Accumulation of phenolic compounds in birch leaves is changed by elevated carbon dioxide and ozone
Abstract
Atmospheric change may affect plant phenolic compounds, which play an important part in plant survival. Therefore, we studied the impacts of CO2 and O3 on the accumulation of 27 phenolic compounds in the short-shoot leaves of two European silver birch (Betula pendula Roth) clones (clones 4 and 80). Seven-year-old soil-grown trees were exposed in open-top chambers over three growing seasons to ambient and twice ambient CO2 and O3 concentrations singly and in combination in central Finland.
Elevated CO2 increased the concentration of the phenolic acids (+25%), myricetin glycosides (+18%), catechin derivatives (+13%) and soluble condensed tannins (+19%) by increasing their accumulation in the leaves of the silver birch trees, but decreased the flavone aglycons (−7%) by growth dilution. Elevated O3 increased the concentration of 3,4′-dihydroxypropiophenone 3-β-d-glucoside (+22%), chlorogenic acid (+19%) and flavone aglycons (+4%) by inducing their accumulation possibly as a response to increased oxidative stress in the leaf cells. Nevertheless, this induction of antioxidant phenolic compounds did not seem to protect the birch leaves from detrimental O3 effects on leaf weight and area, but may have even exacerbated them. On the other hand, elevated CO2 did seem to protect the leaves from elevated O3 because all the O3-derived effects on the leaf phenolics and traits were prevented by elevated CO2. The effects of the chamber and elevated CO2 on some compounds changed over time in response to the changes in the leaf traits, which implies that the trees were acclimatizing to the altered environmental conditions. Although the two clones used possessed different composition and concentrations of phenolic compounds, which could be related to their different latitudinal origin and physiological characteristics, they responded similarly to the treatments. However, in some cases the variation in phenolic concentrations caused by genotype or chamber environment was much larger than the changes caused by either elevated CO2 or O3.
Introduction
Plants produce numerous phenolic compounds for different purposes in their tissues. It appears that phenolics play an important role in plant evolution, because most of the known functions emphasize their role as defences and signals. Various phenolic compounds serve, for example, as growth regulators, antioxidants, enzyme inhibitors, pigments and UV light screens, and they interact with herbivores, microbes, fungi and nematodes as chemical signals and toxins (Koes et al., 1994; Cooper-Driver & Bhattacharya, 1998; Seigler, 1998). Hence, the phenolics seem to have evolved in response to abiotic and biotic challenges and thereby, have enabled plant survival under different growth conditions. The different classes of phenolic compounds have appeared sequentially during plant evolution and the composition of compounds varies in different plant families (Koes et al., 1994). In Finland, European white birches (Betula pendula, B. pubescens) have been used as model plants in studies investigating plant–herbivore interactions and the effects of various air pollutants on forest health. Therefore, the phenolic composition or profile of birches and the biosynthetic pathways leading to different compounds are known in general outline (Nurmi et al., 1996; Ossipov et al., 1996, 2003; Keinänen & Julkunen-Tiitto, 1998). These pathways and the compounds found in birch are schematically depicted in Fig. 1. Ossipov et al. (2003) suggested that in mountain birch (B. pubescens ssp. czerepanovii) the hydrolysable tannins are produced from an intermediate of the shikimic acid pathway. In addition, some unexplained correlative connections among phenolic end products synthesized from seemingly distant branches of the metabolic pathway, for example, between dihydroxypropiophenone glucoside and flavone aglycons, have been found (Keinänen et al., 1999a).

Biosynthesis of phenolic compounds in B. pendula leaves. Solid arrows represent direct step in a pathway; dashed arrows represent multiple steps. Abbreviations: E4P, erythrose 4-phosphate; PEP, phosphoenol pyruvate, DHPPG, 3,4′-dihydroxypropiophenone 3-β-d-glucoside; PAL, phenylalanine ammonia-lyase; CA4H, cinnamic acid 4-hydroxylase; 4CL, 4-coumarate:coenzyme A ligase; CHS, chalcone synthase; CHI, chalcone isomerase; FNS, flavone synthase, F3H, flavanone 3-hydroxylase; FLS, flavonol synthase; DFR, dihydroflavonol 4-reductase; LAR, leucoanthocyanidin reductase.
The accumulation of phenolic compounds is controlled by genotype and by factors affecting photosynthesis, such as light and CO2 availability (Koricheva et al., 1998 and references therein). Estimated doubling of the atmospheric CO2 concentration during this century (IPCC, 2001) is increasing the CO2 availability. It has been shown that part of the extra carbon assimilated as a consequence of increased photosynthesis under CO2-enriched atmosphere is directed to the synthesis of phenolic compounds (Koricheva et al., 1998). In young birch seedlings the effects of elevated CO2 on the concentration of phenolic compounds have been positive (total cinnamic acid derivatives, total flavonol glycosides, catechin, condensed tannins, total phenolics), negative (cinnamic acid derivatives, kampferol-, quercetin- and myricetin rhamnosides, apigenin) or nonexistent (3,4′-dihydroxypropiophenone 3-β-d-glucoside (DHPPG), cinnamic acid derivatives, flavone aglycons, flavonol glycosides, total low-molecular-weight compounds, condensed tannins) depending on the level and duration of exposure and other environmental factors (Lavola & Julkunen-Tiitto, 1994; Lavola et al., 1998, 2000; Kuokkanen et al., 2001, 2003).
Parallel to CO2 elevation, anthropogenic air pollution is also increasing in the atmosphere (IPCC, 2001). Ozone is a gaseous air pollutant, which impairs the photosynthesis and growth of plants (De Temmerman et al., 2002). Therefore, one might expect that ozone exposure would decrease the concentration of phenolic compounds by reducing carbon available for phenolic synthesis. On the contrary, studies have shown increases in their concentrations (Koricheva et al., 1998). It is known that ozone activates phenylalanine ammonia-lyase (PAL) and chalcone synthase (CHS) enzymes (Fig. 1) and hence, induces the phenylpropanoid metabolism in plants (Kangasjärvi et al., 1994). Phenolic compounds such as cinnamic acid derivatives, flavonols, flavan-3-ols and tannins have antioxidant activity (Rice-Evans et al., 1997; Hagerman et al., 1998), and possibly protect against reactive oxygen species induced by O3 (De Temmerman et al., 2002). The observed effects of elevated O3 on the concentrations of birch phenolics have been variable. Previous studies show decrease (phenolic glucoside), increase (DHPPG, cinnamic acid derivatives, flavone aglycons, flavonol glycosides, catechin, total phenolics) or no effect (flavonol glycosides, catechin, condensed tannins) (Lavola et al., 1994; Saleem et al., 2001; Yamaji et al., 2003).
Although the individual effects of CO2 and O3 on phenolics have been studied to some extent, there are still few reports describing the effects of co-exposure. Up to date, simultaneous exposure to CO2 and O3 has been investigated with aspen (Lindroth et al., 2001; Kopper & Lindroth, 2003) and pine (Kainulainen et al., 1998; Sallas et al., 2001). Significant interaction of CO2 and O3 has been observed only on aspen: the concentration of condensed tannins was increased in the combined treatment although the individual treatments had no effect (Kopper & Lindroth, 2003). The limitation of these studies is that they describe the effects on condensed tannins and total phenolics, but not on individual low-molecular-weight compounds. Moreover, concentration, which is the function of both the absolute content of the compound (numerator) and the weight of the plant tissue (denominator), has been generally used as a defining measure for treatment effects. However, any factor changing either the chemical content or the plant weight may, thus, change the concentration and this, if not considered, may lead to the misinterpretation of the results (Gianoli et al., 1999; Koricheva, 1999). As plant biomass is known to increase and decrease by elevated CO2 and O3, respectively (e.g. Saxe et al., 1998; Skärby et al., 1998), it is reasonable to expect these changes influence the concentrations of phenolic compounds as well. The effects may be, for example, antagonistic, additive or synergistic, as was the case with condensed tannins in aspen (Kopper & Lindroth, 2003). Furthermore, it has been shown that phenolic compounds are highly compartmentalized (they are found in the vacuole, nucleus and cell wall) and are situated in different cells and tissues (Hutzler et al., 1998). This may also have influence on how the concentrations of phenolics change if elevated CO2 and O3 affect leaf structure, size or weight.
Recent studies have left also other important gaps in the information concerning the responses of the phenolic compounds of deciduous trees to the changing atmospheric conditions. A major part of the data gathered so far has been acquired from potted seedlings or very young saplings, but information concerning the responses of older trees under field conditions is scarce. Many studies have also been short term, with one sampling per season, thus excluding the examination of seasonal changes. Hence, our current understanding of the effects of elevated CO2 and O3 on phenolics during leaf development is poor although many of these compounds are shown to have different seasonal patterns (Laitinen et al., 2002; Riipi et al., 2002). On the other hand, studies with aspen, willow and mountain birch have shown that genotype-dependent quantitative variation in phenolics can be manifold in comparison with treatment or seasonal effects (Julkunen-Tiitto et al., 1993; Suomela et al., 1995; Lindroth & Hwang, 1996; Nurmi et al., 1996). Large qualitative and quantitative variation of phenolic compounds have also been found in natural and clonal populations of European white birches (Keinänen et al., 1998, 1999a; Laitinen et al., 2000, 2002). It may, therefore, be assumed that different birch genotypes could respond differently to CO2 and O3.
In this study, we have examined the long-term effects of ambient and elevated concentrations of CO2, O3 and their combination on the leaf phenolics of two field-growing silver birch clones. For this study, we hypothesize that
- 1
elevated CO2 increases the concentrations of phenolic compounds, but the effects may be compound specific,
- 2
elevated O3 increases the concentration of antioxidant phenolic compounds, such as phenolic acids, flavonol glycosides, catechin derivatives and tannins,
- 3
elevated CO2 increases leaf dry weight, whereas elevated O3 decreases it, which has influence on phenolic concentrations,
- 4
genotypic variation exceeds the influence of treatments in phenolic concentrations, and genotypic difference has influence on responses.
Materials and methods
Experimental design
This study was conducted at an experimental silver birch field site at the Finnish Forest Research Institute Suonenjoki Research Station (62°39′N, 27°03′E, 120 m a.s.l.) in central Finland. The birch plantation was established in 1993 from 1-year-old cloned silver birch saplings, representing 15 clones originating from southern and central Finland (for experimental details, see Mutikainen et al., 2000). Two birch clones, clones 4 and 80 (register numbers V5952 and K1659 in the Finnish forest genetic register, respectively), differing in their ozone sensitivity (Pääkkönen et al., 1997), latitudinal origin (Mutikainen et al., 2000) and physiological characteristics (Riikonen et al., 2003) were selected for this 3-year open-top chamber experiment. At the beginning of 1999, 20 soil-growing trees from both clones were randomly assigned to five different treatments with four replicate trees each. The treatments were outside control (no chamber) (OC), chamber control (ambient air, CC), elevated CO2 (2 × ambient, EC), elevated O3 (2 × ambient, EO) and elevated CO2 and elevated O3 in combination (both 2 × ambient, EC+EO). The cylindrical open-top chambers, each enclosing one tree, were 2.5 m in diameter and 6 m high in 1999 and they were 7.8 m high after the setting of a conical extension in 2000, and had a computer-controlled CO2 and O3 dispensing and monitoring system (for details, see Vapaavuori et al., 2002).
The trees were fumigated for three growing seasons. Our aim was to double the concentration of CO2 in the EC treatments for a 24 h day−1, and to double the ambient O3 concentration in the EO treatments for a 12 h day−1 (08:00–20:00 hours) in 1999 and 2000, and for a 14 h day−1 (08:00–22:00 hours) in 2001. The fumigation periods were May 25–October 4 in 1999, May 4–September 29 in 2000, and May 2–September 27 in 2001. The mean CO2 concentrations, the AOT40 values for the exposure hours, and the AOT00 values are given in Table 1. The trees were fertilized with 22, 33 and 41 kg N ha−1 in 1999, 2000 and 2001, respectively, and were irrigated (5–20 mm) if the soil water content was below 10%, which was regarded as the threshold for nonlimiting water uptake (Vapaavuori et al., 2002).
| Parameter | Treatment | 1999 | 2000 | 2001 |
|---|---|---|---|---|
| AOT00 (ppm h−1) | CC | 49.4 | 66.2 | 66.3 |
| EO | 73.6±1.5 | 97.1±1.8 | 106.8±1.5 | |
| AOT40 (ppm h−1) | CC | 2.8 | 2.5 | 1.7 |
| EO | 20.6±1.3 | 24.4±1.5 | 30.9±1.4 | |
| CO2 (ppm) | CC | 365 | 371 | 373 |
| EC | 651±85 | 720±40 | 729±38 | |
| Temperature sum (degree days) | Ambient | 1390 | 1234 | 1303 |
| Chambers | 1603±52 | 1618±65 | 1701±77 |
- Ozone and CO2 exposures are based on hourly mean values. The ozone exposure is expressed as accumulated values over a threshold of 0 ppb (AOT00) or 40 ppb (AOT40) during the exposure hours (12, 12 and 14 h in 1999, 2000 and 2001, respectively), and the CO2 concentration is expressed as the mean of each growing season. For the EO and EC treatments, the data are the means±SD of 16 chambers and n=1 for the CC treatment. The temperature sum is the mean of all 32 chambers±SD, and the temperature of ambient air was measured at one location within the canopy. CC, chamber control; EC, elevated CO2; EO, elevated O3.
The environmental conditions in the open-top chambers were somewhat different from the ambient conditions during the growing seasons. Despite the forced-air ventilation of the chambers, the daily mean temperature inside them was, on average, 1.7, 2.3 and 2.4°C higher than the daily mean temperature of the ambient air in 1999, 2000 and 2001, respectively. The cumulative temperature sum increased by 380–400 degree days in 2000 and 2001 compared with that of the ambient air (Table 1). The relative humidity in the ambient air was constantly higher than in the chambers in 2000 and 2001, with a maximum RH difference of about 10–15%. The polyethene film (Hytilux 4, Hyplast Ltd, Hogstraten, Belgium) used in the chamber walls possessed a high transmittance of light (91%) at a wavelength of 400–800 nm, but at wavelengths below 400 nm the transmittance decreased sharply, and at 300 nm only 4.3% was transmitted through the film (Vapaavuori et al., 2002). Hence, the chamber wall filtered off most of the UV radiation.
Sampling and chemical analyses
Short-shoot leaf samples were collected from the experimental trees several times during the growing seasons. In 1999 and 2000, the leaves were sampled on June 1, July 5, August 2 and September 6. In 2001, the leaves were sampled on May 22, May 28, June 5, August 2 and September 6. To minimize defoliation within the canopy during the experiment, one leaf was collected from the upper and lower third of the sun-illuminated side of the canopy of each tree. The variation of phenolic levels in the leaves of mountain birch and silver birch has been shown to be high among trees (genotypes) and low within trees (branches, leaves) (Suomela et al., 1995, Laitinen et al., 2002). Therefore, we assumed that the samples would adequately represent the responses of the active photosynthetic part of the canopy.
The leaf samples were air-dried at room temperature after the measurement of leaf area (LI-3050A leaf area meter, LI-COR Inc., Lincoln, NE, USA). Two leaf disks (0.25 cm2) were cut with a cork-borer from both leaves, avoiding the main veins. The disks were pooled (together consisting of 5–10 mg dry mass) and homogenized with an Ultra-Turrax T8 (Janke and Kunkel, Ika-Labortechnik, Staufen, Germany) for 2 min in 500 μL of methanol (HPLC grade) in an Eppendorf vial. Samples were kept at +4°C for 15 min and then centrifuged (13 000 rpm for 3 min; Biofuge pico, Heraeus Instruments, Hamburg, Germany). The supernatant was removed into a 6 mL glass tube, and the residue was further homogenized in 500 μL of methanol for 1 min three more times. The combined extracts were dried under nitrogen and the dry samples were stored at −20°C. Before chemical analysis, the samples from the leaves collected in 1999 and 2000 were dissolved in 1.5 mL of methanol. The samples were then divided into two aliquots, one for the assay of low-molecular-weight phenolics (LMWP) and the other for the assay of condensed tannins. For the LMWP determination a 700 μL aliquot was taken and dried under nitrogen. The sample was dissolved in water : methanol (1 : 1) and was analysed using high-performance liquid chromatography (Agilent 1100 Series HPLC, Palo Alto, CA, USA), which consisted of a quarternary solvent delivery system, an autosampler and a photodiode array detector coupled with an HP data system and a PC. The samples collected in 2001 were dissolved directly in 0.4 mL methanol+0.4 mL water and analysed using HPLC.
Autoinjected samples of 20 μL in volume were separated on a HP Hypersil ODS column (4.6mm × 60 mm, 3 μm particles) and were run at 31°C. The elution gradient with the solvents A (aqueous 1.5% tetrahydrofuran and 0.25% orthophosphoric acid) and B (MeOH) was 0–5 min 100% of A, 5–10 min 15% of B in A, 10–20 min 30% of B in A, 20–30 min 35% of B in A, 30–45 min 50% of B in A and 45–50 min 100% of B. The eluent flow was 2 mL min−1.
Identification of the LMWP was based on their retention times (Table 2) and UV spectra, which were monitored at 220, 270, 280, 320 and 360 nm. The LMWP were quantified on the basis of the commercial standards: picein (Extrasynthese, Genay, France) for the DHPPG; gallic acid (Aldrich, Steinheim, Germany) for the gallotannins; chlorogenic acid (3-caffeoylquinic acid) (Aldrich) for the caffeoylquinic acid (CaQA) and coumarylquinic acid (CoQA) derivatives; (+)-catechin (Aldrich); myricetin-3-rhamnoside (Apin Chemicals Ltd, Abingdon, UK) for the myricetin derivatives; quercetin-3-galactoside (Apin Chemicals Ltd) for the quercetin-3-galactoside and quercetin-3-glucoside/glucuronide, quercetin-3-arabinoside (Roth, Karlsruhe, Germany); quercetin-3-rhamnoside (Apin Chemicals Ltd); luteolin (Roth) for the luteolin derivatives and apigenin (Roth) for the apigenin derivatives.
| Group | Compound | Retention time (min) | LC/MS molecular ions (m/z) |
|---|---|---|---|
| DHPPG | 3.3 | 351* | |
| Hydrolysable tannins | Pentagalloylglucose | 20.5 | 964* |
| Phenolic acids | Neochlorogenic acid | 3.6 | 355†, 377* |
| Chlorogenic acid | 8.1 | 355†, 377* | |
| Caffeoylquinic acid derivative 1 | 20.0 | ‡ | |
| Caffeoylquinic acid derivative 2 | 23.3 | ‡ | |
| Coumaroylquinic acid derivative 1 | 4.0 | ‡ | |
| Coumaroylquinic acid derivative 2 | 5.7 | ‡ | |
| Coumaroylquinic acid derivative 3 | 10.5 | ‡ | |
| Coumaroylquinic acid derivative 4 | 10.9 | ‡ | |
| Coumaroylquinic acid derivative 5 | 25.1 | ‡ | |
| Coumaroylquinic acid derivative 6 | 29.8 | ‡ | |
| Flavone aglycons | Apigenin | 30.7 | 271† |
| Apigenin derivative 1 | 28.2 | ‡ | |
| Apigenin derivative 2 | 31.0 | ‡ | |
| Apigenin derivative 3 | 40.2 | ‡ | |
| Apigenin derivative 4 | 42.4 | ‡ | |
| Luteolin derivative 1 | 32.5 | ‡ | |
| Luteolin derivative 2 | 33.0 | ‡ | |
| Luteolin derivative 3 | 35.2 | ‡ | |
| Flavonol glycosides | Myricetin-3-galactoside | 15.6 | 481†, 503* |
| Myricetin-3-glucoside | 15.8 | 481†, 503* | |
| Myricetin-3-glucuronide | 15.8 | 495†, 517* | |
| Myricetin-3-rhamnoside | 17.9 | 487* | |
| Quercetin-3-galactoside | 18.3 | 465†, 487* | |
| Quercetin-3-glucoside | 18.9 | 465†, 487* | |
| Quercetin-3-glucuronide | 18.9 | 479†, 501* | |
| Quercetin-3-arabinopyranoside | 19.6 | 435†, 457* | |
| Quercetin-3-arabinofuranoside | 21.0 | 435†, 457* | |
| Quercetin-3-rhamnoside | 21.6 | 449†, 471* | |
| Kaempferol glycoside | 23.8 | ‡ | |
| Catechin derivatives | Gallocatechin | 2.9 | ‡ |
| (+)-catechin | 8.0 | 291† |
- * [M+Na]+; † [M+H]+ ‡ Unidentified molecular ion, tentative identification based on diode array detector spectrum. DHPPG, 3,4′-dihydroxypropiophenone 3-β-D-glucoside.
Half of the compounds were also identified using HPLC/API-ES mass spectrometry (HP 1100 Series LC/MSD, Hewlett-Packard, Palo Alto, CA, USA). Autoinjected samples of 8–20 μL in volume were separated in a HP Hypersil ODS column (2.1mm × 100 mm, 3 μm particles). The elution gradient with the solvents A (aqueous 1.5% THF and 0.25% acetic acid) and B (MeOH) was 0–5 min 100% of A, 5–10 min 15% of B in A, 10–20 min 30% of B in A, 20–40 min 50% of B in A and 40–60 min 100% of B. For the electrospray ionization the capillary was set at 4000 V, the nebulizer pressure was 30 psig and the drying gas (N2) temperature was 350°C with a flow of 12 L min−1. The fragmentation voltage was set at 80 V. The compound fragments found in the mass spectrometer are shown in Table 2.
Methanol extractable soluble condensed tannins (40–500 μL aliquot of extract) and nonextractable cell-wall-bound condensed tannins (1–2 mg of air-dried extraction residue powder) were determined using a butanol–HCl test (Porter et al., 1986; Hagerman, 1995). Purified tannin from the leaves of B. nana (L.) was used as a standard. The extraction and purification of condensed tannins from the leaves of B. nana was done as described by Juntheikki et al. (1996). The background interference in the spectrophotometric assay was corrected using a blank sample.
Of the 33 compounds identified from the birch leaf samples (Table 2), altogether 19, 21 and 27 compounds were measured in 1999, 2000 and 2001, respectively. CoQA derivative 5 and luteolin derivative 3 were measured in 2000–2001. CaQA derivative 2, CoQA derivative 6, apigenin derivative 4, luteolin derivatives 1 and 2, and cell-wall-bound condensed tannins were measured only in 2001. Quercetin-3-arabinofuranoside was not detected from the samples of clone 80. Luteolin derivatives 1 and 2 in clone 4, kaempferol glycoside and apigenin derivative 1 were not measured because they were detected only in trace amounts in some or all of the samples. Myricetin-3-rhamnoside in clone 80, apigenin, CoQA derivatives 1 and 4 and CaQA derivative 1 were not measured because their peaks overlapped with known or unknown compound(s). The total LMWP were calculated as the sum of all of the compounds analysed with HPLC. The total measured phenolics were calculated as the sum of all measured compounds. The recovery of the phenolic compounds by our method was about 90% for most compounds and was determined by conducting our extraction procedure to a portion of phenolic extract from birch leaves and by comparing the results of both analyses.
Statistical and graphical analyses
The data were analysed with repeated-measures analysis of variance (anovar) (Potvin et al., 1990; von Ende, 2001) using SPSS 10.0 for Windows statistical software (SPSS, Chicago, IL, USA). Time (sampling date) was used as a within-subjects factor, and the CO2, O3 and clone were used as between-subjects factors. Trees in the outside control treatment were excluded from the analysis of the main effects and their interactions. The chamber effect was analysed by comparing the trees in OC and CC treatments in anovar. Data from samples taken in August–September 1999 was omitted owing to an error in the sample extraction.
The normality of the data and the homoscedasticity of the variances were checked, and, if necessary, a log10 or square-root transformation was used to meet the assumptions for the analysis. The circularity of the variance–covariance matrix was tested using Mauchly's test for sphericity. If the assumption of sphericity was not met, the Huynh–Feldt correction was used for the within-subjects effects, as suggested by Potvin et al. (1990). Repeated contrasts were used to test the changes in the CO2, O3 and clone effects among the sampling dates. The main effects were considered significant at α=0.05. An interaction of factors was considered significant at α=0.1, although more liberal α level could have been used (Stehman & Meredith, 1995). When the interaction of between-subjects factors was significant, the main effects were interpreted from profile plots using the estimated marginal means as plot values and with the analysis of simple main effects (SMEs) at α=0.025 (Maxwell & Delaney, 1990; Winer et al., 1991).
Treatment effects were also analysed using a graphical vector analysis of the phenolic data from the 2000 and 2001 samples (the complete leaf dry weight data was available only from the 2000 and 2001 samples) by plotting the relative values of phenolic content (x-axis), phenolic concentration (y-axis) and leaf dry weight (diagonal lines) on a graph (Haase & Rose, 1995; Koricheva, 1999). The values for the CC trees were used as the reference value for the calculation of the relative values. The vectors representing the different treatments were drawn from the CC treatment (reference point: 1, 1, 1) and their direction and magnitude were used to interpret the treatment effects (Table 3, modified from Haase & Rose, 1995; Koricheva, 1999). The induced/reduced accumulation of a given compound could mean increased/decreased synthesis or decreased/increased catabolism, transport or turnover, respectively.
| Content | Concentration | Dry weight | Interpretation |
|---|---|---|---|
| + | 0 | + | Steady increase |
| +/0 | − | + | Dilution effect |
| − | − | +/0/− | Reduced accumulation |
| − | 0 | − | Steady decrease |
| −/0 | + | − | Concentration effect |
| + | + | +/0/− | Induced accumulation |
- Modified from Haase & Rose (1995) and Koricheva (1999).
- +, increase; −, decrease; 0, no change.
Results
Leaf characteristics
Elevated CO2 increased the leaf dry weight by 10% (Table 4), but had no effect on the leaf area, whereas elevated O3 decreased the leaf dry weight by 13% in 2000–2001 and also decreased the leaf area by 10% in 2001 (time × O3: P=0.071). On average, the leaf dry weight was 22% greater in clone 80 compared with clone 4 during 2000–2001. The leaf area was 19% greater in clone 80 in 2000, but the difference was not significant in 2001 (time × clone: P=0.05). The leaf dry weight was 58% and the leaf area was 42% greater in the CC trees compared with the OC trees in 2001, but the difference was not significant in 2000 (time × chamber: P<0.001).
| Clone | OC | CC | EC | EO | EC+EO | |
|---|---|---|---|---|---|---|
| Dry weight | ||||||
| 2000 | 4 | 118.8±24.9 | 120.1±12.9 | 141.6±19.6 | 115.2±15.5 | 142.0±7.8 |
| 80 | 131.6±13.9 | 148.8±22.3 | 172.5±11.0 | 135.9±18.4 | 167.3±11.5 | |
| Pooled | 125.2±19.9 | 134.4±22.8 | 157.1±22.1 | 125.6±19.3 | 154.6±16.3 | |
| 2001 | 4 | 72.2±10.3 | 114.2±23.5 | 127.0±12.0 | 94.6±10.9 | 125.6±7.1 |
| 80 | 85.6±5.0 | 135.8±20.2 | 137.3±23.0 | 108.3±10.2 | 124.0±19.4 | |
| Pooled | 78.9±10.4 | 125.0±23.4 | 132.1±17.8 | 101.5±12.2 | 124.8±13.5 | |
| Area | ||||||
| 2000 | 4 | 16.8±2.1 | 17.2±0.9 | 18.0±1.7 | 16.3±2.4 | 17.8±1.1 |
| 80 | 20.0±0.8 | 20.5±2.0 | 20.6±1.1 | 19.1±2.2 | 20.8±2.1 | |
| Pooled | 18.4±2.3 | 18.8±2.3 | 19.3±1.9 | 17.7±2.6 | 19.3±2.3 | |
| 2001 | 4 | 14.3±1.5 | 21.4±3.2 | 21.2±1.9 | 18.5±1.2 | 21.4±0.8 |
| 80 | 17.4±1.5 | 23.4±1.6 | 23.3±2.5 | 20.2±2.8 | 21.0±3.5 | |
| Pooled | 15.8±2.2 | 22.4±2.6 | 22.2±2.4 | 19.4±2.2 | 21.2±2.4 | |
| CO2 | O3 | Clone | ||||
| Dry weight | ↑*** | ↓* | 80>4*** | |||
| Area | ns | ↓* | 80>4** | |||
- The treatments were chamber control (CC), elevated CO2 (EC), elevated O3 (EO) and elevated CO2 and O3 (EC+EO). Number of replicates in each treatment were four for individual clone data (N=4) and eight for pooled data (N=8).
Individual and interactive effects of elevated CO2 and O3
Elevated CO2 increased the concentration of the total myricetin glycosides by 18%, the total catechin derivatives by 13%, soluble condensed tannins by 19% and the total phenolics by 12% (Tables 5 and 6), as a result of induced accumulation of these compounds (Fig. 2). However, the 25% increase in phenolic acids (total CaQA and CoQA, neochlorogenic acid, CoQA derivative 2) was significant only in clone 4 (SME: P<0.01). In addition, the concentrations of the total phenolic acids and the total CoQA were increased by CO2 enrichment only in 2000 and in May 2001 (time × CO2: P=0.001 and P<0.001, respectively). Moreover, increased concentrations of neochlorogenic acid, myricetin glycosides (excluding myricetin-3-rhamnoside) and soluble condensed tannins were found after the first fumigation year and that of the total phenolics after the second year (time × CO2: P=0.015, P<0.05, P=0.001 and P=0.014, respectively). In contrast, elevated CO2 did not have effect on PGG (SME: P>0.025 for both clones) or the total LMWP and even decreased the concentration of the total flavone aglycons by 7% and quercetin-3-rhamnoside by 10% (Tables 5 and 6). In addition, elevated CO2 decreased the concentrations of some of the quercetin glycosides in clone 80, but the effect was significant only in quercetin-3-glucoside and glucuronide (SME: P=0.016) (Table 5). Vector analysis showed that the accumulation of PGG and the total LMWP followed the increase in leaf dry weight (steady increase, data not shown). The flavone aglycones were diluted (Fig. 3c) and the accumulation of quercetin-3-rhamnoside was reduced (data not shown) in response to elevated CO2.
| CO2 | O3 | Clone | CO2× O3 | CO2×clone | O3×clone | CO2× O3×clone | Chamber | |
|---|---|---|---|---|---|---|---|---|
| Pentagalloylglucose | ns | ns | 80>4**** | ns | ** | ns | ns | OC>CC** |
| DHPPG | ↓*** | ↑*** | 4>80**** | ** | ns | ns | ns | OC>CC**** |
| Phenolic acids | ↑**** | ↑* | 80>4**** | *** | *** | ns | ns | ns |
| Caffeoylquinic acids | ns | ns | ns | *** | * | ns | ns | ns |
| Neochlorogenic acid | ↑** | ns | ns | ** | ** | ns | ns | ns |
| Chlorogenic acid | ns | ↑** | ns | *** | ns | ns | ns | ns |
| Derivative 2 | ns | ns | 80>4**** | ns | ns | ns | ns | ns |
| Coumarylquinic acids | ↑**** | ns | 80>4**** | *** | *** | ns | ns | 80: CC>OC***** |
| Derivative 2 | ↑**** | ns | 80>4**** | ns | *** | ns | ns | CC>OC*** |
| Derivative 3 | ns | ns | 80>4**** | * | ns | ns | ns | ns |
| Derivative 5 | ↑**** | ns | 80>4**** | ** | * | ns | ns | ns |
| Derivative 6 | ns | ns | 80>4**** | ** | ns | ns | ns | 80: CC>OC***** |
| Flavone aglycons | ↓*** | ↑** | 4>80**** | ns | ns | * | ns | OC>CC** |
| Apigenin derivatives | ↓*** | ↑** | 4>80*** | * | ns | ns | ns | OC>CC*** |
| Derivative 2 | ↓*** | ↑* | 80>4**** | ns | ns | ns | ns | OC>CC*** |
| Derivative 3 | ↓*** | ↑** | 4>80**** | ns | ns | * | ns | ns |
| Derivative 4 | ns | ns | 80>4**** | ns | ns | ns | ns | OC>CC** |
| Luteolin derivatives | ↓** | ↑** | 80>4**** | ns | ns | ns | ns | 4: OC>CC***** |
| Derivative 1 | ns | ns | – | ns | – | – | – | ns |
| Derivative 2 | ns | ns | – | ns | – | – | – | ns |
| Derivative 3 | ↓** | ↑* | 80>4**** | ns | ns | ns | ns | 4: OC>CC***** |
| Flavonol glycosides | ns | ns | 80>4*** | * | ns | ns | ns | OC>CC**** |
| Myricetin glycosides | ↑** | ns | 80>4**** | * | ns | ns | ns | OC>CC*** |
| M-3-galactoside | ↑** | ns | 80>4**** | ns | ns | ns | ns | OC>CC*** |
| M-3-glucoside and glucuronide | ↑** | ns | 80>4**** | ns | ns | ns | ns | OC>CC* |
| M-3-rhamnoside | ns | ns | – | ns | – | – | – | ns |
| Quercetin glycosides | ns | ↑* | ns | ** | ** | ns | ns | OC>CC**** |
| Q-3-galactoside | ns | ns | 80>4**** | * | ** | * | ns | OC>CC**** |
| Q-3-glucoside and glucuronide | ns | ns | ns | ns | ** | * | ns | OC>CC**** |
| Q-3-arabinopyranoside | ns | ns | 80>4**** | ns | ** | * | ns | OC>CC**** |
| Q-3-arabinofuranoside | ns | ns | – | ns | – | – | – | OC>CC**** |
| Q-3-rhamnoside | ↓*** | ns | 4>80**** | ns | ns | ns | * | OC>CC**** |
| Catechin derivatives | ↑*** | ns | 4>80*** | ns | ns | ns | ns | ns |
| (+)-catechin | ↑*** | ns | 4>80**** | ns | ns | ns | ns | ns |
| Gallocatechin | ↑** | ns | ns | ns | ns | ns | ns | CC>OC* |
| LMWP | ns | ↑** | ns | *** | ns | ns | ns | OC>CC**** |
| Condensed tannins | ↑*** | ↑** | 4>80**** | ns | ns | ns | ns | CC>OC** |
| Soluble | ↑*** | ↑* | 4>80**** | ns | ns | ns | ns | CC>OC** |
| Cell wall bound | ns | ns | ns | ns | ns | ns | ns | CC>OC** |
| Total measured phenolics | ↑** | ↑*** | 4>80**** | * | ns | ns | ns | OC>CC** |
- ↑, increase; ↓, decrease; >, greater than; 80, clone 80; 4, clone 4; OC, outside control; CC, chamber control; -, not measured in both clones; ns, not significant. * P<0.1; ** P<0.05; *** P<0.01; **** P<0.001; ***** P<0.025 in SME.
| Compound/group | Clone | OC | CC | EC | EO | EC+EO |
|---|---|---|---|---|---|---|
| PGG | 4 | 0.12±0.10 | 0.11±0.09 | 0.13±0.10 | 0.11±0.09 | 0.11±0.09 |
| 80 | 0.31±0.33 | 0.16±0.14 | 0.16±0.13 | 0.19±0.16 | 0.19±0.23 | |
| Pooled | 0.22±0.26 | 0.14±0.12 | 0.14±0.11 | 0.15±0.14 | 0.15±0.18 | |
| DHPPG | 4 | 15.0±6.9 | 11.2±6.1 | 10.2±5.4 | 13.2±6.4 | 10.0±4.9 |
| 80 | 7.3±4.4 | 5.4±3.5 | 5.4±3.4 | 7.0±4.2 | 5.9±3.5 | |
| Pooled | 11.1±7.0 | 8.3±5.7 | 7.8±5.1 | 10.1±6.3 | 7.9±4.7 | |
| Phenolic acids | 4 | 9.4±4.6 | 8.9±3.9 | 11.4±5.5 | 9.8±4.5 | 10.9±4.9 |
| 80 | 10.7±4.8 | 11.8±4.7 | 13.6±5.9 | 13.4±5.4 | 12.8±5.8 | |
| Pooled | 10.1±4.7 | 10.4±4.6 | 12.5±5.8 | 11.6±5.3 | 11.9±5.4 | |
| Caffeoylquinic acids | 4 | 3.0±1.0 | 2.6±0.8 | 3.1±1.2 | 2.9±0.8 | 2.9±1.0 |
| 80 | 2.6±0.8 | 2.5±0.6 | 2.7±1.1 | 3.1±0.9 | 2.7±0.9 | |
| Pooled | 2.8±0.9 | 2.5±0.7 | 2.9±1.1 | 3.0±0.9 | 2.8±0.9 | |
| Neochlorogenic acid | 4 | 1.02±0.46 | 0.85±0.34 | 1.23±0.52 | 0.88±0.30 | 1.02±0.42 |
| 80 | 0.91±0.36 | 0.85±0.26 | 0.99±039 | 1.01±0.33 | 0.92±0.31 | |
| Pooled | 0.96±0.41 | 0.85±0.30 | 1.11±0.47 | 0.95±0.32 | 0.97±0.37 | |
| Chlorogenic acid | 4 | 1.9±0.6 | 1.7±0.5 | 1.9±0.7 | 1.9±0.6 | 1.8±0.6 |
| 80 | 1.7±0.5 | 1.6±0.4 | 1.8±0.6 | 2.0±0.6 | 1.7±0.6 | |
| Pooled | 1.8±0.6 | 1.6±0.5 | 1.8±0.7 | 2.0±0.6 | 1.8±0.6 | |
| Derivative 2 | 4 | 0.12±0.05 | 0.09±0.04 | 0.10±0.04 | 0.10±0.04 | 0.09±0.02 |
| 80 | 0.12±0.04 | 0.11±0.03 | 0.13±0.04 | 0.14±0.04 | 0.13±0.04 | |
| Pooled | 0.12±0.04 | 0.10±0.04 | 0.11±0.04 | 0.12±0.04 | 0.11±0.04 | |
| Coumaroylquinic acids | 4 | 6.5±3.7 | 6.4±3.2 | 8.2±4.4 | 6.9±3.7 | 8.1±4.0 |
| 80 | 8.1±4.1 | 9.3±4.3 | 10.8±5.0 | 10.3±4.7 | 10.1±5.0 | |
| Pooled | 7.3±4.0 | 7.8±4.0 | 9.5±4.9 | 8.6±4.5 | 9.1±4.6 | |
| Derivative 2 | 4 | 3.8±1.7 | 4.1±1.4 | 5.7±2.4 | 4.2±1.5 | 5.5±1.9 |
| 80 | 4.7±2.1 | 5.7±2.0 | 6.7±2.4 | 6.2±2.2 | 6.3±2.3 | |
| Pooled | 4.3±1.9 | 4.9±1.9 | 6.2±2.4 | 5.2±2.1 | 5.9±2.1 | |
| Derivative 3 | 4 | 2.2±1.9 | 1.9±1.6 | 2.0±1.7 | 2.3±2.0 | 2.1±1.8 |
| 80 | 2.9±2.1 | 3.0±2.3 | 3.4±2.6 | 3.5±2.6 | 3.3±2.7 | |
| Pooled | 2.6±2.0 | 2.5±2.0 | 2.7±2.3 | 2.9±2.4 | 2.7±2.3 | |
| Derivative 5 | 4 | 0.51±0.40 | 0.39±0.33 | 0.59±0.42 | 0.46±0.38 | 0.58±0.40 |
| 80 | 0.52±0.26 | 0.56±0.34 | 0.72±0.36 | 0.60±0.29 | 0.64±0.36 | |
| Pooled | 0.51±0.33 | 0.47±0.35 | 0.65±0.39 | 0.53±0.34 | 0.61±0.38 | |
| Derivative 6 | 4 | 0.07±0.04 | 0.07±0.04 | 0.08±0.05 | 0.09±0.06 | 0.08±0.05 |
| 80 | 0.09±0.03 | 0.15±0.06 | 0.17±0.07 | 0.17±0.06 | 0.16±0.09 | |
| Pooled | 0.08±0.04 | 0.11±0.07 | 0.13±0.08 | 0.13±0.07 | 0.12±0.08 | |
| Flavone aglycons | 4 | 2.8±1.3 | 2.4±1.2 | 2.2±1.1 | 2.6±1.1 | 2.1±1.0 |
| 80 | 2.6±1.6 | 2.2±1.2 | 2.0±1.1 | 2.7±1.4 | 2.2±1.2 | |
| Pooled | 2.7±1.5 | 2.3±1.2 | 2.1±1.1 | 2.6±1.2 | 2.2±1.1 | |
| Apigenin derivatives | 4 | 2.6±1.3 | 2.3±1.2 | 2.1±1.1 | 2.4±1.1 | 2.0±1.0 |
| 80 | 2.2±1.6 | 1.8±1.0 | 1.7±0.9 | 2.3±1.3 | 1.9±1.1 | |
| Pooled | 2.4±1.4 | 2.1±1.1 | 1.9±1.0 | 2.4±1.2 | 2.0±1.0 | |
| Derivative 2 | 4 | 0.51±0.31 | 0.36±0.26 | 0.32±0.23 | 0.39±0.23 | 0.30±0.20 |
| 80 | 0.62±0.50 | 0.48±0.30 | 0.43±0.27 | 0.59±0.37 | 0.47±0.30 | |
| Pooled | 0.57±0.42 | 0.42±0.28 | 0.37±0.25 | 0.49±0.32 | 0.39±0.27 | |
| Derivative 3 | 4 | 2.0±1.0 | 1.9±1.0 | 1.8±0.9 | 2.0±0.9 | 1.7±0.8 |
| 80 | 1.5±1.1 | 1.3±0.7 | 1.2±0.7 | 1.6±0.9 | 1.3±0.8 | |
| Pooled | 1.7±1.1 | 1.6±0.92 | 1.5±0.8 | 1.8±0.9 | 1.5±0.8 | |
| Derivative 4 | 4 | 0.13±0.08 | 0.11±0.06 | 0.09±0.04 | 0.10±0.05 | 0.09±0.04 |
| 80 | 0.20±.013 | 0.17±0.09 | 0.18±0.10 | 0.19±0.10 | 0.18±0.10 | |
| Pooled | 0.16±0.11 | 0.14±0.08 | 0.13±0.09 | 0.15±0.09 | 0.13±0.09 | |
| Luteolin derivatives | 4 | 0.25±0.18 | 0.14±0.12 | 0.13±0.10 | 0.17±0.14 | 0.13±0.10 |
| 80 | 0.44±0.50 | 0.40±0.47 | 0.41±0.43 | 0.50±0.51 | 0.39±0.40 | |
| Pooled | 0.35±0.39 | 0.27±0.36 | 0.26±0.34 | 0.34±0.40 | 0.26±0.32 | |
| Derivative 1 | 4 | – | – | – | – | – |
| 80 | 0.18±0.12 | 0.16±0.10 | 0.15±0.09 | 0.17±0.10 | 0.15±0.10 | |
| Pooled | 0.18±0.12 | 0.16±0.10 | 0.15±0.09 | 0.17±0.10 | 0.15±0.10 | |
| Derivative 2 | 4 | – | – | – | – | – |
| 80 | 0.21±0.16 | 0.20±0.15 | 0.21±0.14 | 0.25±0.14 | 0.19±0.12 | |
| Pooled | 0.21±0.16 | 0.20±0.15 | 0.21±0.14 | 0.25±0.14 | 0.19±0.12 | |
| Derivative 3 | 4 | 0.25±0.18 | 0.14±0.12 | 0.13±0.10 | 0.17±0.14 | 0.13±0.10 |
| 80 | 0.23±0.23 | 0.21±0.21 | 0.20±0.19 | 0.27±0.25 | 0.20±0.17 | |
| Pooled | 0.24±0.21 | 0.17±0.17 | 0.16±0.15 | 0.22±0.20 | 0.16±0.14 | |
| Flavonol glycosides | 4 | 33.7±9.3 | 20.0±7.9 | 22.2±7.9 | 20.9±7.3 | 20.6±6.6 |
| 80 | 32.5±10.6 | 21.8±7.4 | 22.6±8.0 | 24.9±8.8 | 23.1±8.4 | |
| Pooled | 33.1±9.9 | 20.9±7.6 | 22.4±7.9 | 22.9±8.3 | 21.8±7.6 | |
| Myricetin glycosides | 4 | 6.3±2.9 | 4.9±2.3 | 6.2±2.4 | 5.3±2.3 | 5.5±1.9 |
| 80 | 8.6±4.0 | 7.3±2.8 | 8.2±2.9 | 8.5±3.9 | 8.5±3.5 | |
| Pooled | 7.5±3.7 | 6.1±2.8 | 7.2±2.8 | 6.9±3.5 | 7.0±3.1 | |
| M-3-galactoside | 4 | 3.6±1.5 | 2.5±1.2 | 3.3±1.3 | 2.7±1.1 | 2.9±1.0 |
| 80 | 6.6±3.0 | 5.5±2.1 | 6.3±2.3 | 6.5±2.9 | 6.4±2.6 | |
| Pooled | 5.1±2.8 | 4.0±2.3 | 4.8±2.3 | 4.6±2.9 | 4.7±2.6 | |
| M-3-glucoside and -glucuronide | 4 | 1.0±0.5 | 0.8±0.3 | 1.0±0.4 | 0.9±0.3 | 0.9±0.3 |
| 80 | 1.9±0.8 | 1.7±0.6 | 1.8±0.6 | 1.9±0.8 | 1.9±0.7 | |
| Pooled | 1.4±0.8 | 1.2±0.6 | 1.4±0.6 | 1.4±0.8 | 1.4±0.7 | |
| M-3-rhamnoside | 4 | 1.7±1.0 | 1.6±0.7 | 1.9±0.8 | 1.7±0.8 | 1.7±0.7 |
| 80 | – | – | – | – | – | |
| Pooled | 1.7±1.0 | 1.6±0.7 | 1.9±0.8 | 1.7±0.8 | 1.7±0.7 | |
| Quercetin glycosides | 4 | 27.4±7.1 | 15.0±6.0 | 16.0±5.8 | 15.5±5.4 | 15.1±5.0 |
| 80 | 23.8±6.9 | 14.4±5.0 | 14.4±5.3 | 16.4±5.4 | 14.6±5.2 | |
| Pooled | 25.6±7.2 | 14.7±5.5 | 15.2±5.6 | 16.0±5.4 | 14.8±5.1 | |
| Q-3-galactoside | 4 | 13.0±3.5 | 6.8±3.0 | 7.4±2.6 | 6.9±2.4 | 6.9±2.3 |
| 80 | 14.5±4.5 | 8.5±3.2 | 8.7±3.4 | 9.8±3.5 | 8.7±3.4 | |
| Pooled | 13.7±4.1 | 7.7±3.2 | 8.1±3.1 | 8.3±3.3 | 7.8±3.0 | |
| Q-3-glucoside and -glucuronide | 4 | 4.9±1.2 | 2.6±1.0 | 2.8±0.9 | 2.7±0.8 | 2.6±0.8 |
| 80 | 4.4±1.0 | 2.8±0.8 | 2.6±0.8 | 3.1±0.9 | 2.7±0.8 | |
| Pooled | 4.7±1.1 | 2.7±0.9 | 2.7±0.9 | 2.9±0.9 | 2.7±0.8 | |
| Q-3-arabinopyranoside | 4 | 3.1±0.8 | 1.7±0.7 | 1.9±0.6 | 1.7±0.6 | 1.8±0.6 |
| 80 | 4.0±1.2 | 2.5±0.8 | 2.6±0.9 | 2.9±0.9 | 2.6±0.9 | |
| Pooled | 3.5±1.1 | 2.1±0.9 | 2.2±0.8 | 2.3±1.0 | 2.2±0.8 | |
| Q-3-arabinofuranoside | 4 | 4.1±1.0 | 2.4±0.9 | 2.5±1.0 | 2.5±0.9 | 2.4±0.8 |
| 80 | – | – | – | – | – | |
| Pooled | 4.1±1.0 | 2.4±0.9 | 2.5±1.0 | 2.5±0.9 | 2.4±0.8 | |
| Q-3-rhamnoside | 4 | 2.3±0.9 | 1.5±0.7 | 1.4±0.8 | 1.8±0.9 | 1.4±0.7 |
| 80 | 1.0±0.5 | 0.6±0.4 | 0.5±0.3 | 0.6±0.3 | 0.6±0.3 | |
| Pooled | 1.6±1.0 | 1.1±0.7 | 1.0±0.8 | 1.2±0.9 | 1.0±0.7 | |
| Catechin derivatives | 4 | 2.4±0.8 | 2.8±1.3 | 3.0±0.9 | 2.7±0.9 | 3.2±1.0 |
| 80 | 2.3±1.1 | 2.4±0.9 | 2.7±1.0 | 2.4±0.9 | 2.8±1.2 | |
| Pooled | 2.3±1.0 | 2.6±1.1 | 2.9±0.9 | 2.6±0.9 | 3.0±1.1 | |
| (+)-catechin | 4 | 1.5±0.6 | 1.8±0.9 | 1.9±0.7 | 1.7±0.7 | 2.0±0.7 |
| 80 | 1.3±0.6 | 1.3±0.5 | 1.5±0.6 | 1.4±0.6 | 1.6±0.8 | |
| Pooled | 1.4±0.6 | 1.5±0.8 | 1.7±0.7 | 1.5±0.7 | 1.8±0.8 | |
| Gallocatechin | 4 | 0.9±0.5 | 1.1±0.5 | 1.1±0.4 | 1.0±0.4 | 1.2±0.5 |
| 80 | 1.0±0.7 | 1.1±0.5 | 1.2±0.5 | 1.0±0.4 | 1.1±0.6 | |
| Pooled | 1.0±0.6 | 1.1±0.5 | 1.1±0.5 | 1.0±0.4 | 1.2±0.5 | |
| LMWP | 4 | 63.4±20.3 | 45.4±16.7 | 49.2±18.4 | 49.3±17.4 | 47.0±16.6 |
| 80 | 55.6±19.2 | 43.7±14.4 | 46.5±16.5 | 50.7±17.3 | 46.9±17.2 | |
| Pooled | 59.5±20.0 | 44.5±15.5 | 47.8±17.4 | 50.0±17.3 | 46.9±16.8 | |
| Condensed tannins | 4 | 82.2±24.9 | 93.7±26.8 | 108.2±30.8 | 104.7±26.7 | 112.5±27.8 |
| 80 | 76.9±29.2 | 82.9±26.1 | 93.5±24.5 | 90.8±23.5 | 92.7±24.5 | |
| Pooled | 79.6±27.1 | 88.8±26.8 | 100.9±28.6 | 97.8±25.9 | 102.6±27.9 | |
| Soluble | 4 | 66.1±20.0 | 76.5±20.6 | 90.4±22.0 | 86.7±25.7 | 93.8±19.2 |
| 80 | 59.0±16.8 | 64.5±18.6 | 75.5±14.2 | 72.8±19.7 | 74.8±16.4 | |
| Pooled | 62.5±18.7 | 70.5±20.4 | 83.0±19.9 | 79.7±23.8 | 84.3±20.2 | |
| Cell wall bound | 4 | 35.5±10.1 | 37.9±9.6 | 39.1±10.3 | 39.7±10.0 | 41.0±11.3 |
| 80 | 39.5±10.0 | 40.5±10.9 | 39.6±11.7 | 39.6±10.1 | 39.2±12.7 | |
| Pooled | 37.5±10.1 | 39.2±10.2 | 39.4±10.9 | 39.7±10.0 | 40.1±11.9 | |
| Total measured phenolics | 4 | 145.6±32.1 | 139.1±32.1 | 157.4±37.5 | 154.0±30.8 | 159.4±35.7 |
| 80 | 132.6±35.3 | 126.6±29.9 | 140.3±33.9 | 141.4±30.6 | 139.6±32.7 | |
| Pooled | 139.1±34.2 | 132.9±31.4 | 148.9±36.6 | 147.7±31.2 | 149.5±35.5 |

Effects of treatments on the relative concentration and content of flavonol glycosides, catechin derivatives and condensed tannins, and the relative leaf dry weight in the short-shoot leaves of silver birch in 2000 and 2001. The values for the CC trees were used for the calculation of the relative values and the CC treatment was used as a reference point (1,1,1). For the interpretation of the vectors, see the instructions in Table 3. Abbreviations: OC=outside control, CC=chamber control, EC=elevated CO2, EO=elevated O3, EC+EO=elevated CO2 and O3 in combination.
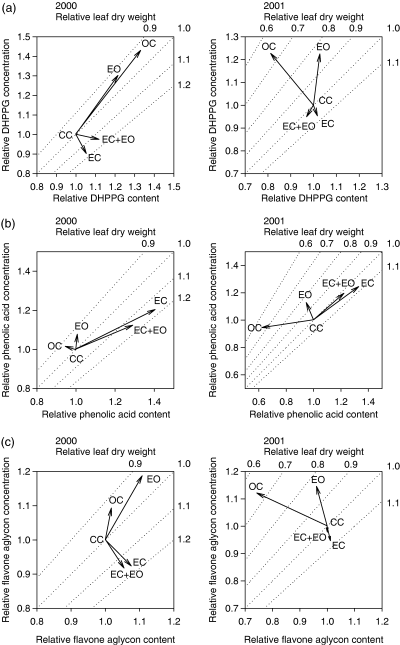
Effects of treatments on the relative concentration and content of 3,4′-dihydroxypropiophenone 3-β-d-glucoside (DHPPG), phenolic acids and flavone aglycons, and the relative leaf dry weight in the short-shoot leaves of silver birch in 2000 and 2001. The values for the CC trees were used for the calculation of the relative values, and the CC treatment was used as a reference point (1,1,1). The interpretation of the vectors follows the instructions in Table 3. Abbreviations as in Fig. 2.
Elevated O3 increased the concentration of DHPPG by 22%, the total phenolic acids by 13%, chlorogenic acid by 19%, the total LMWP by 12%, the total condensed tannins by 13% and the total phenolics by 11% (Tables 5 and 6). However, the increase in the concentration of soluble condensed tannins was only marginally significant, and cell-wall-bound condensed tannins were not affected at all. Moreover, the 11% increase in the concentration of the total flavone aglycons and apigenin derivative 3 was significant only in clone 80 (P=0.005 and 0.003, respectively) (Tables 5 and 6). The O3 effect on concentration was consistent in most of the compounds throughout the experiment, although the total quercetin glycosides were not affected in clone 4 in 2000 (time × O3× clone: P=0.049). The O3 effect on the total measured phenolics was variable in 2000–2001 showing both increase and no effect (time × O3: P=0.028). According to the vector analysis, elevated O3 induced the accumulation of DHPPG and the flavone aglycons in 2000, but this induction was changed to concentration effect in 2001 (Fig. 3a, c). Elevated O3 increased the total concentration of the phenolic acids by concentration effect in both years (Fig. 3b), although the accumulation of chlorogenic acid was induced in 2000 (data not shown).
The effects of elevated O3 on phenolic concentrations were adjusted by elevated CO2 and vice versa, especially in DHPPG and phenolic acids (CO2× O3 interaction, Table 5). The SME analysis showed that elevated O3 increased DHPPG in ambient CO2, but the effect was prevented by elevated CO2 (P<0.001 and P=0.001, respectively) (Fig. 4a). The results of the SME analyses were similar for chlorogenic acid (P=0.008 and 0.001) (Fig. 4c) and for the total apigenin derivatives (P=0.007 and 0.001) (Fig. 5d). Elevated CO2 increased neochlorogenic acid and the total myricetin glycosides only in ambient O3 (SME: P=0.002 and 0.006, respectively) (4, 5), whereas it increased CoQA derivative 5 in both ambient and elevated O3 (SME: P<0.001 and P=0.023) (Fig. 4e). Elevated O3 increased CoQA derivative 3, the total quercetin glycosides and the total LMWP only in ambient CO2 (SME: P=0.022, 0.009 and 0.001, respectively) (4, 5). Elevated CO2 and O3 increased the concentrations of the phenolic acids, the CaQA and the total phenolics singly, but not in combination (SME: P<0.001 and P=0.001; P=0.004 and 0.002; P=0.002 and 0.01, respectively) (Fig. 5a, b, i). Elevated CO2 increased the concentration of the total CoQA in both O3 levels, but the effect of elevated O3 was prevented by elevated CO2 (P<0.001, P=0.014 and 0.001) (Fig. 5c).
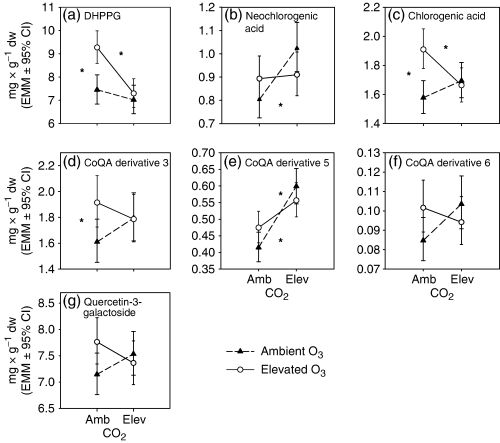
Three-year mean concentrations (mg g−1 dry weight, back-transformed estimated marginal means [EMM]±95% confidence interval) of individual phenolic compounds in different CO2- and O3-concentrations. The significant simple main effect (P<0.025) of elevated O3 in ambient or elevated CO2 is indicated with an asterisk on the right or left, respectively. The significant simple main effect (P<0.025) of elevated CO2 in ambient or elevated O3 is indicated with an asterisk on the low or high position, respectively.
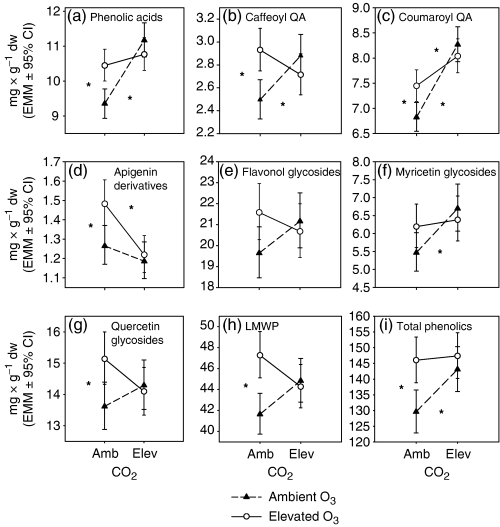
Three-year mean concentrations (mg g−1 dry weight, back-transformed estimated marginal means [EMM]±95% confidence interval) of phenolic groups in different CO2 and O3 concentrations. Explanations of the significant simple main effects as in Fig. 4.
Clone effect
Clone 80 had 9–120% higher concentrations of PGG, phenolic acids, some flavone aglycons, and flavonol glycosides compared with clone 4 (Tables 5 and 6). On the other hand, clone 80 had up to 52% lower concentrations of DHPPG, the total apigenin and catechin derivatives, condensed tannins and the total measured phenolics compared with clone 4 (Tables 5 and 6). On the basis of differences in leaf dry weight between the clones, it seems that in clone 80, the accumulation of some of the compounds was increased and some were most likely diluted when compared with clone 4.
Also, for many phenolics (PGG, total flavone aglycons, total quercetin glycosides and total catechin derivatives) there was a significant time × clone interaction (P<0.001) indicating that the difference between the clones was not consistent over time. For example, there was no difference in the concentration of the total flavone aglycons between the clones in the early season (May–June).
Chamber effect
The concentrations of PGG, DHPPG, and the total flavone aglycons, flavonol glycosides and phenolics decreased by 4–37% in the CC trees in comparison with the OC trees (Tables 5 and 6). The vector analysis showed that the accumulation of DHPPG and the total flavonol glycosides was dramatically reduced in 2000 but they were diluted in 2001 (2, 3) whereas the total flavone aglycons were diluted in both years (Fig. 3c). Conversely, the concentrations of some of the CoQA derivatives, gallocatechin and condensed tannins increased by 14–67% (Tables 5 and 6) owing to induced accumulation of these compounds in the CC trees (2, 3).
This chamber effect was not consistent in all of the measured compounds. The concentration of PGG was higher in the OC trees only in 2001 (time × chamber: P=0.003). The difference between the OC and CC trees in the concentration of DHPPG was not observed in August–September 2001 (time × chamber: P<0.001). The concentrations of the flavone aglycons followed similar (luteolin derivatives) or closely similar patterns (there was a slight variation among the apigenin derivatives) to that of DHPPG (time × chamber: P<0.001).
Discussion
In this study we investigated, in detail, the responses of various phenolic compounds in the leaves of birch saplings to changing atmospheric conditions (CO2 and O3) in order to asses how the accumulation of these compounds is affected in a long run. We found that excess carbon availability did not exclusively increase the concentrations of all the assayed compounds, but even decreased the concentration of some compounds. Likewise, elevated O3 did not increase all potential antioxidant compounds. The vector analysis used in analysing the data proved to be a useful tool in interpretation of the observed concentration effects.
Elevated CO2 and O3 modify leaf traits and phenolic accumulation
Changes in the leaf dry weight under CO2 and O3 fumigation followed the trends found in earlier experiments (Saxe et al., 1998; Skärby et al., 1998) and therefore, the denominator of concentration parameter was changed by the treatments, as was expected. It seems that increased leaf dry weight under CO2 enrichment was at least partly because of increased starch concentration (Riikonen et al., 2004), because neither the area nor the thickness of individual leaves was affected (Oksanen et al., 2005). In contrast, O3 decreased the dry weight, the area (only in 2001) and the thickness (Oksanen et al., 2005) of the leaves, but did not affect the starch concentration (Riikonen et al., 2004).
The effects of elevated CO2 on the concentrations of different compounds were variable. The unresponsiveness of hydrolysable tannin (PGG) in our study is in accordance with the results of Koricheva et al. (1998). Increases in the concentrations of some phenolic acids, catechin derivatives and soluble condensed tannins and decreases in flavone aglycons and quercetin-3-rhamnoside are in accordance with earlier studies with young silver birch seedlings (e.g. Lavola & Julkunen-Tiitto, 1994; Lavola et al., 2000; Kuokkanen et al., 2003). The contents of different compounds had increased by ca. 10–40% under CO2 in almost every compound group (2, 3), which indicates that extra carbon is shunted to a wide array of compounds along the phenylpropanoid pathway. For example, PGG content per leaf was increased ca. 25% as a result of steady increase in the accumulation (data not shown), which shows that hydrolysable tannins are responsive to carbohydrate availability (Koricheva et al., 1998), although there was no change in concentration. In contrast, the contents of DHPPG and flavone aglycons were not changed under elevated CO2. Valkama et al. (2003, 2004) showed that glandular trichomes excrete the epicuticular flavone aglycons of birch leaves in the early development phase of leaf. The observed growth dilution of surface flavonoids in early and late leaf development phases (Valkama et al., 2004) explains the observed dilution effect of flavone aglycons under elevated CO2 in our study (Fig. 3c).
The effect of elevated CO2 on phenolic concentrations was consistent throughout the experiment in most of the compounds analysed. The similarities in the CO2-induced pattern in phenolic acids, myricetin glycosides and soluble condensed tannins hint that the allocation of carbon to these compounds may be linked. Indeed, Seigler (1998) noted that myricetin tends to be associated with condensed tannins in woody plants. We observed transient increase in the concentration of phenolic acids that may indicate that the trees were acclimatizing to CO2-enriched atmosphere by directing the carbon from phenolic acids to myricetin glycosides and soluble condensed tannins.
It has been suggested that the regulation of quercetin-3-rhamnoside is independent from the other glycosides of quercetin (Keinänen et al., 1998, 1999a). These authors noted that flavonol rhamnosides are found in higher proportions in seedlings than in saplings or mature trees. The reduced accumulation of quercetin-3-rhamnoside under elevated CO2 may indicate that the physiological ageing of our trees was accelerated. This is supported by the results of Riikonen et al. (2004) that showed that trees under EC and EC+EO treatments produced seeds but those under CC treatment did not.
Our finding that elevated O3 increased the concentration of DHPPG, the phenolic acids, the flavone aglycons, condensed tannins and the total phenolics is in accordance with recent studies with birch saplings (Saleem et al., 2001; Yamaji et al., 2003). However, vector analysis showed that this O3-derived induction led to induced accumulation only in DHPPG and flavone aglycons while flavonol glycosides and condensed tannins were merely concentrated as a result of decrease in leaf dry weight (2, 3). Hence, our results do not support the hypothesis that hydrolysable and condensed tannins function as antioxidants in birch leaves. Chlorogenic acid, a strong antioxidant in plants (Grace et al., 1998), was also induced (figure not shown), although the phenolic acids as a whole were only concentrated (Fig. 3). On the basis of the structure of DHPPG, having one hydroxyl group in an aromatic ring (Mori et al., 1992), this compound could have antioxidant activity similar to cinnamic acid derivatives. It is known that ozone enters leaves through the stomata that are located on the lower sides of birch leaves where the density of glandular trichomes producing epicuticular flavonoids is also higher (Valkama et al., 2003). Flavone aglycons have antioxidant activity (e.g. Rice-Evans et al., 1997) and they might react directly with O3 deposited on leaf surface.
The unresponsiveness of the total phenolics to O3 at certain phases of leaf development may be related to physiological differences between the leaves of the CC and EO trees. Chronic ozone exposure (≤100 ppb, long duration) generally accelerates the senescence of leaves (Pell et al., 1997), also seen in this field study (Riikonen et al., 2004), and causes accumulating growth reductions in birch over time (Oksanen & Saleem, 1999). These carry-over effects that in our experimental trees decreased leaf biomass and area (Riikonen et al., 2004) imply that the induced accumulation of antioxidant compounds (phenolics, enzymes) may not have been adequate to prevent the deleterious effects of the O3. Chronic O3 exposure is also known to impair the accumulation of assimilates and sucrose transport and thus decreases the plant carbon gain (Dizengremel, 2001). When this defect is combined with the increased production of phenolic compounds, which have a high metabolic cost (Gershenzon, 1994), it is possible that the increase in detoxification or protection mechanisms under O3 exposure is achieved at the expense of leaf growth (Dizengremel, 2001).
Our results show that elevated CO2 prevents the effects of elevated O3 on the birch leaf phenolics and other leaf traits. The interaction of CO2 and O3 shows that O3-derived induction of the phenolic concentration disappears under elevated CO2, and that the phenolic concentration in the EC+EO treatment follows that of the EC treatment. These effects are strongly connected to changes in leaf dry mass, as has been discussed above. At first glance, it seems that the elevating CO2 concentration in the atmosphere may ameliorate the harmful effects of the chronic ozone exposure on birch trees in the future. Our experiment, however, may have underestimated the treatment effects owing to the chamber effect (Eichelmann et al., 2004; Riikonen et al., 2004). The effects of O3 could have been more severe especially on clone 4, which proved to be more sensitive to ozone (Eichelmann et al., 2004; Riikonen et al., 2004).
Genotype controls phenolic production
The genotype is an important source of variation in phenolics in natural and clonal populations of birches (Keinänen et al., 1999a; Laitinen et al., 2000, 2002), and the identification of birch species and clones is possible according to their phenolic profiles (Keinänen et al., 1999b). In this study the clone effect was significant as was expected. The LMWP levels, but not those of condensed tannins, found in clone 80, were comparable with data with younger saplings of the same clone (clone 4 has not been studied earlier) (Keinänen et al., 1999a). Our observations indicate that the differences in chemical composition between the clones during the growing seasons are related to the phenological differences in leaf area and dry weight, both characteristics being greater in clone 80. Clone 80 is physiologically more active and has shorter growth period, expressed as earlier leaf senescence, in comparison with clone 4 (Riikonen et al., 2003). The different origins of the clones may explain these observed phenological and physiological differences: clone 80 is of a more northerly origin than clone 4 (Mutikainen et al., 2000), and may be genetically more fit to the prevailing photoperiod and thermal regimes in Suonenjoki field site than clone 4.
Chamber environment modifies phenolic metabolism
The environmental conditions inside the chambers were close to those predicted to prevail at the end of this century (Vapaavuori et al., 2002) and therefore, the results obtained from this study are valid in modelling the effects of atmospheric change in future conditions. Our observations show that the chamber conditions increased leaf growth, possibly because of lower light regime that promotes birch leaves to grow larger and thinner compared with nonshaded leaves (e.g. Messier & Puttonen, 1994). Although we collected the leaf samples from south side of the canopy, the effect of shading by chamber wall or self-shading cannot be ruled out. Nevertheless, the effect of shading remains unsolved because we do not have data on the light attenuation inside the chambers. We also found that the leaves in the CC trees were thicker compared with the OC trees (Oksanen et al., 2005; Repo et al., 2004), which does not support the shading hypothesis. It seems conceivable that the increased temperature has enhanced leaf growth. Studies made by Eichelmann et al. (2004) of the photosynthetic parameters of the leaves support this hypothesis, and these authors suggest that the CC trees have grown under less stressful conditions than the OC trees, thus possibly permitting enhanced leaf growth.
Earlier studies have shown that shading decreases the concentrations of phenolic compounds because of lower rate of photosynthesis (e.g. Koricheva et al., 1998). In our study, however, the leaves did not suffer from decreased carbon availability because they grew better in the CC trees.
The results show that the accumulation of DHPPG and the flavonol glycosides was higher in the OC trees than in the CC trees in 2000 (2, 3). These compounds are among the most abundant phenolics in birch leaves (Table 6) and their accumulation was affected the most in our study. It is known that UVB radiation induces the key enzymes, PAL and CHS, of the phenylpropanoid and flavonoid pathways (Fig. 1) (Jansen et al., 1998), resulting in the accumulation of UVB-absorbing compounds especially in the leaf epidermis (Burchard et al., 2000, Searles et al., 2001). Studies involving young birch seedlings have shown that enhanced UVB radiation increases the concentration of flavonol glycosides, but the effects on other compounds have been variable (Lavola et al., 1997, 1998, 2000; de la Rosa et al., 2001; Kostina et al., 2001). DHPPG has an absorption maximum in the UV region below 300 nm (Lavola et al., 1997) and it could, therefore, act as UV screen, but may also have antioxidant activity against the radicals produced by UVB radiation. The fact that the PE film covering the chambers almost completely absorbed the UV light appears to explain the observed reduction in the accumulation of the flavonol glycosides, and the reduction of DHPPG may suggest its UVB reactivity. On the contrary, the accumulation of condensed tannins, their catechin derivative precursors and some CoQA derivatives was induced in the CC trees. It could be that carbon directed to the phenylpropanoid pathway might have been shunted to the aforementioned compounds when the need for UV-screening compounds was diminished. The reallocation may not have been complete because the total phenolics were diluted instead of accumulating at steady state. The reduced accumulation of phenolics in the CC trees in 2000 was changed to a dilution effect as a result of an increase in the leaf dry weight in 2001 (2, 3). In conjunction with the observed accumulation patterns of epicuticular flavone aglycons these results indicate that the phenolic metabolism in chamber trees was adjusted in response to leaf enlargement, thus leading to the disappearance of the chamber effect. A 3-year study with birch seedlings growing under enhanced UVB radiation has also shown that the stimulation of phenolic compounds disappears when the experiment lasts sufficiently long (Tegelberg et al., 2001). These observations underscore the importance of multiyear studies in investigations concerning the effects of atmospheric change on plant chemistry.
Conclusions
The results show clearly that phenolic concentration in birch leaves is influenced by changes in the leaf biomass, and thus suggest that content-based expression is a better indicator for carbon allocation to phenolics. Excess fixed carbon seems to be directed towards the accumulation of some low-molecular-weight phenolic compounds and condensed tannins in birch leaves grown in an CO2-enriched atmosphere. On the other hand, chronic ozone exposure seems to have induced at least some potent antioxidant compounds, which, however, could not adequately protect leaves from harmful ozone effects and may even have exacerbated them. In addition to effects on photosynthesis, elevating O3 concentration in the troposphere could be detrimental to birches by directing the allocation of their resources to the production of phenolic compounds instead of growth. It appears, however, that this O3 effect was prevented by elevated CO2 under the experimental conditions of our study.
The results also show the importance of genotype and environment as modifiers of the phenolic metabolism in birch: in some cases the effects could be greater in comparison with treatment effects. The observed acclimatization to the chamber environment and the altering responses of some of the compounds to the CO2 treatment not only emphasize the need for long-term experiments, but also indicate that the myriad genotypes of birch trees may have potential to adapt to gradually changing atmospheric conditions. The high chemical resolution used in the present study not only helps to assess the possible connections between compounds along the phenolic pathway but also addresses the need for integration of leaf biochemical and anatomical studies. More research on the function and localization of different compounds is needed before we are able to fully understand the ecological and evolutionary consequences of the observed changes.
Acknowledgements
This study was funded by the Academy of Finland (project nos. 40924 and 47074), the European Commission (ERBIC15CT980102), the University of Kuopio, the Finnish Cultural Foundation, the Emil Aaltonen Foundation and the Graduate School in Forest Sciences. We gratefully acknowledge the assistance of the technical staff at the FFRI (METLA) Suonenjoki Research Station, and we thank Mrs Sinikka Sorsa for the phenolic analyses and Mr Jaakko Heinonen for the statistical guidance that he provided.




