenarios ranged from 135 to 170 g C m−2 yr−1, with a mean sum of 154 g C m−2 yr−1and standard deviation of 7 g C m−2 yr−1. The distribution of NEP sums was normal (Shapiro–Wilke, P=0.6818), with skewness of 0.25 and kurtosis of 0.09.
Annual Re and GEP are given for both forests in Table 5. The tropical storms in 2004 caused a reduction in Re and GEP in both ecosystems. Interestingly, the compensatory reduction of these fluxes resulted in very little difference in NEP with respect to the other years. In the ACMF, Re and GEP had an asynchronous seasonal pattern where GEP generally outpaced Re in the spring and early summer (Fig. 8c), which helps to explain why C gain was greatest during this period. The asynchronous pattern was also present in DT, but it was considerably less pronounced (Fig. 8c).
Discussion
Error analyses
Recent studies have reported the distribution of random flux errors at diverse flux sites (Hollinger & Richardson, 2005; Richardson et al., 2006, 2008). The σ(δ) reported for forested sites in these studies ranged from 2.4 to 4.3; both ACMF and Donaldson Tract σ(δ) was 2.6. Most sites also report higher σ(δ) under high radiation conditions compared with that under low radiation, and during the growing season vs. winter, as we also observed in our data (Table 2). These patterns are consistent with a scaling of random error with flux magnitude, with δ being lowest near fluxes of 0.0, and increasing as fluxes become more negative or more positive (Richardson et al., 2006).
Equations (2) and (4) have been demonstrated to perform well as gap-filling models for a wide range of forests (Falge et al., 2001; Moffat et al., 2007). In our case, the gap analysis for 2005 data established uncertainty boundaries within 12% for annual NEP and indicated that no major bias was introduced by the distribution of gaps. Therefore, we assumed that estimates of annual NEP for the other measurement years likely have a gap-filling error similar in magnitude.
Net CO2 exchange
NEEday was a strong curvilinear function of PPFD throughout each year due to the relatively mild winters and year-round physiological activity of pines in northern Florida (Martin, 2000; McGarvey et al., 2004). The magnitude of maximum summertime NEEday of the ACMF (−16 to −18 μmol CO2 m−2 s−1, dotted lines in Fig. 3) was ∼10 μmol CO2 m−2 s−1 lower than the adjacent DT plantation (Clark et al., 2004) and ∼5 μmol CO2 m−2 s−1 lower than an adjacent rotation-aged plantation (Clark et al., 1999). The less negative NEEday of the older ACMF stand likely was a result of higher maintenance respiration rates relative to net canopy assimilation and lower ecosystem LAI and stocking density relative to the plantations (Clark et al., 1999). However, ACMF maximum NEEday was similar to the global value (ca. −18.0 μmol CO2 m−2 s−1) reported for a wide range of temperate coniferous forests (Ruimy et al., 1995; Falge et al., 2002).
On a half-hourly time scale, PPFD was the dominant control over NEEday explaining 30–80% of its variation. However, there were important secondary controls when both ACMF and DT were under water stress. After VPD exceeded 2.0 kPa, the magnitude of NEEday in the ACMF decreased by as much as 5 μmol CO2 m−2 s−1. We have previously observed stomatal closure at VPD of 1.5–2.0 kPa across a range of soil water contents which is consistent with this pattern (Powell et al., 2005). A similar type of analysis conducted for DT also showed a decrease in mean NEEday of 5 μmol CO2 m−2 s−1 at PPFD of 1500 μmol m−2 s−1 when VPD exceeded 2.0 kPa for both drought and nondrought conditions (Clark et al., 2004). These results agree with findings of an old-growth Ponderosa pine forest in Oregon, which also experiences periods of extreme water stress and high VPD, where NEEday became increasingly suppressed with increasing VPD (Anthoni et al., 2002).
There was an important contrast in how the ACMF and plantations responded to the severe drought. Although the relative reduction in NEEday (i.e. 5 μmol CO2 m−2 s−1) was the same for both ecosystems during periods of high VPD, there was also a reduction in maximum NEEday during drought compared with nondrought periods at DT (e.g. Fig. 2 in Clark et al., 2004). Maximum NEEday at the ACMF, however, was similar between drought periods in 2001 compared with 2005 (e.g. dotted lines for My01 and Jy01 compared with My05 and Jy05 in Fig. 3); these contrasting responses were also reflected in differing patterns of WUE response to VPD in the two stands in dry and wet years (Fig. 5).
Residuals plotted against air temperature showed a broad optimum between 10 and 30 °C. This response can be explained by integrating the temperature responses of photosynthesis and ecosystem respiration. At the leaf level, Teskey et al. (1994) showed that slash pine net photosynthesis responded linearly and positively to temperature across a range of air temperature from 10 to 35 °C. We see similar responses in GPP, the sum of ecosystem photosynthesis. At a half-hourly time scale in this study, GPP declined linearly with air temperature (GPP=0.72–0.44Ta, n=18 186, R2=0.23, where GPP is the gross primary production in μmol CO2 m−2 s−1 and Ta is the air temperature in °C). When this linear response is combined with the weakly nonlinear response of NEEnight to temperature over the range of 10–25 °C (Fig. 5b), the result is a broad temperature optimum for NEEres.
In coniferous forests, global values for Fsat and α are −32.4 μmol CO2 m−2 s−1 and −0.024 μmol CO2 μmol photon−1, respectively (Ruimy et al., 1995). Although seasonality was weak in this ecosystem, Fsat was greatest in magnitude during growing season months when precipitation was not limiting (i.e. October 2001 and May–October 2005; Table 3) and similar to values reported for the adjacent rotation-aged plantation (−26.5 mol CO2 m−2 s−1; Clark et al., 1999). The variation in ACMF's quantum efficiency (−0.021 to −0.047) was less than the range reported for adjacent pine plantations [−0.025 to −0.075 (excluding clearcut values); Clark et al., 2004] and similar to that reported for a mixed-temperate maritime forest (−0.029 to −0.044; Aubinet et al., 2001) and a Scots pine forest (−0.011 to −0.047; Zha et al., 2004).
There was a statistical difference in the sensitivity of NEEnight to Ta when SWC of 5.5% was used as a threshold for dividing between wet and dry conditions (Table 4, Fig. 5). However, there is probably not any biological significance of this SWC value alone. This relationship more likely operates along a nonlinear soil moisture continuum as has been found in other studies (Reichstein et al., 2002). Unfortunately, our soil moisture sensor was damaged for 2004 and 2005, and thus our relatively small number of data points precluded us from further partitioning the data to better define this relationship for the ACMF. Under wet soil conditions, summertime NEEnight at this site (6.8 μmol CO2 m−2 s−1 at 25 °C) was similar to DT and rotation-aged (24-year-old) pine plantation NEEnight (range: 6.4–6.7 μmol CO2 m−2 s−1 at 25 °C; Clark et al., 1999, 2004). Similarly, under dry soil conditions (SWC<5.5% in the present study), summertime NEEnight (4.6 μmol CO2 m−2 s−1) was similar to that of DT (Clark et al., 2004).
Understory C fluxes
Night-time measurements of understory fluxes have been found to be less reliable than daytime fluxes in open canopy forests due to a build up of a strong inversion layer (Misson et al., 2007). To overcome this problem, understory data were screened using the same 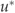 threshold as the canopy (0.2 m s−1) to ensure turbulent conditions. Nevertheless, the broader frequency distribution of night-time NEEu : NEE and large number of values greater than 1 (Fig. 7b) was evidence that the canopy and understory were not always well coupled even after the
threshold as the canopy (0.2 m s−1) to ensure turbulent conditions. Nevertheless, the broader frequency distribution of night-time NEEu : NEE and large number of values greater than 1 (Fig. 7b) was evidence that the canopy and understory were not always well coupled even after the 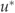 filter was applied.
filter was applied.
During the summertime, midday NEEu at the ACMF accounted for 27% of NEE, which was on the higher end of values reported by Misson et al. (2007) for a broad range of evergreen and deciduous forests (range: −54% to 36%, negative indicates that respiration dominates). During the summertime, night-time NEEu comprised 85% of NEE, which was also on the upper end of values reported for other pine forests (range: 35–65%; Misson et al., 2007). The proportion of 24-h mean half-hour NEE attributed to understory fluxes in the current study (∼25–35%; Fig. 6) was a bit higher than the estimate of 21% for a 23-year-old pine flatwoods plantation simulated by Golkin & Ewel (1984). Periodic ceptometer measurements indicated that 30–60% of incident PPFD penetrated the canopy of this stand (data not shown), suggesting that understory CO2 assimilation capacity was relatively high. In contrast, only 18–42% of incident PPFD penetrates the canopy in adjacent pine plantations (Gholz et al., 1991), indicating that the CO2 assimilation capacity of plantation understories is lower. Our results were similar to those of a boreal pine forest with a similar understory radiation environment, which accounted for 20–30% of NEE (Baldocchi & Vogel, 1996). However, our results do not support the hypothesis that NEEu would become a greater part of NEE as stresses imposed by VPD and SWC increased, as has been found in other studies of coniferous forests (e.g. Black & Kelliher, 1989). Rather, the relationship between NEE and NEEu was insensitive to changes in above-canopy VPD and soil moisture. This may be the result of the more open canopy resulting in the understory being subjected to similar stresses as the canopy trees.
Annual ecosystem C balance and partitioning
Annual NEP based on eddy covariance was greater than mensurational measurements in our study by 56 and 82 g C m−2 yr−1 for 2000–2001 and 2001–2002, respectively. The differences may be accounted for by unmeasured C translocation to coarse root carbohydrate storage pools. In addition to eddy covariance measurement errors, assumptions associated with the biomass measurements should be considered. First, our assumption that fine roots and soil organic matter were in steady state may be invalid. Second, although the years overlapped for the mensurational and eddy covariance measurements, they were not the same exact intervals. Third, designating June as the anniversary for eddy covariance during the first 2 years separated two halves of a growing cycle and may be problematic because labile C storage and use can differ between years (Sampson et al., 2001).
Spring to early summer appears to be the most intense period of C accumulation across a range of ecosystems within the Florida landscape [i.e. natural and intensively managed pine forests, cypress wetlands and scrub oak (Fig. 7a; Clark et al., 1999, 2004; Powell et al., 2006)], keeping in mind that maximum C gain may be decreased or shift to later in the summer in extremely dry springs. Nevertheless, this suggests that the asynchronous patterns of Re and GEP are similar across species and management practices in this region. A major contrast between the natural and managed stands was that the ACMF was essentially C neutral from late summer until the following spring (Fig. 8a), while the plantations were strong C sinks throughout the year (8, 6 in Clark et al., 2004).
Global climate change is expected to manifest in this region with increased tropical storm activity and more dramatic swings between very dry and very wet conditions (Twilley et al., 2001). Until this study, it was poorly understood how mature, less intensively managed pine flatwoods would respond to future weather scenarios. Our results demonstrate that both photosynthesis and respiration will be affected in different ways. For example, water stress caused both premature needlefall (Fig. 1e) and stomatal closure (Fig. 5), which resulted in reduced canopy photosynthesis (i.e. Fsat in Table 3). At the same time, soil respiration was reduced by dry soil conditions and presumably reduced substrate as a result of lowered photosynthesis (Hogberg et al., 2001; Bhupinderpal-Singh et al., 2003; Johnsen et al., 2007). Or in terms of the hurricane, GEP was reduced by needle loss, while Re was thought to be reduced by anoxic conditions in the inundated soils coupled with a reduction in substrate due to needle loss. The compensatory effect of both reduced GEP and Re in the face of either drought or tropical storms results in NEP values that are very similar to ‘average’ years. Our results are supported by Clark et al. (2004) and Li et al. (2007) who found similar compensatory effects of GEP and Re on NEP for a pine plantation exposed to drought and a Florida scrub-oak exposed to a hurricane, respectively.
The ACMF sequestered ∼700 g C m−2 over the four measurement years of the study. In comparison, the highly productive DT pine plantation sequestered ∼2500 g C m−2 over the same 4-year period. Additionally, a nearby rotation-aged slash (24-year-old) pine plantation and a loblolly pine plantation in the North Carolina piedmont both had annual NEP estimates of the same order of magnitude as DT annual NEP (Clark et al., 1999, 2004; Juang et al., 2006). The lower Re to GEP ratio for the plantations compared with the ACMF (Table 5) suggests that the higher GEP in the plantations was driven by higher LAI and a smaller fraction of photosynthate allocated to maintenance respiration. After the rotation-aged plantation was harvested, a large amount of C was released (e.g. −1269 and −882 g C m−2 yr−1) over the subsequent 2 years (Clark et al., 2004). These large differences highlight the importance of stand age and management regime for stand-level C balance in this region.
Implications of these management regimes on C sequestration in the long term can be assessed using simple calculations of cumulative NEP for the ACMF stand and for a generic slash pine plantation in the region over a 24-year period, which is a typical plantation rotation length. We used plantation calculations of NEP for regenerating, mid-rotation, and rotation-aged stands published by Clark et al. (1999, 2004) and Binford et al. (2006) that started after the harvest of a previous stand. Using the average NEP for the ACMF over the 4 years yields 4140 g C m−2 after 24 years vs. 9900 g C m−2 for the plantation. This calculation leaves two important uncertainties unaddressed. Timber harvesting removes most of the accumulated C from the plantations at the end of each rotation, and the fate of harvested C must be tracked with life cycle analysis (Skog & Nicholson, 1998; Ross & Evans, 2004). For the ACMF stand, periodic prescribed fire will return some proportion of understory and forest floor C to the atmosphere at each fire cycle; research on this topic is ongoing at this site.
The dynamics of landscape-level C sequestration is a function of interactions among environmental conditions, site-specific edaphic characteristics, and land management (e.g. evergreen pine vs. deciduous cypress, age or time since disturbance, soil type and topography, tree age demographics, season and year; Gholz et al., 1991; Liu, 1998; Binford et al., 2006). Intensively managed plantations change from a net C source to a net C sink as early as 3 years following harvest (Gholz & Fisher, 1982; Thornton et al., 2002; Clark et al., 2004), and rapidly develop high rates of C sequestration (Clark et al., 2004) that are offset by periodic timber harvesting. These large changes do not occur in older, less intensively managed pine stands, such as the ACMF, because canopy cover, stand structure, and biomass are largely maintained over time. Therefore, arguably one of the largest uncertainties in forecasting regional C budgets lies with our ability to predict how present management practices might change with future ownership. Finally, this study demonstrated that prolonged severe drought had little effect on NEP, which highlights that the effects of projected changes in climate on coastal plain pine forest NEP are likely to be small relative to those of land use and management change.
Acknowledgements
This research was supported in part by the Biological and Environmental Research (BER) Program, U.S. Department of Energy, through the Southeast Regional Center (SERC) of the National Institute for Global Environmental Change (NIGEC) and the National Institute for Climatic Change Research (NICCR); National Science Foundation award no. 0344029; the NASA Land Cover and Land Use Change (LCLUC) Program; the U.S. Department of Agriculture Forest Service; and the University of Florida School of Forest Resources and Conservation. This paper was partially based on work supported by the National Science Foundation while Henry L. Gholz was working at the Foundation. Any opinion, findings, and conclusions expressed here are those of the authors and do not necessarily reflect the views of the Foundation. We thank Dr Jennifer Jacobs, Ryan Atwood, Jose Luis Hierro, Jennifer Staiger, Antje Moffat, and Julie Graves for their contributions to this work. We are grateful to the Associate Editor and two anonymous referees for their thoughtful and constructive comments.




