Net ecosystem productivity of boreal jack pine stands regenerating from clearcutting under current and future climates
Abstract
Life cycle analysis of climate and disturbance effects on forest net ecosystem productivity (NEP) is necessary to assess changes in forest carbon (C) stocks under current or future climates. Ecosystem models used in such assessments need to undergo well-constrained tests of their hypotheses for climate and disturbance effects on the processes that determine CO2 exchange between forests and the atmosphere. We tested the ability of the model ecosys to simulate diurnal changes in CO2 fluxes under changing air temperatures (Ta) and soil water contents during forest regeneration with eddy covariance measurements over boreal jack pine (Pinus banksiana) stands along a postclearcut chronosequence. Model hypotheses for hydraulic and nutrient constraints on CO2 fixation allowed ecosys to simulate the recovery of C cycling during the transition of boreal jack pine stands from C sources following clearcutting (NEP from −150 to −200 g C m−2 yr−1) to C sinks at maturity (NEP from 20 to 80 g C m−2 yr−1) with large interannual variability. Over a 126-year logging cycle, annualized NEP, C harvest, and net biome productivity (NBP=NEP–harvest removals) of boreal jack pine averaged 47, 33 and 14 g C m−2 yr−1. Under an IPCC SRES climate change scenario, rising Ta exacerbated hydraulic constraints that adversely affected NEP of boreal jack pine after 75 years. These adverse effects were avoided in the model by replacing the boreal jack pine ecotype with one adapted to warmer Ta. This replacement raised annualized NEP, C harvest, and NBP to 81, 56 and 25 g C m−2 yr−1 during a 126-year logging cycle under the same climate change scenario.
Introduction
Net ecosystem productivity (NEP) of boreal forest stands is strongly affected by the time course of recovery following stand-replacing disturbances (fire, logging). Such disturbances must be accounted for when projecting changes in forest carbon (C) stocks under hypothesized changes in climate. Forest ecosystems have been found to lose C for 10–20 years after disturbance (Cohen et al., 1996; Schulze et al., 1999; Janisch & Harmon, 2002; Litvak et al., 2003; Humphreys et al., 2005) because heterotrophic and autotrophic respiration (Rh and Ra) exceeded CO2 fixation (= gross primary productivity GPP) (Amiro, 2001; Rannik et al., 2002; Pypker & Fredeen, 2002a, b; Kowalski et al., 2003). During this period, Rh was raised by decomposition of forest residue left after disturbance, while GPP was constrained by small leaf area while photosynthetic capacity was being rebuilt. Eventually GPP rose as forest leaf area recovered, while Rh declined as residue left after disturbance was depleted, so that forests started to regain C. Net primary productivity (NPP = GPP−Ra) reached a maximum about 20–40 years after stand-replacing disturbances (Amiro et al., 1999; Song & Woodcock, 2003), but declined gradually thereafter. Over time litterfall and hence Rh rose, so that NEP (= NPP−Rh) also declined with advancing age after maximum NPP was reached (Litvak et al., 2003).
Because forest age strongly affects NEP, there is a need to understand the time course of stand-level NEP following disturbance so as better to model the contribution of disturbance and postdisturbance recovery to national projections of forest C inventories. Age effects on GPP have been modelled from growth curves (Li et al., 2002; Peng et al., 2002) or allometric relationships (Song & Woodcock, 2003) of different forest types derived from existing inventories of wood volume. These modelled effects require assumptions about the time course and limits of forest growth that may not be valid under site conditions and climates different than those under which these models were developed. A more robust approach to modelling age effects on forest productivity would require the explicit simulation of the hydraulic and nutrient processes that are believed to determine GPP, Ra and Rh.
The recovery of GPP after logging and fire has been modelled from changing nutrient uptake caused by different time scales for decomposition of fine, nonwoody litter and coarse woody litter (Grant et al., 2006b). During the first few years after logging and fire, rapid decomposition of fine litter with comparatively small C : nutrient ratios caused a transient net mineralization of nutrients in the model. This mineralization could stimulate early regeneration of leaf area index (LAI) and a rise in GPP, mostly from herbaceous pioneer species (assart effect as in Kimmins, 2004). However, slower decomposition of large amounts of coarse woody litter with larger C : nutrient ratios gradually immobilized soil nutrients thereafter (Schimel & Firestone, 1989), slowing plant nutrient uptake and reducing foliar nutrient concentrations (Bradley et al., 2002), and thereby slowing the recovery of GPP (Kimmins, 2004). This constraint on nutrient uptake was eventually relieved after∼30 years as the coarse woody litter was oxidized or transformed into products with smaller C : nutrient ratios, allowing gradual nutrient mineralization, and hence more rapid nutrient uptake and GPP.
Declines in NPP several decades after disturbance have been modelled from hydraulic limitations caused by increasing path length for water uptake in taller trees, forcing lower canopy water potentials (ψc) to maintain transpiration (Grant et al., 2006a, b). Lower ψc in taller trees was modelled from larger gravitational potential differences and lower axial hydraulic conductances between soil and canopy (Ryan & Yoder, 1997; Hubbard et al., 1999), although lower axial hydraulic conductances may be partially offset by increased sapwood area (Becker et al., 2000). Lower ψc reduced stomatal conductance (gc) and hence GPP in the model, especially under higher vapour pressure deficit (D), without reducing the maintenance component (Rm) of Ra, so that NPP declined during later forest growth (Grant et al., 2006a, b).
In this study, we test whether hydraulic and nutrient constraints on GPP as simulated in the ecosystem model ecosys (Grant, 2001) could explain changes in hourly CO2 and energy exchange and in annual and centennial NEP measured in boreal jack pine (Pinus banksiana) stands of different ages following clearcutting under a boreal continental climate in central Saskatchewan. Hydraulic constraints are expected to be particularly severe in this ecosystem because of the low water holding capacity and rapid drainage of the soils on which boreal jack pine grows in central Saskatchewan. This test extends earlier work modelling regeneration of highly productive Douglas-fir stands under a temperate coastal climate (Grant et al., 2006b) to regeneration of low-productivity boreal pine stands under a cold continental climate. We then use this model to project changes in these constraints and hence in net ecosystem and biome productivity (NBP = NEP−C removals from clearcutting) under projected changes in boreal climate.
Model development
A comprehensive description of ecosys with a detailed listing of inputs, outputs, governing equations, parameters, results and references can be found in Grant (2001). A more detailed description of model algorithms and parameters most relevant to simulating forest age effects on NEP is given in Grant et al. (2006b). These algorithms are described briefly below:
Energy exchange
Canopy energy exchange in ecosys is calculated from an hourly two-stage convergence solution for the transfer of water and heat through a multilayered multipopulation soil–root–canopy system. The first stage of this solution requires convergence to a value of canopy temperature Tc for each plant population at which the first-order closure of the canopy energy balance (net radiation, sensible heat flux, latent heat flux and change in heat storage) is achieved [Eqs. (A1–A15) in Grant et al., 1999b]. These fluxes are controlled by aerodynamic (ga) and stomatal (gc) conductances. Two controlling mechanisms are postulated for gc:
- 1
At the leaf level, a maximum leaf conductance gl is calculated for each leaf surface that allows an initial ratio of intercellular to atmospheric CO2 concentration Ci : Ca to be maintained at carboxylation rates (Farquhar et al., 1980) calculated from mesophyll CO2 concentration (Cm, the aqueous counterpart of Ci) under ambient irradiance, Tc, Ca and full turgor. This ratio will be allowed to vary when canopy water status is solved at a later stage in the calculations as described in ‘GPP’ below. Leaf carboxylation rates from this model are then used with the initial Ci : Ca to calculate maximum gl which is then aggregated by leaf surface area to maximum gc for use in the energy balance convergence scheme (Grant et al., 1999b, 2006a, b).
- 2
At the canopy level, gc is then reduced from that at full turgor through an exponential function of canopy turgor potential ψT determined from total ψc and osmotic ψ water potentials generated during convergence for transpiration vs. water uptake [Eqs. (A32–A37) in Grant et al., 1999b; Eqs. (B1–B4) in Grant et al., 2006b]. The calculation of ψc is desribed in the next subsection.
Water relations
After convergence for Tc is achieved, the difference between canopy transpiration E from the energy balance and total water uptake U from all rooted layers in the soil is tested against the difference between canopy water content from the previous hour and that from the current hour (Grant et al., 1999b, 2006b). This difference is minimized by adjusting ψc which determines transpiration through ψT, and hence gc [Eqs. (A24–A25) in Grant et al., 1999b; Eqs. (B1–B4) in Grant et al., 2006b], and uptake through the potential differences with root water potential ψr, and soil water potential ψs across soil–root radial hydraulic resistance Ωs and root–canopy radial and axial hydraulic resistances Ωr and Ωa in each rooted soil layer [Eqs. (A32–A37) in Grant et al., 1999b; Eqs. (B5–B12) in Grant et al., 2006b).
GPP
After successful convergence for Tc and ψc, leaf carboxylation rates are adjusted from those calculated under full ψT to those under ambient ψT. This adjustment is required by the decrease in gc from its maximum value (calculated in ‘Energy exchange’) to that at ambient ψT (calculated in ‘Water relations’). The adjustment is achieved through a convergence solution for Ci at which the diffusion rate of gaseous CO2 between Ca and Ci through gl [Eqs. (A48–A53) in Grant et al., 1999b] equals the carboxylation rate of aqueous CO2 at Cm (described in ‘Energy exchange’) [Eqs. (A38–A47) in Grant et al., 1999b; Eqs. (C1–C11) in Grant et al., 2006b]. The CO2 fixation rate of each leaf surface at convergence is added to arrive at a value for GPP by each tiller (or branch) of each plant population (i.e. species or cohort) in the model. The CO2 fixation product is stored in a nonstructural C pool for translocation to growing organs.
Nutrient uptake and translocation
Uptake of nitrogen (N) and phosphorous (P) is calculated by solving for solution [NH4+], [NO3−] and [H2PO4−] at root and mycorrhizal surfaces at which radial transport by mass flow and diffusion from the soil solution to these surfaces equals active uptake by the surfaces [Eq. (A36) in Grant, 1998; Grant et al., 2006b]. Solution N and P concentrations are controlled by precipitation, adsorption and ion pairing reactions [Eqs. (A1–A55) in Grant & Heaney, 1997; Grant et al., 2004a], vertical and horizontal solute transport (Grant & Heaney, 1997) and microbial activity including mineralization, nitrification, denitrification, volatilization and N2 fixation described in ‘Heterotrophic respiration’ below. Products of N and P uptake are stored in nonstructural pools for translocation to growing organs.
Nonstructural C is coupled with nonstructural N and P to drive growth of shoots, roots and mycorrhizae according to organ-specific C : N : P ratios. Coupling requires the transfer of nonstructural C, N and P among shoots, roots and mycorrhizae [Eqs. (18–24) in Grant, 1998). These transfers are driven by concentration differences in nonstructural C, N and P (Brugge & Thornley, 1985).
Low ratios of nonstructural N or P to nonstructural C in branch or tiller pools indicate excess CO2 fixation with respect to N or P uptake for shoot growth. Such ratios in the model have two effects:
- 1
They reduce specific activities of rubisco and chlorophyll (i.e. deactivation), thereby simulating the suppression of CO2 fixation by leaf nonstructural carbohydrate accumulation widely reported in the literature (Bowes, 1991; Stitt, 1991).
- 2
They reduce the structural N : C and P : C ratios at which leaves are formed because the nonstructural C, N and P pools are the substrates for organ growth. Lower structural ratios cause a proportional reduction in areal concentrations of rubisco and chlorophyll, reducing leaf CO2 fixation.
Autotrophic respiration
The nonstructural C pool in each branch is also used for autotrophic respiration Ra [Eqs. (A26–A31) in Grant et al., 1999b; Eqs. (C12–C16) in Grant et al., 2006b]. Ra is first used to meet requirements for maintenance respiration Rm then any excess is used for growth respiration Rg to drive biosynthesis according to organ-specific growth yields. Allocation of C to aboveground biosynthesis of foliage, twigs, branches, boles and reproductive material is directed by phenology-dependent partitioning coefficients (e.g. Weinstein et al., 1991) driven by temperature and photoperiod. Allocation of C to biosynthesis of roots is governed by a functional equilibrium between shoot and root nonstructural pools. Low nonstructural C or rapid Rm may cause Ra to become less than Rm, in which case the shortfall is made up through respiration of remobilizable protein C withdrawn from foliar and twig C at each node. The remaining structural C at the node is dropped from the branch and added to the soil surface as litter [Eqs. (C17–C19) in Grant et al., 2006b]. Environmental constraints such as nutrient, heat or water stress that lower nonstructural C and hence Ra, or that raise Rm, will therefore hasten litterfall from the plant.
Heterotrophic respiration
Organic transformations in ecosys occur in five organic matter – microbe complexes i [coarse woody litter, fine nonwoody litter, animal manure, particulate organic matter (POM), and humus] in each soil layer. Each complex consists of five organic states: solid organic matter, dissolved organic matter, sorbed organic matter, microbial biomass, and microbial residues, among which C, N and P are transformed. Coarse woody and fine nonwoody litterfall are partitioned into carbohydrate, protein, cellulose and lignin components (Trofymow et al., 1995), each of which is of differing vulnerability to hydrolysis by heterotrophic decomposers. Hydrolysis rates are controlled by soil temperature Ts through an Arrhenius function [Eq. (A5) in Grant et al., 2006b], and by soil water content θ through its effect on aqueous microbial concentrations and thereby on specific activity [Eqs. (A3–A4) in Grant et al., 2006b], in surface residue and in a spatially resolved soil profile.
Heterotrophic respiration Rh in each soil layer is the sum of that by all heterotrophic microbial populations n in each substrate-microbe complex [Eq. (A11) in Grant et al., 2006b]. Total Rh for all soil layers drives CO2 emission from the soil surface through volatilization and diffusion in gaseous and aqueous phases. Heterotrophic oxidation rates may be constrained by microbial nutrient concentrations [Eq. (A12)], and by θ [Eq. (A3)], Ts [Eq. (A13, A18)], DOC [Eq. (A13)] and O2 [Eqs. (A14–A16), all in Grant et al., 2006b]. Further details about the calculation of Rh may be found in Grant (2001), Grant et al. (1993a, b, 2006b).
Experimental sites
The Southern Old Jack Pine (SOJP) site in 2004 was an 85-year-old jack pine forest regenerated from fire on an excessively drained Eutrocrept overlying a glacial till south of Narrow Hills Provincial Park, Saskatchewan, Canada near the southern limit of the boreal forest. Site and soil data at SOJP are given in Tables 1 and 2. Eddy covariance (EC) techniques used to measure mass and energy exchange at SOJP were described in Griffis et al. (2003) and Kljun et al. (2006). Gap-filling techniques to replace rejected EC CO2 fluxes were described in Barr et al. (2002), using a threshold friction velocity of 0.35 m s−1. Soil respiration was measured continuously with an infrared gas analyser connected to five 65 L transparent automated soil chambers (Griffis et al., 2004). Soil water contents through the rooting zone were measured continuously by automated TDR probes.
| Site characteristics | HJP02 | HJP94 | HJP75 | SOJP |
|---|---|---|---|---|
| Latitude | 53.945°N | 53.908°N | 53.876°N | 53.916°N |
| Longitude | 104.649°W | 104.656°W | 104.645°W | 104.692°W |
| Elevation (m) | 579 | 580 | 534 | 579 |
| Mean annual precipitation (mm) | 467 | 467 | 467 | 390–405 |
| Mean annual temperature (°C) | 0.4 | 0.4 | 0.4 | 1.5 |
| Dominant vegetation | None | Jack pine | Jack pine | Jack pine |
| Age in 2004 | 2 | 10 | 29 | 85 |
| Understory vegetation | Lichen grasses herbs | Grasses shrub | Lichen green alder | Sparse green alder |
| Horizon | LFH | Ae | AB | Bm | C1 | C2 | Ck | Ck |
|---|---|---|---|---|---|---|---|---|
| Depth to bottom (m) | 0.04 | 0.06 | 0.10 | 0.38 | 0.89 | 1.17 | 1.69 | 3.0 |
| Bulk Density (Mg m−3) | 0.24 | 1.23 | 1.45 | 1.48 | 1.52 | 1.60 | 1.60 | 1.60 |
| Field Capacity (m3 m−3) | 0.21 | 0.062 | 0.064 | 0.048 | 0.038 | 0.024 | 0.027 | 0.026 |
| Wilting Point (m3 m−3) | 0.07 | 0.033 | 0.038 | 0.027 | 0.024 | 0.014 | 0.016 | 0.016 |
| Ksat (mm h−1) | 420 | 620 | 620 | 830 | 980 | 1130 | 1130 | 1130 |
| Sand (g kg−1) | – | 943 | 934 | 939 | 963 | 975 | 975 | 976 |
| Silt (g kg−1) | – | 29 | 39 | 33 | 19 | 10 | 8 | 10 |
| Clay (g kg−1) | – | 28 | 27 | 28 | 18 | 15 | 17 | 14 |
| Coarse fragments (m3 m−3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| pH | 4.7 | 5.3 | 5.5 | 5.8 | 5.8 | 5.9 | 6.1 | 6.2 |
| CEC (cmol(+) kg−1) | 501 | 46 | 40 | 22 | 21 | 16 | 13 | 16 |
| Organic C (g kg−1) | 250.7 | 9.95 | 6.43 | 1.30 | 0.23 | 0.17 | 0.17 | 0.17 |
| Total N (g Mg−1) | 4827 | 430 | 290 | 130 | 120 | 60 | 80 | 70 |
| Total P (g Mg−1) | 473 | 150 | 200 | 200 | 190 | 120 | 140 | 190 |
- *Anderson D (1998) BOREAS TE-01 Soils Data over the SSA Tower Sites in Raster Format, Available online at [http://www-eosdis.ornl.gov/] from the ORNL Distributed Active Archive Center, Oak Ridge National Laboratory, Oak Ridge, TN, USA.
EC flux towers were also installed in three jack pine sites near SOJP that had regenerated naturally after clearcutting in late 2000 and scarification in 2002 (HJP02), and after clearcutting in 1994 (HJP94) and 1975 (HJP75). Before clearcutting, these sites had been under mature jack pine stands that had regenerated after wildfires many years earlier. Soils at these sites were similar to that at SOJP (Table 1), except that surface organic layers were mostly incorporated into the upper mineral soil by scarification after logging. EC instrumentation and techniques were the same at HJP02, HJP75 and SOJP (Kidson, 2006). EC instrumentation and techniques at HJP94 were described in Iwashita et al. (2005). Closed-path infrared gas analysers were used at all sites.
Model experiments
Model evaluation
Ecosys was initialized with the soil and topographic properties given for SOJP in Tables 1 and 2, and with above- and belowground residues estimated to correspond to those left after a stand-replacing fire (e.g. Grant et al., 2006a). The rooting zone consisted of the soil horizons described in Table 2, with subdivision into nine soil layers to increase spatial resolution. Two additional soil layers with properties the same as those of the lowest rooted layer were modelled below the rooting zone. The lower soil boundary was set so that the water content in the lowest soil layer was maintained near field capacity. These additional layers enabled water transfer modelled in the rooting zone to be largely independent of assumptions about drainage through the lower boundary.
Ecosys was then run for three 126-year cycles, each of which ended with clearcutting in which 0.1, 0.1 and 0.6 of foliar, nonfoliar nonwoody, and coarse woody aboveground phytomass respectively above a height of 0.5 m was removed on 30 June. This cycle length was selected to allow modelling of forest productivity during regeneration, maturation and postmaturation growth stages. To simulate disturbance effects on surface litter during clearcutting, a small fraction (0.05) of surface woody and nonwoody litter was incorporated into the soil LFH horizon after simulated clearcutting. All fine nonwoody and coarse woody root phytomass in the model at clearcutting was added as litter to the soil layers in which they had been growing.
Jack pine was germinated at a density of 2 m−2 on 1 April of the year after initialization and each clearcut, and nonvascular vegetation (e.g. moss as in Grant et al., 2001a) was germinated at a density of 100 m−2 to simulate the surface vegetation that competed with jack pine for nutrients and water at most of the chronosequence sites (Table 1). These two plant populations used common attributes for CO2 fixation and C allocation by C3 perennial species (Appendices B and C of Grant et al., 2006b). In ecosys, these attributes include two key inputs for plant thermal adaptation: (1) one that defines thermal requirements to complete growth stages within the annual phenological cycle that controls aboveground C allocation among foliage, stem and reproductive material (e.g. Weinstein et al., 1991) and (2) one that determines values of temperature sensitivity functions for photosynthesis (Arrhenius) and maintenance respiration (exponential). These inputs were set to values for boreal ecotypes used in earlier testing of CO2 and energy exchange over boreal forests (Grant et al., 1999a, 2001a, b). The rooting system of jack pine was modelled with large primary root axes (Grant, 1998), creating a strong primary root sink that enabled roots to reach the lowest horizon boundary at a depth of 3 m (Table 2) about 20 years after seeding. Both populations regenerated without further management in the model, except for annual self-thinnings of jack pine that allowed the model to simulate a gradual decline in tree density from 2.0 m−2 at germination to 0.1 m−2 after 50 years, consistent with declines observed in jack pine stands. All self-thinned phytomass was added to above- and belowground residues.
Each logging cycle was run under repeated 11-year sequences (1994–2004 in Table 3) of hourly averaged weather data (shortwave radiation, Ta, humidity, wind speed and precipitation). Concentrations of NH4+ and NO3− in precipitation were set to 0.15 and 0.60 g N m−3, respectively to give average wet deposition rates of ca. 0.3 g N m−2 yr−1, and atmospheric NH3 concentration was set at 0.0025 μmol mol−1 to give average dry deposition rates of ca. 0.03 g N m−2 yr−1. Hourly CO2 fluxes and daily and annual NEP modelled from site weather data were compared with measured half-hourly fluxes and with total daily and annual fluxes derived through gap filling (Barr et al., 2002). These comparisons were made in years 2–3 after the first clearcut, which corresponded to 2003 and 2004 at HJP02, in years 8–11 after the first clearcut, which corresponded to 2001–2004 at HJP94, in year 29 after the first clearcut, which corresponded to 2004 at HJP75, and in years 78–81 after model initialization following fire, which corresponded to 2001–2004 at SOJP. The weather data under which the model was run were recorded 10 m above the SOJP forest canopy, but were replaced by those recorded above the HJP02, HJP94 and HJP75 sites during years when model results were compared with EC fluxes recorded over the younger stands.
| Year | Average temperature (°C) | Precipitation (mm) |
|---|---|---|
| 1994 | 0.48 | 436 |
| 1995 | 0.28 | 445 |
| 1996 | −1.03 | 565 |
| 1997 | 2.42 | 425 |
| 1998 | 3.21 | 421 |
| 1999 | 2.78 | 480 |
| 2000 | 0.96 | 379 |
| 2001 | 2.98 | 307 |
| 2002 | 0.74 | 429 |
| 2003 | 1.39 | 262 |
| 2004 | −0.10 | 719 |
Model predictions
Climate represented by the hourly averaged weather data used in the Model Evaluation is likely to change during the next century. To examine the effects of climate change on jack pine productivity, the model run described above was also conducted under Ca, Ta and precipitation events that were incremented hourly after the first clearcut at rates corresponding to those in the IPCC SRES A1B scenario (IPCC, 2001), and in the average projections of the climate change scenarios in Berthelot et al. (2005) (Table 4). This model run was intended to demonstrate model sensitivity to climate change. A fuller examination of climate change effects on jack pine productivity should include a wider range of climate and disturbance scenarios.
| Variable | Years After Clearcutting | |||||
|---|---|---|---|---|---|---|
| Season | 25 | 50 | 75 | 100 | 125 | |
| C a(μmol mol−1) | 409 | 452 | 499 | 552 | 609 | |
| P a (increase %) | 0.625 | 1.25 | 1.875 | 2.5 | 3.125 | |
| Maximum Ta (+°C) | January–March | 0.625 | 1.25 | 1.875 | 2.5 | 3.125 |
| April–June | 0.75 | 1.50 | 2.25 | 3.0 | 3.75 | |
| July–September | 0.375 | 0.75 | 1.125 | 1.5 | 1.875 | |
| October–December | 0.50 | 1.0 | 1.50 | 2.0 | 2.5 | |
| Maximum Ta (+°C) | January–March | 0.875 | 1.75 | 2.625 | 3.5 | 4.375 |
| April–June | 1.0 | 2.0 | 3.0 | 4.0 | 5.0 | |
| July–September | 0.625 | 1.25 | 1.875 | 2.5 | 3.125 | |
| October–December | 0.75 | 1.50 | 2.25 | 3.0 | 3.75 | |
- These rises were calculated hourly and added to existing weather data.
There is concern that current tree varieties may not be well adapted to higher temperatures expected during climate change, and so should be replaced at planting by varieties adapted to warmer climates. To examine possible impacts of adaptation on productivity, the climate change run was also conducted with the attributes for thermal adaptation of the boreal jack pine ecotype replaced after the first clearcut by those of a cold temperate ecotype. This changed thermal adaptation represented an ecotype intermediate between the boreal ecotype and a cool temperate ecotype used in Grant et al. (2006b) to model CO2 exchange and growth over a Douglas-fir forest in British Columbia. This change involved (1) increasing the thermal requirements to complete growth stages so that timing of these stages, and of associated changes in aboveground C allocation, during climate change coincided approximately with those modelled under current climate, and (2) displacing temperature functions for photosynthesis (Berry & Björkman, 1980) and respiration (Gifford, 1995) upwards by 0.75 °C, thereby reducing their values at lower temperatures, but increasing them at higher temperatures.
Results
Diurnal CO2 exchange
Differences in precipitation recorded at SOJP during 2001–2004 caused θ measured and modelled in the LFH and upper part of the mineral soil (0–15 cm) to remain near field capacity for most of 2001, 2002 and 2004 except during brief drying cycles, but declined to wilting point in 2003 from mid-July onwards (Fig. 1). Modelled θ remained within 0.02 m3 m−3 of measured values except when the soil was frozen, at which time modelled θ, which excluded ice, was lower. Both modelled and measured θ declined rapidly between rainfall events during all years because of the low water holding capacity and high hydraulic conductivity of the sandy soil at the jack pine sites (Table 2).
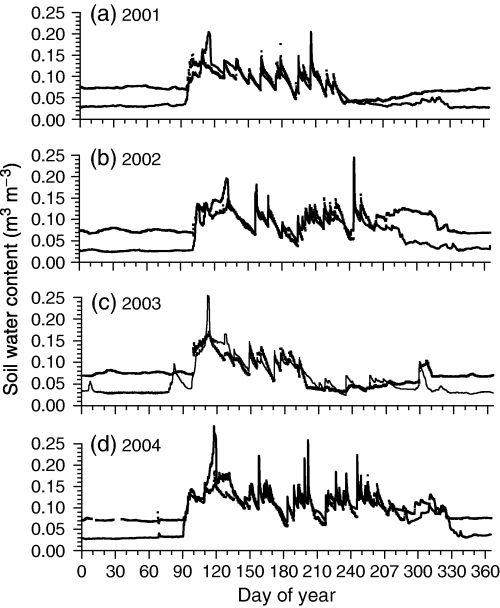
Water contents measured (symbols) and modeled (lines) during 2001–2004 in the upper 0.15 m of the soil profile under an 80-year-old jack pine stand (SOJP).
These soil attributes could cause productivity at these sites to be affected by hydraulic constraints if rainfall events are infrequent. These constraints were apparent during soil drying under high Ta and D from DOY 228–234 (16–22 August) 2003 (Fig. 2). During this period, rapid midday declines in CO2 influxes were measured and modelled at HJP94 and SOJP (Fig. 3b and c), especially when Ta > 25 °C and D > 2 kPa on DOY 228, 230, 231 and 234. Hydraulic constraints on CO2 fixation were alleviated by rainfall early in DOY 235 (Fig. 2), so that CO2 influxes at HJP94 and SOJP were greater from DOY 235 to 237 (23–25 August) 2003 (Fig. 3). CO2 influxes modelled and measured at HJP02 remained small and unaffected by rainfall (Fig. 3a).

(a) Radiation, air temperature, (b) vapour pressure deficit (D), wind speed and precipitation measured at Southern Old Jack Pine from DOY 228 to 237 (16–25 August) 2003.
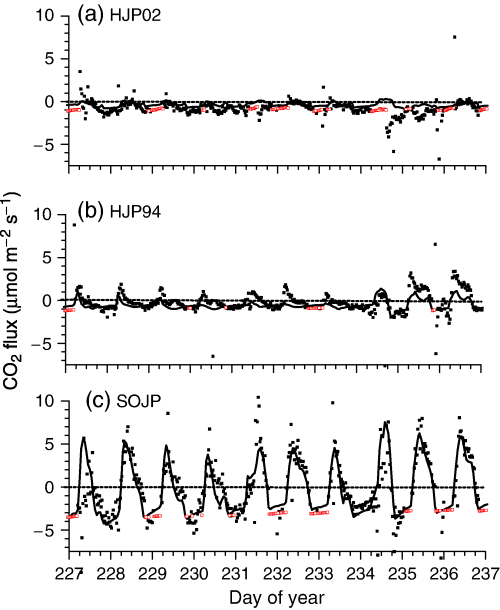
CO2 fluxes measured (closed symbols), gap-filled (open symbols) and modeled (lines) during DOY 228–237 (16–25 August) 2003 over regenerating jack pine sites clearcut in (a) 2002 (HJP02), (b) 1994 (HJP94), and (c) an adjacent mature jack pine stand burned in 1919 [Southern Old Jack Pine (SOJP)].
Soil drying slowed CO2 fixation and hence Ra (‘Autotrophic respiration’), and slowed decomposition and Rh (‘Heterotrophic respiration’). Ecosystem CO2 effluxes declined during soil drying at SOJP (e.g. DOY 228 vs. 234 in Fig. 3c). Soil CO2 effluxes also declined with θ during DOY 228–234 in 2003 (Fig. 4). These effluxes were partially offset by CO2 uptake from surface vegetation during daytimes, although uptake also declined with soil drying. Soil CO2 effluxes rose briefly following rainfall on DOY 235, as did CO2 uptake, but declined thereafter as soil drying continued.
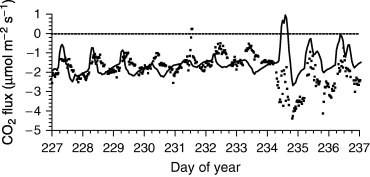
CO2 fluxes measured (closed symbols) and modeled (lines) during DOY 228–237 (16–25 August) 2003 over the vegetated soil surface at Southern Old Jack Pine.
Hydraulic constraints on CO2 fixation were further studied during soil drying under changing Ta and D from DOY 177 to 183 (25 June–1 July) 2004 (Fig. 5). Hydraulic constraints on CO2 fixation were apparent in the midafternoon declines in CO2 influxes measured and modelled during DOY 179 and 180 at HJP94, HJP75 and SOJP when Ta > 25 °C and D > 2 kPa (Fig. 6b–d). CO2 influxes at HJP02 remained low and unaffected by Ta and D (Fig. 6a).
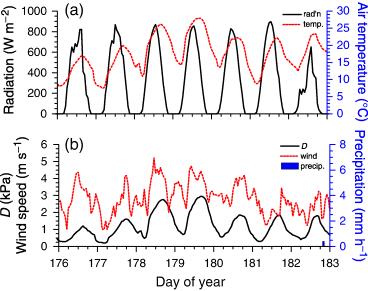
(a) Radiation, air temperature, (b) vapour pressure deficit (D), wind speed and precipitation measured at Southern Old Jack Pine from DOY 177 to 183 (25 June to 1 July) 2004.
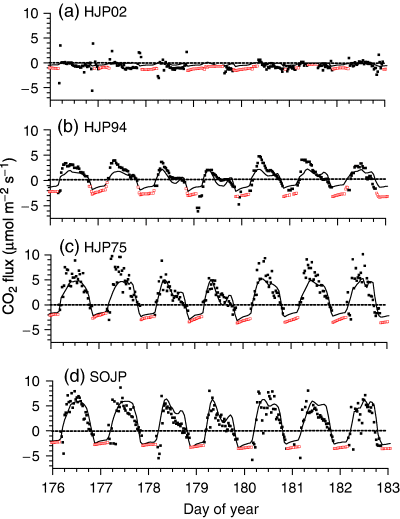
CO2 fluxes measured (closed symbols), gap-filled (open symbols) and modeled (lines) during DOY 177 to 183 (25 June to 1 July) 2004 over regenerating jack pine sites clearcut in (a) 2002 (HJP02), (b) 1994 (HJP94), (c) 1975 (HJP75), and (d) an adjacent mature jack pine stand burned in 1919 [Southern Old Jack Pine (SOJP)].
Some of the variation in EC measurements of CO2 fluxes was not explained by the model. Root mean squares for differences (RMSD) between measured and modelled CO2 fluxes during all of 2003 and 2004 rose with the magnitude of CO2 fluxes from 0.65 μmol m−2 s−1 at HJP02 to 1.40 μmol m−2 s−1 at HJP94 to 1.75 μmol m−2 s−1 at HJP75 and SOJP. Richardson et al. (2006) estimated that random errors of EC measurements at a range of forest sites rose from ca. 0.6 μmol m−2 s−1 for CO2 fluxes of 0 μmol m−2 s−1 to ca. 3.6 μmol m−2 s−1 for CO2 fluxes of +10 (influxes) or −5 (effluxes) μmol m−2 s−1, the largest fluxes measured in this study. The RMSD at HJP02, where CO2 fluxes were small, was comparable with random errors for near zero CO2 fluxes estimated by Richardson et al. (2006). The RMSD at HJP75 and SOJP was comparable with the middle of the range in random errors estimated for the CO2 fluxes measured in this study, and similar to an estimate of 2.4 and 1.5 μmol m−2 s−1 for random errors in annual CO2 influxes and effluxes respectively over a boreal transition spruce/hemlock forest (Richardson et al., 2006). These comparisons indicate that much of the variation in measured CO2 fluxes not explained by the model (e.g. 3, 6) may be attributed to uncertainty in EC measurements. This attribution is also suggested by the narrow range of 1.8–1.9 μmol m−2 s−1 in RMSDs reported by several other ecosystem models during a model intercomparison at SOJP (Grant et al., 2005).
Seasonal NEP
Daily NEP measured and modelled at HJP02 during 2004 remained below zero (net C source) during the entire year (Fig. 7b) because GPP remained smaller than Ra+Rh (Fig. 6a). Daily NEP measured and modelled at HJP94 during the same year was slightly above zero (net C sink) during late May and June, but remained near or slightly below zero during the rest of the growing season (Fig. 7c). NEP measured and modelled at HJP75 and SOJP during 2004 rose above zero during late April, reached maximum values during June, but declined gradually thereafter to zero by mid-October (Fig. 7d and e). Larger NEP at SOJP and HJP75 vs. HJP94 and HJP02 was caused by larger CO2 influxes vs. effluxes (3, 6) modelled and measured at the older sites. Declines in NEP modelled after June at all sites were attributed to rising CO2 effluxes from warming soils that increasingly offset CO2 influxes as the growing season progressed. NEP at all sites was adversely affected by warm weather between DOY 195 and 205 (Fig. 7).
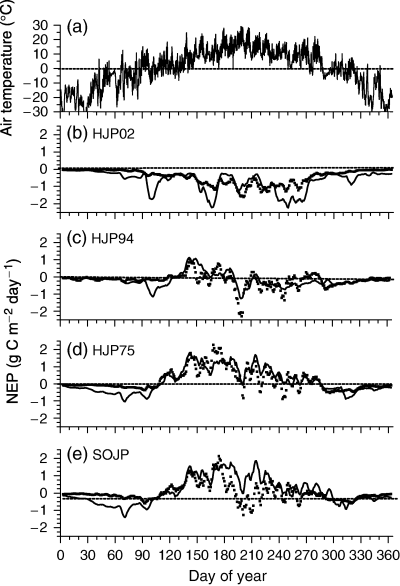
(a) Air temperatures, and (b–e) 5-day moving averages of net ecosystem productivity (NEP) calculated from gap-filled eddy covariance fluxes (symbols) and from ecosys (lines) in 2004 over regenerating jack pine sites clearcut in (b) 2002 (HJP02), (c) 1994 (HJP94), (d) 1975 (HJP75) and (e) an adjacent mature jack pine stand burned in 1919 (SOJP).
Annual NEP
During the first 2 years after clearcutting, NPP in the model was small in comparison with Rh so that annual NEP (= sum of all CO2 influxes and effluxes) averaged −180 g C m−2 yr−1 (net C source) (Table 5; Fig. 8a). GPP in the model was similar to that derived from gap-filled EC fluxes during these 2 years, but Re in the model rose with microbial growth on litter left after clearcutting more than did Re derived from EC. Howard et al. (2004) calculated NPP and Rh of 90 and 270 g C m−2 yr−1 respectively during the first year after logging in this jack pine chronosequence.
| Age (year) | Site | GPP | R a | NPP | Litterfall | Δwood | ΔSoil | R h | R e | NEP | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Above | Below | Above | Below | ||||||||||
| g C m−2 yr−1 | |||||||||||||
| 1 (2003) | HJP 02 | E | 60 | 214 | −154 | ||||||||
| S | 55 | 22 | 8 | 23 | 9 | 5 | 11 | −137 | 151 | 181 | −126 | ||
| 2 (2004) | HJP 02 | E | 69 | 226 | −157 | ||||||||
| S | 59 | 16 | 11 | 22 | 6 | 8 | 8 | −258 | 268 | 295 | −236 | ||
| 7 (2001) | HJP 94 | E | 290 | 358 | −68 | ||||||||
| S | 230 | 22 | 84 | 124 | 7 | 110 | 5 | −54 | 171 | 277 | −47 | ||
| 8 (2002) | HJP 94 | E | 261 | 328 | −67 | ||||||||
| S | 240 | 22 | 87 | 131 | 11 | 112 | 6 | −73 | 188 | 297 | −57 | ||
| 9 (2003) | HJP 94 | E | 306 | 15±7 | 324 | −18 | |||||||
| S | 195 | 20 | 75 | 100 | 9 | 108 | 3 | −43 | 168 | 263 | −68 | ||
| 10 (2004) | HJP 94 | E | 394 | 7±2 | 459 | −65 | |||||||
| S | 258 | 21 | 91 | 146 | 7 | 100 | 7 | −127 | 222 | 334 | −76 | ||
| 29 (2004) | HJP 75 | E | 564 | 74±6 | 493 | +71 | |||||||
| S | 573 | 196 | 100 | 277 | 64 | 113 | 64 | −53 | 227 | 523 | +50 | ||
| 76 (2000) | SOJP | E* | 683 | 62±6 | 605 | +78 | |||||||
| S | 793 | 267 | 187 | 339 | 57 | 185 | 70 | +5 | 256 | 710 | +83 | ||
| 77 (2001) | SOJP | E* | 717 | 46±4 | 676 | +41 | |||||||
| S | 776 | 293 | 183 | 300 | 72 | 197 | 62 | +52 | 216 | 692 | +84 | ||
| 78 (2002) | SOJP | E* | 603 | 87±11 | 626 | −23 | |||||||
| S | 621 | 236 | 163 | 222 | 82 | 155 | 63 | −2 | 204 | 603 | +18 | ||
| 79 (2003) | SOJP | E* | 653 | 66±7 | 624 | +29 | |||||||
| S | 656 | 242 | 156 | 258 | 61 | 157 | 66 | +16 | 222 | 620 | +36 | ||
| 80 (2004) | SOJP | E | 694 | 50±4 | 618 | +77 | |||||||
| S | 634 | 186 | 152 | 296 | 47 | 147 | 56 | −70 | 251 | 589 | +45 | ||
- * From Griffis et al. (2003) and Kljun et al. (2006).
- NEP, net ecosystem productivity; SOJP, Southern Old Jack Pine; GPP, gross primary productivity; NPP, net primary productivity.
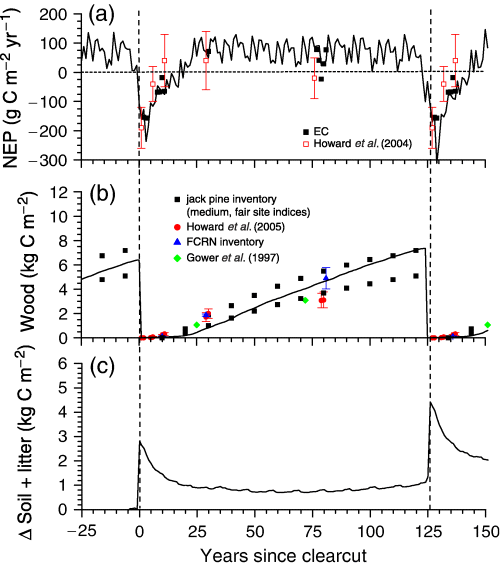
(a) Annual net ecosystem productivity (NEP) calculated from gap-filled eddy covariance fluxes (EC) and from C stock measurements by Howard et al. (2004) (symbols) along a jack pine chronosequence vs. NEP modelled during a 126-year ecosys logging cycle (lines), (b) wood C growth derived from forest inventory measurements (Alberta Forest Service, 1985), and from C stock measurements in Howard et al. (2004) and this study (FCRN) (symbols) along a jack pine chronosequence vs. wood C modelled during a 126-year ecosys logging cycle (lines), and (c) change in soil plus litter C modelled during a 126-year ecosys logging cycle under current climate. Vertical dashed lines indicate times of clearcutting.
Subsequent rises in GPP and declines in Rh caused NEP in the model to increase to ca. −50 g C m−2 yr−1 (net C source) between 7 and 10 years after clearcutting (Table 5; Fig. 8a). Howard et al. (2004) also calculated rises in NPP and declines in Rh to 130 and 170 g C m−2 yr−1 respectively after 5 years, and to 270 and 210 g C m−2 yr−1 respectively after 10 years. They estimated that boreal jack pine stands likely remained C sources for 7 years after clearcutting before becoming C sinks. The model run in this study indicated that jack pine stands would remain C sources for 15–20 years after clearcutting (Fig. 8a).
GPP in the model began to rise more rapidly after 10 years while Rh rose little, so that NEP approached mature values of 50 g C m−2 yr−1 after 29 years (Table 5; Fig. 8a). GPP rose more slowly thereafter, so that NEP remained at average values of 53±29 g C m−2 yr−1 interannual variability under 2000–2004 weather. NEP declined very gradually thereafter (Fig. 8a) from slight declines in stem axial conductance caused by slowly increasing bole height (Grant et al., 2006b), as observed experimentally in pine by Mencuccini & Grace (1996).
R a rose with respect to GPP during modelled forest growth, so that ratios of NPP : GPP declined from 0.50 to 0.55 during the first decade of growth to 0.40–0.45 after 80 years. These ratios were within the range of those derived in diverse forest ecosystems by Waring et al. (1998), but were larger than one of 0.31 derived from chamber measurements at SOJP in 1994 by Ryan et al. (1997). In the mature jack pine, 0.40–0.45 of modelled NPP was attributed to trees above ground (approximated by Δwood C+ aboveground litterfall C in Table 5, both of which were corroborated by measurements) and the remainder was attributed to surface vegetation or belowground. Gower et al. (1997) estimated from biometric measurements that 0.46 of NPP was allocated below ground at SOJP during 1994, but these measurements did not account for exudation, which contributed to belowground NPP in the model. Aboveground NPP in the model drove annual wood growth (Table 5) that was consistent with mean annual increments derived from wood growth curves for boreal jack pine at medium-productivity sites (Alberta Forest Service, 1985). These increments reached peak values of ca. 75 g C m−2 yr−1 60 years after stand establishment, and declined gradually thereafter.
Most modelled NEP was stored in wood C (Fig. 8b) and soil C (Fig. 8c) because losses of C as DOC (∼0.2 g C m−2 yr−1) and CH4 (0 g C m−2 yr−1) were negligible. Wood C growth modeled after the first clearcut was similar to that estimated from biometric measurements or from allometric calculations used in wood inventory assessments of jack pine sites with site indices similar to that at SOJP (Fig. 8b). Soil+litter C declined during the first 20 years after clearcutting while litter left from the previous forest decomposed (Fig. 8c), during which time NEP was negative (Fig. 8a; Table 5). Soil+litter C stabilized ca. 50 years after clearcutting at values that rose by ca. 1.6 kg C m−2 in successive logging cycles or 13 g C m−2 yr−1 (Fig. 8c). This rise was partly attributed to a small increase in ecosystem N stocks that slightly raised ecosystem productivity during successive logging cycles because N removals in wood during logging events were smaller than N inputs from atmospheric deposition and biological fixation during the intervening 126 years of growth in the absence of fire. This increase in modelled N stocks would be sensitive to logging frequency, and would be adversely affected by fire. Annualized NEP, C removal by clearcutting, and net biome productivity (NBP =∑NEP−C removal) averaged 47, 33 and 14 g C m−2 yr−1 respectively during the 126-year logging cycle.
Changes in NEP and C stocks with climate change
After 70 years of climate change, rising Ta (average+1.9 °C from Table 4) hastened thawing and evapotranspiration (‘Energy exchange’), so that θ rose and declined earlier than under current climate (Fig. 9a). Earlier declines in θ combined with higher Ca (Table 4) and D (Fig. 9b) to lower gc during soil drying, especially during midafternoons under higher D (Fig. 9c). Lower gc offset the effects of higher Ca on GPP so that CO2 influxes modelled under climate change declined with respect to those under current climate as D rose (Fig. 9d). At the same time, soil warming raised CO2 effluxes above those modelled under current climate, so that higher D between precipitation events had an adverse impact on NEP during climate change.
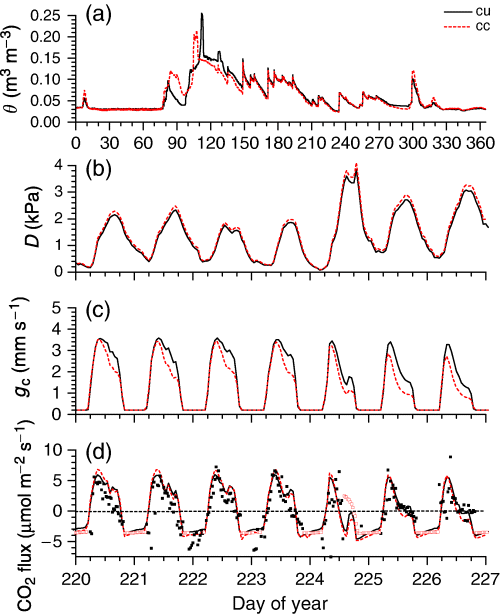
(a) Soil water content (θ), (b) vapour pressure deficit (D), (c) canopy stomatal conductance (gc), and (d) CO2 fluxes measured (closed symbols), gap-filled (open symbols) and modeled (lines) during (a) 2003 and (b–d) DOY 221–227 (20–26 July) 2003 under current climate (cu), and after 70 years of climate change (cc).
Annual NEP of boreal jack pine modelled under climate change gradually rose above that under current climate over time after clearcutting, especially during years with larger NEP (Fig. 10a). However, the adverse effects of rising Ta and D on GPP vs. Re (e.g. Fig. 9d) hastened declines in NEP more than 75 years after clearcutting. These declines led to the dieback of boreal jack pine in the model after 100 years of climate change as GPP eventually failed to offset Ra, depleting nonstructural C and accelerating litterfall (‘Autotrophic respiration’). At this time jack pine boles were transferred to standing dead, the remains of which were harvested at the end of the modelled logging cycle, and jack pine was reseeded. However, substantial losses of ecosystem C were modelled during jack pine regeneration following dieback (Fig. 10a).
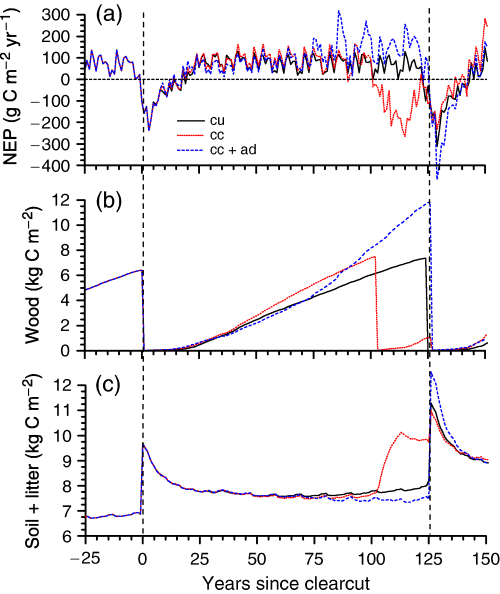
(a) Annual net ecosystem productivity (NEP), (b) wood carbon (C) and (c) soil plus litter C modelled during a 126-year ecosys logging cycle under current climate (cu), climate change (cc) (Table 4), and climate change+adapted tree variety (cc+ad) (see text). Vertical dashed lines indicate times of clearcutting.
Replacing the boreal pine ecotype with the cold temperate pine ecotype in the model lowered NEP during the first 50 years of climate change, but raised it thereafter (Fig. 10a). This later gain occurred as the temperature sensitivity of GPP in the temperate ecotype became better adapted to rising Ta, enabling GPP to offset Ra and so avoiding dieback. As productivity of the temperate pine rose, productivity of the understory declined, causing further gains in modelled NEP as understory nutrients were released. Annualized NEP, C removal by clearcutting, and net biome productivity (NBP =∑NEP−C removal) averaged 81, 56 and 25 g C m−2 yr−1 respectively during the 126-year logging cycle under climate change.
Climate change accelerated wood growth of boreal jack pine until dieback after 100 years (Fig. 10b), following which standing dead wood contributed to rises in soil+litter C (Fig. 10c). Wood growth of the cold temperate pine ecotype was slower than that of the boreal pine ecotype during the first 75 years of climate change, but surpassed it thereafter. Gains in wood C under climate change were partially offset by losses in soil+litter C (Fig. 10c).
Discussion
Hydraulic constraints on forest NEP
NEP of jack pine stands was affected at diurnal time scales by hydraulic constraints imposed by declines in θ and rises in D between rainfall events (3, 6) on this rapidly drained soil with low water holding capacity (Table 2). In the model, declines in θ forced declines in ψs, rises in Ωs and Ωa, and hence declines in ψc to meet transpirational demands (‘Water relations’), particularly when these demands were raised by large D (e.g. DOY 179–180 in Fig. 6). Values of ψc modeled in conifers were more sensitive to θ and D than were those in other plant functional types because of the larger root and stem axial resistances used to calculate Ωa in conifers (Grant et al., 2006b). Strong sensitivity of gc to D in conifers has often been observed in other studies (e.g. Saugier et al., 1997; Grelle et al., 1999; Ohta et al., 2001; Chen et al., 2002), and may explain the positive correlation between δ13C and potential evapotranspiration found at SOJP by Brooks et al. (1998).
Declines in ψc imposed by hydraulic constraints forced declines in ψT and eventually in gc (e.g. Fig. 9c), which imposed nonstomatal (ψT) and stomatal (gc) limitations on CO2 fixation (‘GPP’). Declines in CO2 fixation under higher D, combined with rises in Ra+Rh under higher Ta and Ts, caused earlier declines in daytime CO2 influxes and rises in night-time CO2 effluxes to be modelled and measured during warming events (e.g. DOY 228 in Fig. 3c and DOY 179–180 in Fig. 6c). Daytime CO2 influxes rapidly returned to higher values with less-pronounced mid-day declines when hydraulic constraints on CO2 fixation were alleviated by lower D during subsequent cooling (e.g. DOY 236–237 in Fig. 3b and c and DOY 181–183 in Fig. 6c and d). The imposition and alleviation of these constraints on CO2 fixation was clearly apparent in EC measurements of CO2 influxes when D rose above or fell below 2 kPa. Declines in CO2 influxes with higher D, combined with rises in CO2 effluxes with higher Ta, were also apparent as sharp declines in daily NEP from the model or from gap-filled EC fluxes whenever maximum daily Ta exceeded 25 °C (e.g. DOY 195–205 in Fig. 7). The adverse impacts of warming on CO2 influxes caused by hydraulic constraints and accelerated respiration in conifers has been observed in several other studies (Griffis et al., 2003; Morgenstern et al., 2004; Grant et al., 2005, 2006b).
Hydraulic constraints in the model rose gradually with stand age because Ωa increased with longer axial path lengths for water uptake as trees grew taller (Grant et al., 2006b), reaching 13 m in the 75-year-old stand at SOJP. Mencuccini & Grace (1996) found that hydraulic conductance and NPP of Scots pine attained maximum values in a stand of 7.7 m height, and declined in stands of greater heights. The gradual rise in hydraulic constraints, particularly on this rapidly drained soil with low water holding capacity, caused the very gradual decline in NEP and wood growth modelled more than 50 years after clearcutting (Fig. 8a and b). This decline was consistent with smaller wood growth increments for boreal jack pine stands older than 60 years calculated from allometric relationships (Alberta Forest Service, 1985).
Nutrient constraints on forest NEP
CO2 influxes remained small during the first decade after clearcutting, even when hydraulic constraints were not present (3, 6). During this period, GPP was limited by root N uptake which in turn was limited by competition with microbial populations for soil mineral N (‘Heterotrophic respiration’) during decomposition of fine nonwoody and coarse woody litter with high C : N ratios (ca. 40 : 1 and 250 : 1, respectively) left after clearcutting. Ecosystem productivity is frequently found to be strongly limited by N during regeneration after logging (e.g. Schimel & Firestone, 1989; Kimmins, 2004). Consequently, annual NEP from the model and from EC measurements remained below zero for at least 10 years after harvesting (Table 5; Fig. 8a). In sensitivity tests of the model, NEP declined further and rose more slowly after clearcutting when larger amounts of litter remained on site. This model behaviour was consistent with the observation by Janisch & Harmon (2002) that the regeneration of disturbed coniferous sites in northwestern USA was delayed by large amounts of coarse woody debris left after disturbance. Although the amounts of litter left on site after clearcutting in the model were generally consistent with litter C stock measurements at the jack pine chronosequence, these amounts will depend on harvesting practices, for which better estimates of wood and foliar litter will be needed in the future.
After clearcutting, some of the litter decomposition products in the model gradually accumulated in microbial residue pools with lower C : N ratios (Grant et al., 1993a, b). The mineralization of these pools eventually alleviated competition for soil mineral N among root and microbial populations, allowing sustained rises in GPP that enabled NEP to reach maximum values between 25 and 50 years after clearcutting. These values were consistent with those estimated from EC fluxes and C stock measurements 29 and 76–80 years after clearcutting (Table 5; Fig. 8a). However, jack pine growth remained N-limited throughout the logging cycle, as indicated by foliar C : N ratios of 45–50 modelled and measured in mature stands. These ratios remained well above the threshold of 30 above which N is considered limiting in conifers (Erisman et al., 1998). Growth of boreal jack pine stands in Canada with soil and foliar C : N ratios similar to those at SOJP have been found to increase sharply with N fertilizer application (Morrison & Foster, 1977). The sensitivity of GPP to N indicates the importance of representing N constraints on GPP when modelling boreal forest response to climate change, especially because higher soil temperatures accelerate N mineralization in boreal forest ecosystems (Verburg, 2004).
Climate change effects on forest NEP: Hydraulic constraints
The gradual rise in Ca during climate change (Table 4) accelerated CO2 fixation modelled in boreal jack pine by increasing Ci and hence carboxylation rates, thereby raising CO2 influxes (e.g. DOY 221–222 in Fig. 9d). This rise also accelerated CO2 fixation by reducing gc (Fig. 9c) and hence transpiration, thereby raising ψc and lowering nonstomatal limitations to CO2 fixation at a given ψs (Grant et al., 2004b). However, the rise in Ta during climate change (Table 4) offset the effects of reduced gc on transpiration by raising D (Fig. 9b), so that evapotranspiration rose and θ declined (Fig. 9a). Rising Ta also increased the frequency and intensity of warming events and their adverse impacts on jack pine CO2 fixation caused by hydraulic limitations under higher D (e.g. DOY 226–227 in Fig. 9d). These warming events lowered nonstructural C in the model by slowing contributions from CO2 fixation and accelerating removals by Rm, thereby hastening litterfall and needle turnover and lowering LAI (‘Autotrophic respiration’). Aboveground litterfall rates were comparable with measured rates under current climate (Table 5), and gave an annual turnover of foliage and twigs of ∼0.3. The more rapid litterfall modelled during climate change was consistent with the frequent observation that accelerated litterfall in pine follows high temperatures (Kouki & Hokkanen, 1992) or high evapotranspiration rates (Berg & Meentemeyer, 2001).
During litterfall in the model, remobilized C from senescent material sustained nonstructural C and hence Ra, while litterfall reduced foliar C and hence Rm, thereby rebalancing contributions to and removals from nonstructural C. Litterfall was thus used in the model to lower foliar mass and area of jack pine under rising D so that respiration requirements remained in balance with primary productivity. The model thus simulated the lower relative foliar mass found in pine growing in climates with higher D (Callaway et al., 1994; DeLucia et al., 2000) or in fir growing under higher Ta (Olszyk et al., 1998).
As climate change progressed, C gains modelled under lower Ta and D gradually failed to offset C losses modelled under higher Ta and D (Fig. 9). This model behaviour was consistent with the findings from EC measurements that above-average summer Ta was correlated with decreased net C uptake (Hollinger et al., 2004), from tree ring analyses that above-average summer Ta adversely affected growth of spruce and pine (Dang & Lieffers, 1988; Oleksyn et al., 1998; Hofgaard et al., 1999), and from growth chambers that elevated Ta reduced height and foliar mass of conifer seedlings (Olszyk et al., 1998). The model thus combined a positive response of NEP to increases in lower Ta with a negative response of NEP to increases in higher Ta (Fig. 9). These model results may explain the findings of Wilmking et al. (2004) that growth of Alaskan conifers tended to respond positively to warmer springs and negatively to warmer summers.
Declines in foliar mass from accelerated litterfall under continually rising Ta eventually caused failure of the boreal jack pine ecotype after 100 years of climate change (Fig. 8a and b), and its replacement by other vegetation. Loss of conifer dominance during climate change in boreal ecosystems has been predicted by biogeochemical models (e.g. Potter, 2004) and by dynamic vegetation models (e.g. Kirilenko & Solomon, 1998). The ability to model rapid change in vegetation caused by mortality of dominant woodland species is an important attribute of models used to project climate change impacts on vegetation dynamics (Allen & Breshears, 1998).
Replacing the boreal ecotype with a cold temperate ecotype reduced jack pine productivity during the first 75 years of climate change, but greatly improved it thereafter (Fig. 10a and b). This improvement was attributed to:
- 1
The upward displacement of the temperature sensitivity functions for photosynthesis and respiration by 0.75 °C. Although temperature sensitivity functions of diverse plant species are known to be affected by zones of thermal adaptation (e.g. Larcher, 2001), the physiological bases for this adaptation by different ecotypes of key forest species has received limited attention. Smets et al. (2006) showed differences in temperature sensitivity of CO2 fixation among different varieties of lodgepole pine from British Columbia that were as large at the difference assumed here between boreal and cold temperate jack pine ecotypes. Forestry managers are already concerned about adaptation to future temperature regimes of tree ecotypes now being replanted (e.g. Spittlehouse, 2005). The nature of this adaptation requires further elucidation if confidence in modelling ecotype adaptation to climate change is to be improved.
- 2
Offsetting the acceleration of seasonal phenology from rising Ta in the boreal ecotype, and thereby rebalancing C allocation among foliage, twigs, branches, boles, reproductive material and roots. Oleksyn et al. (1998) clearly demonstrated that completion of seasonal phenology required more thermal time in temperate than in boreal pine ecotypes, and was accelerated in all ecotypes by increases in Ta, causing shorter growing seasons. These findings suggest that an improved understanding of climatic controls on seasonal C allocation in trees is needed for climate change studies.
Climate change effects on forest NEP: nutrient constraints
In N-limited ecosystems, gains in CO2 fixation during climate change can only be sustained by commensurate gains in root N uptake. Under gradual climate change in the model, root N uptake rose almost proportionately with CO2 fixation, so that foliar C : N ratios by which carboxylation rates were governed rose only marginally (e.g. from 42.7 in July of the 100th year under current climate to 44.1 in July of the 100th year under climate change). The model thus simulated only to a limited extent the downregulation of CO2 fixation caused by lower foliar N contents frequently observed in trees under elevated vs. ambient Ca and common Ta (e.g. Medlyn et al., 1999). This rise in root N uptake was modelled because:
- 1
The gradual rise in litterfall and Ts modelled during climate change accelerated Rh and thereby soil C and N mineralization (‘Heterotrophic respiration’), apparent from lower soil+litter C (Fig. 10c) and associated N modelled under climate change. This accelerated mineralization resulted in a net transfer of N from soil to vegetation, as has been found in several soil warming experiments (Shaver et al., 2000).
- 2
More rapid Rh modelled during climate change also drove more rapid nonsymbiotic N2 fixation, the mineralized product of which contributed to N availability for root uptake. This model behaviour is consistent with findings that long-term exposure to elevated Ca raised soil organic C and total N (Gahrooee, 1998; Ross et al., 2000), indicating that soil N inputs rose over time.
- 3
Slightly larger foliar C : N ratios modelled under elevated Ca forced greater transfers of nonstructural C from shoots to roots in the model, increasing root growth and hence N uptake (‘Nutrient uptake and translocation’), thereby rebalancing root N uptake with CO2 fixation (Grant, 1998). This model behaviour simulated the reallocation of phytomass from shoots to roots under elevated Ca that has been found experimentally in pine (Callaway et al., 1994; DeLucia et al., 2000) and other tree species (Norby et al., 2004).
These three responses to rising Ca and Ta allowed the model to simulate a continuous rise in NEP and wood growth during climate change. These responses simulated the coupling of C and N cycles in soil–plant systems that was proposed by Eamus & Jarvis (1989) and Idso & Idso (1994) to moderate N limitations under long-term increases in Ca.
Summary
- 1
NEP of boreal jack pine in the model rose from −150 to −50 g C m−2 yr−1 during the first 10 years after clearcutting, and reached mature values of 53±29 g C m−2 yr−1 by 50 years after clearcutting, with large interannual variability caused by Ta and precipitation (Table 5; Fig. 8a).
- 2
Annualized NEP, C harvest, and NBP of boreal jack pine averaged 47, 33 and 14 g C m−2 yr−1 respectively during a 126-year logging cycle under current climate.
- 3
NEP of boreal jack pine was adversely affected by Ta > 25 °C and D > 2 kPa (3, 6, 7).
- 4
NEP of boreal jack pine was adversely affected during climate change by rising Ta (Fig. 9), which accelerated litterfall and raised Ra+Rh. These adverse effects caused failure of boreal jack pine after 100 years of climate change (Fig. 10).
- 5
The adverse effects of rising Ta on jack pine productivity during climate change were avoided by replacing the boreal jack pine ecotype with a cool temperate jack pine ecotype at planting (Fig. 10). The physiological bases for this avoidance needs further study.
- 6
This replacement allowed annualized NEP, C harvest, and NBP to average 81, 56 and 25 g C m−2 yr−1 during a 126-year logging cycle under changing climate.
Acknowledgements
The Fluxnet-Canada Research Network is funded by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Foundation for Climate and Atmospheric Sciences (CFCAS), and the Biological Implications of CO2 Policy in Canada (BIOCAP). Computational facilities for ecosys were provided by the Multimedia Advanced Computational Initiative (MACI) at the Universities of Alberta and Calgary. We acknowledge the contributions Andrew Sauter, Rick Ketler, Shawn O'Neill, Don Zuiker, Stephanie Thompson, Dominic Lessard and Dan Finch, who provided laboratory, field and data management support; Natascha Kljun, Praveena Krishnan and Sheila McQueen, who quality assured the EC data; Joe Eley, Charmaine Hrynkiw, Dell Bayne, Natasha Neumann, Erin Thompson and Steve Enns, who were responsible for the meteorological measurements and data management.




