Competition increasingly dominates the responsiveness of juvenile beech and spruce to elevated CO2 and/or O3 concentrations throughout two subsequent growing seasons
Abstract
Saplings of Fagus sylvatica and Picea abies were grown in mono- and mixed cultures in a 2-year phytotron study under all four combinations of ambient and elevated ozone (O3) and carbon dioxide (CO2) concentrations. The hypotheses tested were (1) that the competitiveness of beech rather than spruce is negatively affected by the exposure to enhanced O3 concentrations, (2) spruce benefits from the increase of resource availability (elevated CO2) in the mixed culture and (3) that the responsiveness of plants to CO2 and O3 depends on the type of competition (i.e. intra vs. interspecific).
Beech displayed a competitive disadvantage when growing in mixture with spruce: after two growing seasons under interspecific competition, beech showed significant reductions in leaf gas exchange, biomass development and crown volume as compared with beech plants growing in monoculture. In competition with spruce, beech appeared to be nitrogen (N)-limited, whereas spruce tended to benefit in terms of its plant N status.
The responsiveness of the juvenile trees to the atmospheric treatments differed between species and was dominated by the type of competition: spruce growth benefited from elevated CO2 concentrations, while beech growth suffered from the enhanced O3 regime. In general, interspecific competition enhanced these atmospheric treatment effects, supporting our hypotheses. Significant differences in root : shoot biomass ratio between the type of competition under both elevated O3 and CO2 were not caused by readjustments of biomass partitioning, but were dependent on tree size.
Our study stresses that competition is an important factor driving plant development, and suggests that the knowledge about responses of plants to elevated CO2 and/or O3, acquired from plants growing in monoculture, may not be transferred to plants grown under interspecific competition as typically found in the field.
Introduction
The success of an individual plant depends on its ability to acquire resources from external pools shared with its neighbors (Küppers, 1985; Tremmel & Bazzaz, 1995; Grams et al., 2002). Plants must invest these resources into the size, morphology and physiological activity of leaves and fine roots, which in turn are involved in the process of resource acquisition. In addition, supporting structures like shoot axes and coarse roots are required, as their size and architecture determine the positioning of leaves and fine roots in the above- and belowground space, fostering a plant's ability to explore the environment for resource availability (Küppers, 1984; Umeki, 1995; Lemaire & Millard, 1999; Suzuki, 2002). As biomass partitioning and plant architecture determine competitive success, they may change in response to neighboring plants (Weiner & Fishman, 1994; Tremmel & Bazzaz, 1995; Aerts, 1999; Aphalo et al., 1999). Hence, the competitive ability of an individual plant should be analyzed considering its response to competing neighbors. The present study addresses the responses of juvenile beech (Fagus sylvatica L.) and spruce (Picea abies (L.) Karst.) to interspecific competition in terms of biomass partitioning, crown architecture and the associated, whole-plant nitrogen (N) status. These two species, of major economic relevance in Central-European forestry, were of particular interest because of their contrasting growth dynamics and canopy habit (evergreen conifer vs. deciduous angiosperm tree; cf. Yokozawa et al., 1996). To induce changes in growth and resource allocation and, thus, more readily distinguish responses that drive competitiveness (Matyssek et al., 2002), plants were exposed to enhanced ozone (O3) and carbon dioxide (CO2) concentrations.
Overall, growth of spruce benefits from elevated CO2 supply (Mortensen, 1994; Liu et al., 2004), in particular, when growing in competition with beech (Grams et al., 2002; Kozovits et al., 2005). On the other hand, juvenile beech was found to be more sensitive to enhanced O3 concentrations than spruce saplings (Barnes et al., 1995; Lippert et al., 1996). However in beech, elevated CO2 compensates for adverse O3 effects (Grams et al., 1999), but the complex interactions between O3 and elevated CO2 (cf. Karnosky et al., 2003) are hardly investigated in a competition experiment.
Therefore, we hypothesized that (1) because of their contrasting O3 susceptibility, the competitiveness of beech rather than spruce is negatively affected by the prolonged exposure to elevated O3 regime, whereas (2) spruce profits from the increase of resource availability (elevated CO2) in the mixed culture and that (3) the responses of plants to CO2 and O3 depend on the type of competition (i.e. intra- vs. interspecific; cf. Grams et al., 2002).
In the present study, we proposed that plants are able to adjust their resource allocation in response to competition under the CO2/O3 regimes in a way that strengthens their competitiveness. The concept by Timmer & Morrow (1984) was adapted to examine N nutrition as a determinant of competitive interaction. Finally, effects by the gaseous regimes, competition or ontogeny were distinguished according to the allometric analysis proposed by Müller et al. (2000).
Materials and methods
Plants and treatments
In the spring of 1998, 2-year-old seedlings of European beech (F. sylvatica L., seed source 810–24 Freising) and 3-year-old Norway spruce (P. abies (L.) Karst., seed source 840–27 Altötting) were selected for uniform height (about 0.2 m) and transplanted to containers (0.7 × 0.4 × 0.3 m3; volume: 84 L). In central Europe, both species are often found on soils with similar rooting depth (i.e. 0.3 m, Ellenberg, 1996). The planting containers were filled with untreated forest soil (dystric cambisol, Ah-B horizon; see Kreutzer et al., 1991) from a beech stand in Höglwald Forest near Augsburg, Germany. At the end of the experiment, more than 90% of root tips were colonized by mycorrhizal fungi (S. Raidl and R. Agerer, personal communication, University Munich). Twenty trees (arranged in rows of 4 × 5 individuals) were planted into each of 32 containers, resulting in plant densities typical for the natural offspring of both species (Fig. 1b). Sixteen containers comprised 1 : 1 beech/spruce mixtures and eight containers comprised each monocultures of spruce or beech. Being aware of potential edge effects, data were collected only from the six central individuals of each container.

(a) Experimental setup in the phytotrons of the GSF – National Research Center for Environment and Health (adapted from Liu et al., 2004). Each phytotron comprised four Plexiglas subchambers (for individual O3 fumigation) at either ambient (Phytotron 3 and 4) or elevated (Phytotron 1 and 2) CO2 concentrations. Two planting containers, either two mixed cultures or one monoculture of each species, were placed into each of the four Plexiglas subchambers per phytotron. The experimental setup in chambers 1 and 3 was reproduced in chamber 2 and 4, respectively, with a rotated placement of the containers. (b) Spacing of beech (B) and spruce (S) seedlings in a planting container (mixed culture). The position of the studied trees (six central trees per container) is highlighted.
For 1 year prior to the phytotron study, plants were kept in two climate-controlled greenhouse chambers, which were programmed to track outside climate conditions. Air was purified and set to ambient or elevated (ambient +300 ppm) CO2 concentrations. For the following two growing seasons, plants were transferred into the four walk-in phytotrons (area: 9.5 m2 each) maintained by the GSF – National Research Center for Environment and Health in Neuherberg, near Munich. Each phytotron (at either ambient or elevated CO2) contains four Plexiglas subchambers (volume: 0.9 m3 each; Fig. 1a). Adequate ventilation (a constant air flow of 2.5 m3 min−1) allowed for individual O3 treatment. Plants were exposed to ambient or elevated CO2 concentrations in a factorial combination with ambient or twice-ambient (restricted to <150 ppb) O3 concentrations (Fig. 1a, Table 1). Four CO2/O3 regimes were established in this way: ambient CO2/1 × O3 (gaseous control), ambient CO2/2 × O3 (+O3), elevated CO2/1 × O3 (+CO2) and elevated CO2/2 × O3 (+CO2/+O3). Two planting containers (one monoculture of each species or two mixed cultures) were placed in each of the four Plexiglas chambers per phytotron. Hence, six study plants per species, atmospheric treatment and type of competition (i.e. intra- or interspecific competition) were held in one phytotron. This experimental setup was independently reproduced in a parallel phytotron. Growth of the trees was not significantly affected by phytotron (P=0.487) or Plexiglas subchamber (P=0.306). In addition, plant biomass development was not significantly different in planting containers under the same conditions (P>0.450 for all treatments but mixed cultures at +CO2). In mixed cultures at +CO2, the container effect was closer to statistical significance (P=0.079). Analysis of coefficients of variation indicated tree biomass to be fairly distributed among containers. The absence of container effects and the fair distribution of tree growth within the planting containers allowed for the use of individual plants as experimental units (cf. Liu et al., 2004; Kozovits et al., 2005).
| Month | Day/night | T air (°C) | PPFD (μmol m−2 s−1) | RH (%) | Amb. CO2(ppm) | Elev. O2(ppm) | 1 × O3(ppb) | 2 × O3(ppb) | AOT40 1 × O3(μL L−1 h−1) | AOT40 2 × O3(μL L−1 h−1) | SUM0 1 × O3(μL L−1 h−1) | SUM0 2 × O3(μL L−1 h−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May 1999 | Day | 17.5 | 531.8 | 55.0 | 405.6 | 701.5 | 39.0 | 72.9 | 1.7 | 10.1 | 10.5 | 19.7 |
| Night | 13.0 | 0 | 72.6 | 416.5 | 715.7 | 24.2 | 40.5 | – | – | 3.7 | 6.1 | |
| June 1999 | Day | 19.8 | 526.0 | 61.2 | 407.9 | 701.7 | 36.0 | 70.8 | 2.8 | 17.8 | 18.3 | 36.0 |
| Night | 14.8 | 0 | 82.0 | 430.2 | 725.5 | 15.9 | 33.0 | – | – | 3.7 | 7.6 | |
| July 1999 | Day | 19.7 | 458.0 | 65.6 | 399.0 | 690.4 | 34.2 | 67.6 | 2.0 | 15.2 | 16.8 | 33.3 |
| Night | 15.3 | 0 | 83.4 | 431.2 | 723.9 | 15.4 | 30.3 | – | – | 4.1 | 8.0 | |
| August 1999 | Day | 19.7 | 473.3 | 58.7 | 405.1 | 702.7 | 41.0 | 85.1 | 3.9 | 19.8 | 17.2 | 35.6 |
| Night | 13.7 | 0 | 80.4 | 431.9 | 728.1 | 15.2 | 31.2 | – | – | 5.1 | 10.4 | |
| September 1999 | Day | 15.4 | 408.1 | 67.1 | 418.4 | 714.1 | 21.3 | 47.0 | 0.1 | 4.8 | 7.2 | 15.9 |
| Night | 11.5 | 0 | 85.6 | 449.3 | 746.3 | 8.6 | 23.4 | – | – | 2.9 | 7.8 | |
| May 2000 | Day | 18.3 | 427.0 | 57.0 | 381.3 | 665.2 | 37.2 | 76.5 | 1.3 | 10.4 | 9.9 | 20.4 |
| Night | 13.0 | 0 | 77.0 | 410.5 | 675.8 | 23.1 | 45.6 | – | – | 3.2 | 6.4 | |
| June 2000 | Day | 18.4 | 482.6 | 60.3 | 379.7 | 673.1 | 35.4 | 72.9 | 1.9 | 18.1 | 18.3 | 37.6 |
| Night | 13.4 | 0 | 81.7 | 420.8 | 716.2 | 19.9 | 38.4 | – | – | 3.9 | 7.5 | |
| July 2000 | Day | 20.7 | 452.2 | 62.2 | 384.8 | 683.4 | 40.3 | 71.5 | 3.9 | 18.0 | 20.5 | 36.2 |
| Night | 15.5 | 0 | 81.4 | 410.6 | 706.7 | 21.2 | 33.8 | – | – | 4.8 | 7.7 | |
| August 2000 | Day | 20.1 | 447.2 | 63.2 | 390.9 | 688.2 | 35.0 | 65.0 | 2.1 | 14.1 | 17.2 | 32.2 |
| Night | 15.1 | 0 | 84.4 | 439.5 | 736.2 | 19.4 | 36.2 | – | – | 4.3 | 7.7 |
Climatic conditions
In the phytotrons, the climatic conditions and O3 concentrations measured during the previous years at the study site ‘Kranzberg Forest’ near Freising (Germany, 490 m a.s.l.; see Pretzsch, 2002; Nunn et al., 2002) were reproduced on an hourly basis throughout the seasonal course (Fig. 2). Air and soil temperature, as well as relative humidity, were measured in the phytotrons as described by Payer et al. (1993). A combination of different lamps, glasses and water filters reproduced the light spectrum (photosynthetic active radiation, ultraviolet (UV)-A and UV-B) with high precision (Thiel et al., 1996). Photosynthetic photon flux density (PPFD) was registered with one photodiode above each container (Type G1118, Hamamatsu Ltd., Hamamatsu City, Japan), calibrated prior to installation with an LI-189 unit (LI-190SA quantum sensor, LI-COR Inc., Lincoln, NE, USA).
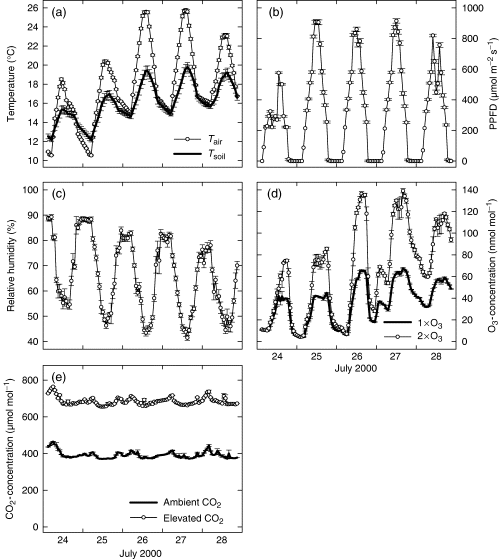
Air and soil temperature (a), photosynthetic photon flux density, PPFD (b), relative humidity, RH (c), O3 (d) and CO2 concentrations (e) as occurring within the 16 Plexiglas subchambers of the four phytotrons from July 24 to 28, 2000. Data are means±SE (n=32 for PPFD, n=16 for temperatures, and n=8 for RH, O3 and CO2 concentrations).
Examples for the daily courses of air and soil temperature, irradiance (PPFD), relative humidity, O3 and CO2 concentrations in the Plexiglas subchambers of the four phytotrons are shown in Fig. 2. Even during hours of high irradiance (e.g. July 25), the cooling systems of the phytotrons effectively reduced air temperature (measured 5–10 cm above the canopy). The monthly mean values of climate conditions in the phytotrons are given in Table 1. The AOT40 by the end of the experiment on August 28 (calculated for daylight hours, according to Fuhrer, 1994) are given in Table 1, and are about five to 10 times higher at +O3 compared with the gaseous control regime. During the winter months of 1998/1999 and 1999/2000, plants were placed into open-top chambers outdoors, where corresponding CO2 regimes were maintained.
Soil moisture of each container was monitored continuously by tensiometers (Model T5, UMS, Munich, Germany), triggering irrigation with deionized water whenever soil water tension reached 350 hPa. Fertilization (1 L of double-concentrated Hoagland solution, Hoagland & Arnon, 1950) was applied six and eight times in the first and second growing season, respectively, in order to maintain tissue nutrient levels similar to those found in trees from Bavarian forests (cf. Kreutzer et al., 1991).
Assessment of plant biomass
Nongreen aboveground biomass of the six central individuals of each container was assessed in March 1999 and 2000 volumetrically by measuring the diameters and lengths of the shoot axes. Biomass to volume relation (measured on comparable plants) was used to convert measured volumetric data into shoot biomass (grams of dry mass (DM)). Aboveground biomass, as determined in March 1999 and March 2000, was regarded to equal that at the end of the growing seasons 1998 and 1999, respectively.
At the end of August 2000, the six central individuals of each container were harvested. For each tree, biomass (DM) of leaves, branches and stem was determined. Beech leaves were separated according to sun, shade and second-flush foliage, and in spruce, current-year branches were separated from older branches. The root biomass of two (randomly chosen) out of the six central plants was determined quantitatively as described earlier (Liu et al., 2004). A square-shaped ground area of 0.013 m2 was assigned to each tree, and a customized metal cutter was used to extract 0.004 m3 of soil underneath that area (down to the bottom of the container). This soil volume was considered to contain a root mass equivalent to that of one individual plant with respect to the amount of root tissue extending out of the cut volume to be similar to the amount intruding from neighboring plants of the same species (see Bengough et al., 2000). Beech and spruce roots were separated from each other based on their characteristic diameter and branching pattern. Soil particles were removed by washing (Oliveira et al., 2000). Subsamples of each plant organ were used for determining the fresh/DM ratio and for analyzing the N status.
Crown volume
The crown volume of individual beech trees was determined at the end of July 1999 and 2000 (when axis length growth was completed) through approximations using cuboid volumes enclosing the shoots. While the crown architecture of beech plants did not develop into any fixed geometrical form, spruce grew in a regular way, resulting in a ‘cylinder+cone’ crown shape. Hence, a ‘cylinder+cone’ model was applied that enclosed the entire, densely branched crown of spruce, accounting for the specific growth pattern in each species (cf. Küppers, 1994). Both volume approximations consider the empty space between the leaves of a branch (as in beech) or between branches (as in spruce) as the occupied crown volume, thereby ensuring a comparable and consistent assessment in each species.
Assessment of leaf gas exchange
Measurements of the net CO2 uptake rate (JCO2) were conducted with an open flow steady-state porometer (CQP130, Walz, Effeltrich, Germany; Schulze et al., 1982). The porometer system was equipped with a differential infrared CO2/H2O gas analyzer and an electrically temperature-controlled cuvette. Measurements were performed at 3- to 4-week intervals throughout the growing seasons of 1999 and 2000 (see the legend of Fig. 4 for climate conditions during measurements). Fully developed sun (n=12) and shade leaves (n=6) of beech, as well as current year (sun, n=12) and older branches (shade, n=6) of spruce, were chosen for these measurements. The net CO2 uptake and transpiration rates were calculated according to the equations of von Caemmerer & Farquhar (1981), based on the one-sided leaf area for beech and the projected leaf area for spruce.

Biomass of stem and shoot axes of beech (a, b) and spruce (c, d) growing in 1998 under ambient and elevated CO2 concentrations in the greenhouse and under the four CO2/O3 regimes in the phytotrons in 1999 and 2000. Monocultures are given as solid and mixed cultures as open symbols. Circles denote the gaseous control regime, triangles +O3, squares +CO2 and rhomboids +CO2/+O3 (means±SE, n=5–12). Measurements were conducted in March 1999 and 2000, and at the end of August 2000, representing the biomass achieved during the growing seasons of 1998, 1999 and 2000, respectively. No biomass differences (at P<0.05) were observed in 1998 in both species and in spruce in 1999. Different letters indicate significant treatment effects between the means of beech or spruce at P<0.05.
N content
Sampled material of each organ was milled after drying at 65°C to constant weight. The total N content was determined using a CHN analyzer (Leco, St Joseph, MI, USA). The product of biomass by N concentration yielded the N content of each organ, which, in total, provided the N content of the entire plant.
Statistical analysis
For differences between treatments and main effects, data are presented as means±one standard error (SE). Treatment main effects and interactions were tested using the SPSS statistic package (Bühl & Zöfel, 2000) assuming a split–split–plot design, with the type of competition (planting containers) within O3 treatments (Plexiglas subchambers), and the O3 treatments within CO2 treatments (phytotrons). Tukey's studentized range test was used to identify differences among treatments within each experimental year (see Table 2). Allometric relationships between root and shoot biomass were analyzed by the general model: ln y=b0+b1 ln x, as derived from the allometric relationship y=b0xb1, where x and y are any two components of the plant structure, and the slope b1 represents the relative change in allocation between components with treatments. To test whether the type of competition and gaseous regimes influenced patterns of biomass partitioning between plant organs or whether the differences observed reflected size-dependent shifts in allocation, an analysis of covariance (ancova) was performed. Natural log-transformed biomass of one organ (dependent variable) was compared with the natural log-transformed biomass of another organ (covariate). Shifts in biomass partitioning were identified by significantly different slopes and/or intercepts between treatments (Müller et al., 2000).
| J CO2 sun | Shoot biomass | Crown volume | Nitrogen | Root/shoot ratio | |||||
|---|---|---|---|---|---|---|---|---|---|
| Sep 30, 1999 | Aug 22, 2000 | 1999 | 2000 | 1999 | 2000 | Content | Conc. | ||
| Beech | |||||||||
| CO2 | 0.713 | 0.083 | 0.459 | 0.091 | 0.777 | 0.012 | 0.164 | 0.017 | 0.305 |
| O3 | 0.265 | 0.002 | 0.057 | 0.309 | 0.652 | 0.687 | 0.040 | 0.005 | 0.319 |
| Comp. | 0.221 | 0.023 | 0.033 | 0.007 | 0.054 | 0.009 | 0.117 | 0.067 | 0.033 |
| CO2× O3 | 0.075 | 0.696 | 0.134 | 0.388 | 0.216 | 0.095 | 0.341 | 0.133 | 0.280 |
| CO2× comp. | 0.748 | 0.994 | 0.007 | 0.547 | 0.088 | 0.124 | 0.494 | 0.271 | 0.446 |
| O3× comp. | 0.989 | 0.185 | 0.680 | 0.050 | 0.237 | 0.581 | 0.346 | 0.159 | 0.381 |
| CO2× O3× comp. | 0.914 | 0.419 | 0.632 | 0.747 | 0.460 | 0.516 | 0.011 | 0.133 | 0.470 |
| Spruce | |||||||||
| CO2 | 0.503 | 0.229 | 0.068 | 0.249 | 0.269 | 0.520 | 0.576 | 0.210 | 0.275 |
| O3 | 0.544 | 0.559 | 0.994 | 0.318 | 0.460 | 0.474 | 0.569 | 0.218 | 0.069 |
| Comp. | 0.115 | 0.376 | 0.524 | 0.065 | 0.075 | 0.026 | 0.218 | 0.096 | 0.043 |
| CO2× O3 | 0.019 | 0.384 | 0.310 | 0.882 | 0.001 | 0.963 | 0.848 | 0.383 | 0.391 |
| CO2× comp. | 0.604 | 0.060 | 0.836 | 0.530 | 0.274 | 0.823 | 0.096 | 0.965 | 0.054 |
| O3× comp. | 0.314 | 0.665 | 0.544 | 0.417 | 0.861 | 0.213 | 0.829 | 0.544 | 0.834 |
| CO2× O3× comp. | 0.368 | 0.329 | 0.119 | 0.217 | 0.358 | 0.728 | 0.203 | 0.023 | 0.324 |
- The main effects for JCO2 were calculated only for the last measured data in 1999 and 2000.
- P-values below 0.050 are given in bold.
Results
Gas exchange
Elevated CO2 stimulated net CO2 uptake rates of beech sun leaves at most measurement dates in 1999, but not so in 2000 (Fig. 3a, b). In the second year, enhanced O3 concentrations reduced the net CO2 uptake (O3 main effect of P=0.002, Table 2). This negative O3 effect was less apparent under elevated CO2, suggesting that elevated CO2 compensated for adverse O3 effects on the net CO2 uptake rate in beech. In 1999, sun leaves of beech showed a similar net CO2 uptake rate in mono- and mixed cultures, although under the elevated CO2, the rates in mixed culture tended to be lower compared with those in monoculture (see the trend lines in Fig. 3b). This negative competition effect became significant in 2000 (P=0.023, Table 2). In contrast, the net CO2 uptake rates of shade leaves (Fig. 3c, d) were not significantly affected by the gaseous regimes or competition.

Net CO2 uptake rate (JCO2) of sun (a, b) and shade (c, d) leaves of beech and, for spruce, of current-year branches (sun crown) after completion of growth (e, f) and of shaded, older than current-year branches (g, h). Assessments were performed under ambient light (PPFD in phytotrons), air temperature and relative humidity at three- to four-week intervals throughout the growing seasons of 1999 and 2000 (ambient CO2 regime: a, c, e, g; elevated CO2 regime: b, d, f, h). Climate conditions in the phytotrons were kept constant throughout the measurements (from 9:00 to 16:00 hours) and can be classified as ‘cool’, ‘intermediate’ and ‘warm’. Air temperatures at canopy level were 17.0±0.3, 22.2±0.3 and 26.0±0.4°C, leaf temperatures of sunlit leaves were 22.8±0.1, 25.8±0.1 and 28.1±0.2 and PPFD at canopy heights 640±15, 720±15 and 850±8 μmol m−2 s−1 at cool, intermediate and warm days, respectively (means±SE; n=16–48). RH was always between 45% and 55%. Cool climate conditions were present in 1999 on August 27, September 16, September 30 and in 2000 on May 15. Intermediate temperatures were present in 1999 on July 15 and in 2000 on June 6, August 1, August 22. Warm climate conditions existed in 1999 on May 6, June 2, June 25, August 6 and in 2000 on July 4. Monocultures are given as solid and mixed cultures as open symbols. Circles denote the gaseous control regime, triangles +O3, squares +CO2 and rhomboids +CO2/+O3 (means±SE, n=6–12). The solid and dotted trend lines represent the mean values of mono- and mixed cultures, respectively.
In both years, current-year branches of spruce had stimulated uptake rates under elevated CO2, in particular, early in the growing seasons (Fig. 3e, f). No consistent effect of enhanced O3 or competition was found. Rates of net CO2 uptake were higher in current-year branches (3.0–14.0 μmol m−2 s−1, Fig. 3e, f) compared with older branches (varying between compensation and 6.0 μmol m12 s−1, Fig. 3g, h). Similar to the situation in current-year branches, elevated CO2 also increased the CO2 uptake rates of older branches, but neither O3 nor competition had a significant effect.
Nongreen aboveground biomass
Development of shoot axes biomass of beech did not show significant CO2 or O3 main effects (Fig. 4, Table 2). In 1999, slight but significant reductions in mixed cultures compared with monocultures were found, in particular, under elevated CO2 (competition main effect of P=0.033). This was a consequence of growth stimulation under elevated CO2 in monoculture but not in mixture with spruce. After two growing seasons, beech suffered from interspecific competition in all gaseous regimes, with a significantly lower biomass in mixed than in monocultures (competition main effect of P=0.007). Spruce profited from the CO2 enhancement (barely significant in 1999: P=0.068) but did not respond to O3 regimes. Differences in the aboveground biomass of spruce between types of competition occurred only in 2000, with the mixed cultures showing more biomass than the monocultures (Fig. 4c, d; competition main effect: P=0.065, Table 2).
At the beginning of the phytotron study in 1998, spruce had, at similar stem height (see Materials and methods), a higher aboveground biomass than beech. However, at the end of the experiment, beech biomass in monocultures was higher (ambient CO2) or similar (elevated CO2) than spruce biomass.
Crown volume
In 1999, the crown volume of beech was not significantly affected by the gaseous treatments but was reduced at +CO2/+O3 in mixture with spruce (Fig. 5a–d, P=0.054). This competition effect was even stronger in the following growing season (competition main effect: P<0.01). In all mixed cultures, irrespective of the gaseous treatment, crown volume was smaller than in 1999, in particular, under +O3. This suggests a higher susceptibility of beech to O3 under interspecific competition. In both growing seasons, spruce was not affected by elevated CO2 or O3, but had higher crown volumes in the mixed than in the monocultures (1999: P=0.075; in 2000: P<0.05).
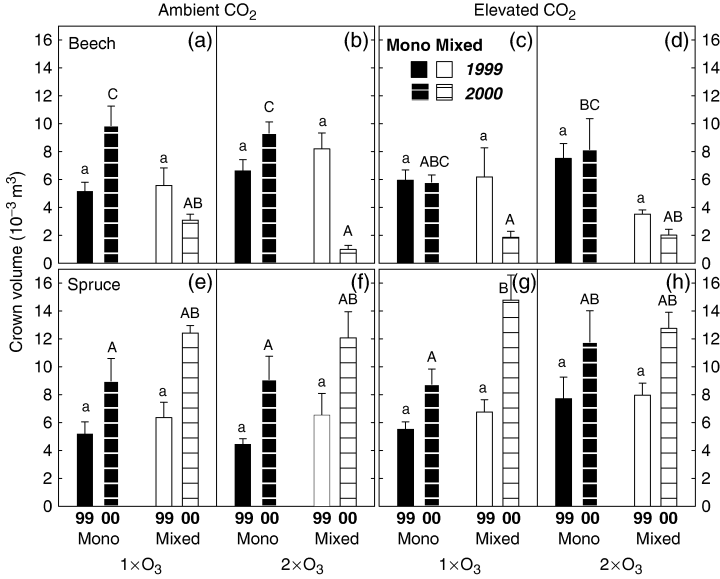
Crown volume (cm3) of beech and spruce under the gaseous control (a, e), +O3 (b, f), +CO2 (c, g) and +CO2/+O3 (d, h) in 1999 and 2000, respectively. Bars without hatching represent the crown volume in 1999, and bars with horizontal hatching denote the volume in 2000. Black and white bars represent mono- and mixed cultures, respectively (means±SE, n=5–12). Different letters indicate significant treatment effects between the means of beech or spruce at P<0.05.
Plant N
In beech, elevated CO2 had a negative and O3 a positive main effect on plant N concentration (P<0.05 and P<0.01, respectively; Table 2, Fig. 6). Compared with the monocultures, beech grown in mixture with spruce under +O3 and +CO2 had a significantly lower N content (see arrows in Fig. 6a). This was associated with a reduced plant biomass in both cases, and, in addition, with a significantly lower N concentration under +O3. Thus, compared with the monocultures, beech appeared to be N-limited under +O3 in the presence of spruce. On the other hand, spruce showed significant enhancement in its whole-plant N content and N concentration under +O3 and +CO2 in the mixed culture. However, the competition main effect was barely significant (P=0.096, see Table 2).

Nitrogen concentration and content of beech (a) and spruce (b) under the different CO2 and O3 regimes (means; n=2–4). Slopes between data points and the origin of the plot give the inverse of whole-plant biomass. For orientation, dotted lines were drawn, which represent whole-plant biomass of 15, 30 and 45 g dry mass (DM) for beech or 40, 50, 60 g DM for spruce. Because of graphical reasons, the average SE of all means of beech or spruce data are given at the bottom of each plot. The arrows indicate significant differences between mono- and mixed cultures (at P<0.05). N content was significantly different in beech under +O3 and +CO2. N concentration was significantly different in beech under +O3, and in spruce under +O3 and +CO2.
Root : shoot biomass ratio
Overall, the beech root/shoot biomass ratio (root/shoot) was not affected by the gaseous treatments. Beech had a higher root/shoot ratio in the mixed as compared with the monocultures, but a significant difference was found only under +O3 (Table 3; competition main effect: P<0.05, Table 2). Also, spruce had a higher root/shoot ratio in the mixed cultures (competition main effect: P<0.05, Table 2), in particular, under elevated CO2 concentrations. Despite the observed differences in root/shoot ratio, biomass partitioning between these two plant compartments was not a result of a regulatory readjustment in resource allocation (Fig. 7). The allometric analysis revealed no significant effects of elevated O3 or CO2 concentrations on root/shoot ratios of beech and spruce (all P-values >0.588 and >0.252, respectively; data not shown). Likewise, the slopes and the intercepts of the linear regressions for mono- and mixed cultures of beech and spruce were not significantly different from each other (all P-values >0.761 and >0.593 for beech and spruce, respectively; Fig. 7). This indicates biomass partitioning to be size dependent (i.e. a consequence of plant size), and hence, to be under ontogenetic control.
| Treatments | Beech | Spruce |
|---|---|---|
| Control | ||
| Monoculture | 0.69±0.10AB | 0.52±0.10A |
| Mixed culture | 0.89±0.12B | 0.52±0.11A |
| +O3 | ||
| Monoculture | 0.54±0.07A | 0.59±0.05A |
| Mixed culture | 0.90±0.06B | 0.57±0.17A |
| +CO2 | ||
| Monoculture | 0.63±0.01AB | 0.49±0.03A |
| Mixed culture | 0.87±0.03AB | 0.68±0.05A |
| +CO2/+O3 | ||
| Monoculture | 0.75±0.11AB | 0.53±0.10A |
| Mixed culture | 0.96±0.15B | 0.68±0.12A |
- Different letters indicate significant treatment effects between the means for beech or spruce at P<0.05.
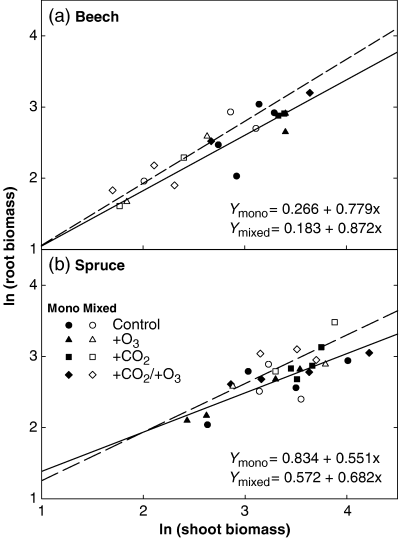
Relationship between log-transformed root and shoot biomass of beech (a) and spruce (b) saplings in mono- (closed symbols) and mixed cultures (open symbols) under the four gaseous regimes. Slopes and intercepts of the regressions for beech (P>0.761 and 0.940, respectively) and for spruce (P>0.593 and 0.754, respectively) did not differ significantly from each other.
Discussion
After two growing seasons under the O3 and CO2 regimes, beech plants growing in competition with spruce developed, with few exceptions, a significantly reduced biomass and crown volume as compared with plants in monoculture. Conversely, spruce tended to benefit from growth in competition with beech.
The type of competition determines responses to CO2/O3 regimes
Elevated CO2 stimulated the growth of beech seedlings only in monoculture and during the first year of the phytotron study. The negative effect of interspecific competition on the biomass development of beech was first observed in 1999, in particular, under elevated CO2, and became larger under all atmospheric treatments during 2000. Reduced responsiveness of beech to elevated CO2 concentration was also observed by Spinnler et al. (2002) under similar growth conditions (beech–spruce mixture, natural acidic soil) in open-top chambers. The authors suggested, in accordance with the present study, that low N availability might limit the response of beech to enhanced CO2 concentrations. In general, absent or moderate biomass increments in response to enhanced CO2 have been observed in plants that grew under ecologically meaningful conditions (Egli et al., 1998; Maurer et al., 1999; Zak et al., 2000; Oren et al., 2001). The competitive disadvantage of beech in interspecific competition was also reflected in its reduced net CO2 uptake rates (cf. Fig. 3). The reduced responsiveness of beech to elevated CO2, in particular, in the mixed culture, confirms hypothesis 3, which predicts the responses of plants to gaseous regimes to be intrinsically subjugated by the type of competition (Grams et al., 2002; Poorter & Navas, 2003; Fuhrer et al., 2003). In contrast to beech, the stronger competitor spruce profited from the CO2 enhancement under interspecific competition by showing an increase in its biomass as compared with spruce plants grown under ambient CO2. Thus, hypothesis 2 that spruce benefits from enhanced CO2 supply was supported. This result also confirms that the positive growth response to elevated CO2 is greater for competitively advantaged trees (McDonald et al., 2002).
As found under elevated CO2 supply, interspecific competition may also modify plant responses to O3. Under enhanced O3, beech in monoculture tended to show an increase in shoot axes biomass as compared with that achieved under the ambient O3 regime. In contrast, plants in mixed culture showed significant reductions in all investigated growth parameters and the lowest net CO2 uptake rate. Fuhrer et al. (2003) summarized results of studies on seminatural vegetation, which show, in accordance with the present findings and with hypothesis 3, that the impact of O3 stress can be enhanced by interspecific competition.
While in 1999, aboveground growth parameters of beech were hardly affected by O3, 1 year later, O3 effects were obvious, in particular, when comparing mono- and mixed cultures (again confirming hypothesis 3). Likewise, Bortier et al. (2001, 2000) did not find O3-induced changes in shoot biomass in beech seedlings within one growing season. With single potted beech seedlings from 12 provenances, even after two seasons of O3 exposure, shoot biomass was not affected (Paludan-Müller et al., 1999). In accordance with other studies on elevated CO2 and O3 levels (Egli et al., 1998; Isebrands et al., 2001), the present results show that it can take more than one growing season before the responses of plants to gaseous treatments become significant.
At the end of the experiment, the constraints provoked by the enhanced O3 regime in beech plant biomass, net CO2 uptake rate and crown volume were partially or totally counteracted by exposure to elevated CO2, in accordance with findings of Grams et al. (1999), Grams & Matyssek (1999) and Volin et al. (1998). In contrast to beech, there was no significant effect of the ‘+O3’ regime on all investigated parameters in spruce, regardless of competition type. These findings confirm hypothesis 1, which claims that beech rather than spruce is negatively affected by the O3 regimes. Spruce, like some other coniferous species, has been considered less sensitive to O3 than deciduous trees (Skärby et al., 1998; Landolt et al., 2000), being aware, however, that the variation of genotypes within a species may strongly determine their responsiveness to O3 and their CO2× O3 interactions (Vanderheyden et al., 2001; Wustman et al., 2001; Karnosky et al., 2003). In accordance with prevailing reports on grasses and legumes (Poorter & Navas, 2003; Bender et al., 2003; Fuhrer et al., 2003), the present study indicates the responses of juvenile beech and spruce plants to elevated CO2 and O3 regimes to be dominated by the type of competition (i.e. intra- or interspecific).
Crown architecture
In 2000, beech in mixed culture developed much smaller crown volumes as compared with those achieved in mono- and mixed culture in 1999. This was a consequence of a lower biomass investment into foliage and current-year axes (Kozovits et al., 2005) in trees under interspecific competition, in particular, under the +O3 regime. Reduction in branching because of O3 was also observed in aspen (Dickson et al., 2001), birch (Matyssek et al., 1992; Maurer & Matyssek, 1997) and poplar (Matyssek et al., 1993). However, in the present study, reduced crown volume in mixed culture did not only occur under elevated O3, but under all atmospheric treatments, characterizing a likely general response of beech to the presence of spruce. The ability of plants to modify crown architecture in response to the neighborhood has been considered a crucial mechanism of plant competition that determines population and community ecology (Küppers, 1985; Tremmel & Bazzaz, 1995; Yokozawa et al., 1996; Umeki, 1997; Grams et al., 2002). Although the red : far red ratio was not measured in the canopies of the present study, it is plausible to assume that light quality was modified in mixed relative to monocultures, being one prerequisite of the changes in the crown architecture of beech (Ballaréet al., 1988; Gilbert et al., 2001).
N status and whole-plant response
Beech favored allocation to roots under interspecific competition (Table 3). This result suggests that beech plants in mixed culture – in addition to aboveground restrictions – might be under limited nutrient conditions (Bloom et al., 1985; Lemaire & Millard, 1999). This indication was corroborated by the lower whole-plant N content (cf. Fig. 6). Conversely, spruce appeared to have an advantage in terms of N uptake in the presence of beech, showing an increase, in parallel to the total biomass, its N concentration (significantly under +O3 and +CO2). At the same time, sugar and starch concentrations in spruce were unaffected or even slightly lower in mixed compared with monocultures (Liu et al., 2004), indicating that growth was not N-limited. According to Kubiske & Godbold (2001), there is some evidence that N uptake per unit of root DM may be stimulated under elevated CO2 in conifers but not so in woody angiosperms. Likewise, Hagedorn et al. (2002) and Wang et al. (2001) found that the presence of spruce in mixed culture may decrease the amount of nutrients available for beech. Thus, in addition to the aboveground situation, beech appears to be a weaker competitor in the belowground compartment too.
Although statistically significant differences in root : shoot biomass ratio (root/shoot) between the types of competition were found (Table 3), the allometric analysis proved that plants do not readjust their internal resource allocation in response to the competition (Fig. 7). The differences observed were a consequence of plant size, and hence, were under ontogenetic control. Beech plants under interspecific competition were in general smaller, and because of this, showed a higher root/shoot ratio as compared with the plants in monoculture. Thus, the treatments influenced plant size, but did not change allocation patterns (Pearsall, 1927; Weiner & Fishman, 1994; Müller et al., 2000).
Conclusions
Beech was more responsive to O3 (confirming hypothesis 1), and elevated CO2 ameliorated the effects of O3 on beech with interspecific competition. Only spruce was able to benefit from the increased CO2 concentration, showing an enhancement in its total biomass, especially with interspecific competition (confirming hypothesis 2). Responses to CO2 and O3 strongly depended on the type of competition (confirming hypothesis 3). The results discussed above indicate that competition is an important factor driving plant development and suggest that the knowledge about responses of plants to CO2 and/or O3 acquired from plants growing in monoculture (or isolation) may not be transferred to plants grown under interspecific competition as typically found in the field.
Acknowledgements
The authors are indebted to Drs H.-D. Payer and H. Seidlitz (GSF – National Research Center for Environment and Health) and their technical staff for their excellent and unstinting support during the experiments. We gratefully acknowledge the skilful technical assistance by Ing. T. Feuerbach, A. Jungermann, P. Kuba and I. Süß. The authors would also like to thank Dr. J. B. Winkler (GSF) for excellent discussions. Drs L. Vivaldi's (University of Brasilia) and H. Scherb's (GSF) statistical advice is highly appreciated. The investigation was funded through SFB 607 ‘Growth and Parasite Defense – Competition for Resources in Economic Plants from Agronomy and Forestry, Projects B5 and B10’ by the ‘Deutsche Forschungsgemeinschaft’ (DFG). Dr A. R. Kozovits was sponsored by a fellowship from DAAD/CAPES.




