
Facilitation and interference among three predators affect their consumption of a stream-dwelling mayfly
Summary
1. We experimentally tested if a multiplicative risk model accurately predicted the consumption of a common mayfly at risk of predation from three predator species in New Zealand streams. Deviations between model predictions and experimental observations were interpreted as indicators of ecologically important interactions between predators.
2. The predators included a drift-feeding fish [brown trout (T), Salmo trutta], a benthivorous fish [galaxiid (G), koaro, Galaxias brevipennis] and a benthic predatory stonefly (S; Stenoperla sp.) with Deleatidium sp. mayflies as prey. Eight treatments with all predator species combinations and a predator-free control were used. Experiments were performed in aquaria with cobbles as predator refuges for mayflies and we measured the proportion of prey consumed after 6 h for both day and night trials.
3. Trout consumed a higher proportion of prey than other predators. For the two predator treatments we found less than expected prey consumption in the galaxiid + trout treatment (G + T) for both day and night trials, whereas a higher than expected proportion of prey was consumed during night time in the stonefly + trout (S + T) treatment.
4. The results indicate interference (G + T) and facilitation (S + T) between predators depending on predator identity and time of day. Thus, to make accurate predictions of interspecific interactions, it is necessary to consider the ecology of individual species and how differences influence the direction and magnitude of interactions.
Introduction
For many years studies were conducted on interactions between single pairs of predator and prey species (Kerfoot & Sih, 1987), although prey in natural systems are faced with several predators that may induce conflicting avoidance behaviour. Interactions among multiple predators can have far more complex effects on prey and community dynamics (Power, 1990; Holt & Polis, 1997; Sih, Englund & Wooster, 1998). Predator foraging mode, prey avoidance behaviour and habitat complexity can influence the outcome of either facilitation or interference interactions between predators, producing deviations from an expected null interaction model. Similar predators can show additive effects when there are few differences in foraging rate (Sokol-Hessner & Schmitz, 2002) or they can interfere with one another and decrease risk of predation (Vance-Chalcraft, Soluk & Ozburn, 2004). Predators with dissimilar foraging behaviour, e.g. one active and one ambush predator, may facilitate each other and enhance predation risk (Soluk & Collins, 1988; Losey & Denno, 1998; Eklöv & VanKooten, 2001).
In natural stream systems vertebrate and invertebrate predators can have different effects on benthic macroinvertebrate prey densities. In many studies, predatory invertebrates are found to have stronger impacts on prey density than fish predators (Wooster, Sih & Englund, 1997), because of differences in anti predator behaviour (Wooster & Sih, 1995), movement rates within and between patches (Sih & Wooster, 1994) and experimental scale (e.g. Englund, 1997). Predatory effects on density can also vary with prey species identity. Dahl & Greenberg (1997) found leeches (Erpobdella octoculata L.) to be more efficient predators on Gammarus pulex L. than brown trout (Salmo trutta L.) and vice versa for Baetis rhodani Pictet.
Predicting multiple predator effects such as changes in prey behaviour or consumption of prey between different predator combinations, is difficult. Predictions depend on the combination of predator and prey species. Non-additive effects (either positive or negative) are more likely to occur for predators and prey that occur naturally together, i.e. when prey recognise all predators as threats (McIntosh & Peckarsky, 1999). A tool for making predictions is the ‘multiplicative risk model’ (Soluk, 1993; Sih et al., 1998). The model is based on expected prey consumption for two-predator combinations calculated from their individual consumption rates (Soluk, 1993). Emergent multiple predator effects are seen as deviations from the expected values, i.e. either facilitation or interference. Many studies in aquatic systems have addressed pair-wise interactions between a vertebrate and an invertebrate predator (e.g. Soluk & Collins, 1988; Krupa & Sih, 1998; Swisher, Soluk & Wahl, 1998). As far as we know, very few have considered three-way predator interactions (but see Huhta et al., 1999) although in nature food webs with multiple vertebrate and invertebrate predators consuming the same prey species (Huhta et al., 1999) and situations with intra-guild predation where one predator also serves as prey (Holt & Polis, 1997), are common. Here we tested the ability of the multiplicative risk model (and extended it to three predators) to predict the outcome of two- and three-way interactions among predators based on single predator performance (Soluk, 1993; Sih et al., 1998).
Consumption patterns of stream dwelling vertebrate and invertebrate predators with different foraging strategies and diurnal activity patterns were experimentally tested. We used one mainly drift-feeding fish (introduced brown trout, S. trutta), one benthivorous fish (native koaro, Galaxias brevipennis Günther) and a benthic predatory stonefly (Stenoperla sp.), which all commonly prey on Deleatidium sp. mayfly nymphs (McIntosh, 2000). Large trout (>15 cm) tend to reduce densities of large predatory invertebrates (Nyström, McIntosh & Winterbourn, 2003) and to fragment galaxiid populations (summarised in Townsend, 2003), whereas Deleatidium sp. mayflies occur in high densities throughout New Zealand, regardless of predator regime (McIntosh, 2000). In a system dominated by small trout (<15 cm), non-lethal interactions, e.g. competition for prey or shelter, between predators are likely to be important and could influence predation effects on prey.
Prey may only respond to the most dangerous predator at the time (McIntosh & Peckarsky, 1999) and different predators may have more or less impact depending on time of day. In a previous study in New Zealand (summarised in Peckarsky, Cooper & McIntosh, 1997) mayflies from trout streams increased daytime movement and drift in the presence of Stenoperla, which might make mayflies more available to visually foraging predators like trout. Interactions between galaxiids and mayflies or trout and mayflies (e.g. McIntosh & Townsend, 1994, 1995), imply that there could be risk enhancement leading to non-additive effects in consumption patterns when the two fish predators are combined. Trout forage mostly during daytime, while galaxiids forage by both day and night (McIntosh & Townsend, 1994, 1995, 1996) and mayfly avoidance of one predator could enhance risk to the other. We expected interference between Stenoperla and galaxiids, as they are both benthic, while we expected facilitation between Stenoperla and trout. Facilitation between galaxiids and trout might occur because they have different foraging modes. Summarising the effects of the two-predator combinations (two positive and one negative), led us to predict facilitation in the three-predator situation. Thus, our main objective was to test how interactions between predators affect their consumption of a stream-dwelling mayfly prey.
Methods
The experiments were conducted at Maxwell Gage Field Station, University of Canterbury, Westport on the West Coast of the South Island, New Zealand between the 13 and 19 February 2005.
Brown trout [T; S. trutta; total length (TL) ± 1 SD; 81.9 ± 7.0 mm], the night-active benthic galaxiid, koaro (G; G. brevipennis; TL ± 1 SD; 53.6 ± 6.28 mm) and Stenoperla sp. nymphs (stonefly, S; TL ± 1 SD; 14.5 ± 1.96 mm) were collected from local streams. Mayfly nymphs of the genus Deleatidium sp. (TL ± 1 SD; 4.1 ± 0.55 mm) are dorso-ventrally flattened slow swimmers that feed on biofilm, especially algae on the substratum and are a ubiquitous component of most unpolluted New Zealand streams. Sizes of organisms were chosen to avoid intra-guild predation and to ensure natural behaviour in a small experimental set up.
The combinations of predators present at each site where organisms were collected differed. However, all streams had several species of fish. All animals were collected using electrofishing (Taylor, McIntosh & Peckarsky, 2001), in three West Coast streams. Trout and Stenoperla sp. from Landing Creek (Easting/Northing, NZ map grid: 24214/59136), koaro from Red Jack Creek (24062/58839) and all Deleatidium sp. from Kerr Stream (241250/59490). Mayflies were collected a few hours prior to experiments, because of a shortage of experimental organisms some fish and Stenoperla sp. had to be randomly reused once. Between experiments the predators were held in separate plastic wading pools (1 m diameter) with continuously aerated water and fed on a daily basis with Deleatidium nymphs.
The experiments were performed in stream tanks (Fig. 1) during both day (15:00 hours to 21:00 hours) and night (22:00 hours to 04:00 hours) to detect differences in consumption between predators because of their specific diurnal activity patterns. Stream tanks consisted of 60 × 40 cm non-transparent, plastic tubs with water circulated (depth 15.5 cm) over five to six cobbles (covered area ±1 SD, 390 ± 58 cm2) using a submersible pump (1200 L h−1) and plexiglass divider (30 × 26 cm). The cobbles were provided for the mayflies to use as a refuge from predators and collected together with the mayflies. All tubs were covered with nets to prevent fish from escaping. Temperature in the tubs ranged between 16.1 and 16.8 °C and pH ranged between 7.2 and 7.5.
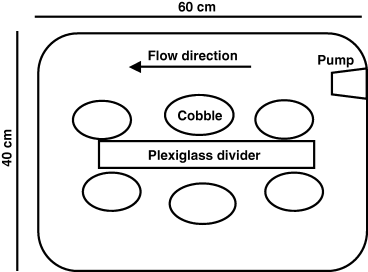
Top view of the experimental set-up showing submersible pump (1200 L h−1), plexiglass divider and cobble placement in a 45 L plastic tub. Cobbles were provided for mayflies to use as a refuge from predators and the divider directed water movement producing circular flow around the tanks.
Eight treatments were randomly assigned to the test aquaria, each replicated four times for both day and night trials (in total 32 aquaria for each trial), included all possible predator combinations and a predator free control. Fish (one trout and/or two koaro, respectively) were added 4 h prior to the start of the experiment to acclimatise. Four stoneflies were added 5 min before the start of the experiment. Thirty mayflies were added to all treatments at the start of the experiment. The experiment ran for 6 h, after which all animals were counted and the number of surviving mayflies was recorded. Densities of predators and prey are within the natural range found in New Zealand streams (K. Olsson, unpubl. data) and fish biomass corresponds to weights used in other experimental studies (Nyström & McIntosh, 2003).
As an index of mayfly behaviour we estimated mayfly activity in all tubs for 5 min before ending the experiment, i.e. any visible mayflies that were drifting, swimming or crawling around in the tubs. At night activity was observed using red light, to minimise influences on mayfly behaviour. Previous studies (McIntosh & Townsend, 1994, 1996) have shown that mayfly behaviour changes little after the first 30 min in a 3–4 h experiment. However, observed activity was low and constant for all treatments.
Data analysis
We calculated the proportion of consumed mayflies for all treatments and corrected for the loss of mayflies that occurred in the control [number of mayflies lost (mean ± SD) day: 1.25 ± 1.25; night: 1.75 ± 1.75].
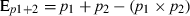 (1)
(1) (2)
(2)where Ep1+2+3 is the expected proportion of prey consumed in the three-predator treatment, where p1, p2 and p3 are the mean observed proportions consumed for the one-predator treatments, respectively. However, in our extended model for the three predator treatment (eqn 2), the first correction should not be made twice for a specific prey individual, hence the term (p1 × p2 × p3).
One sample t-tests (Quinn & Keough, 2002) were performed to examine if differences between observed and expected values for the two- and three-predator treatments deviated from zero, i.e. no deviation implies that the results follow model predictions. On the single predator treatments we performed a two-way anova with treatment and time of day as factors to elucidate predator efficiency and diurnal activity. All proportions were loge-transformed prior to statistical analysis to meet the assumptions of normality and homogeneity of variances and analyses were conducted using SPSS 11.0.3 for Mac OS X.f (SPSS Inc., Chicago, IL, U.S.A.).
Results
A two-way anova on differences in consumption of mayflies between the one-predator treatments, with time and treatment as factors, revealed significant treatment effects (F2,18 = 13.332, P < 0.001) as well as an interaction between time and treatment (F2,18 = 4.252, P = 0.031; Fig. 2), indicating that fish predators were primarily more effective during the day whereas Stenoperla captured most prey at night. Trout consumed more prey than the other two predators (Tukey's HSD: trout > Stenoperla, P < 0.001; trout > galaxiid, P = 0.004). The proportions consumed in the one-predator treatments were combined, according to eqns 1 & 2, to give the expected proportions consumed (Fig. 3). Deviations from the expected proportions consumed (Fig. 4) were significant for the G + T treatment by both day and night and for the S + T night treatment (Table 1). Hence, there were interactive predator effects, where the G + T show interference interactions (lower proportionate consumption than expected) and the S + T treatment facilitation (higher proportionate consumption than expected). For the S + G and S + G + T treatments there was no significant deviation from the expected proportions consumed (Table 1).
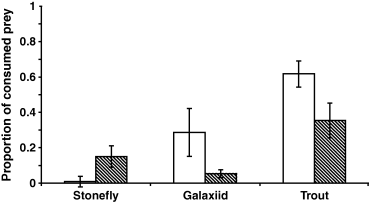
Mean proportion of Deleatidium mayfly prey consumed (± 1 SE) in the one predator treatments during day (open bars) and night (striped bars) trials. Two-way anova with treatment and time as factors had significant treatment effects (F2,18 = 13.332, P < 0.001) and an interaction between time and treatment (F2,18 = 4.252, P = 0.031). Tukey's HSD revealed prey consumption by trout was significantly higher than that of the other two predators (trout > stonefly, P < 0.001; trout > galaxiid, P = 0.004).
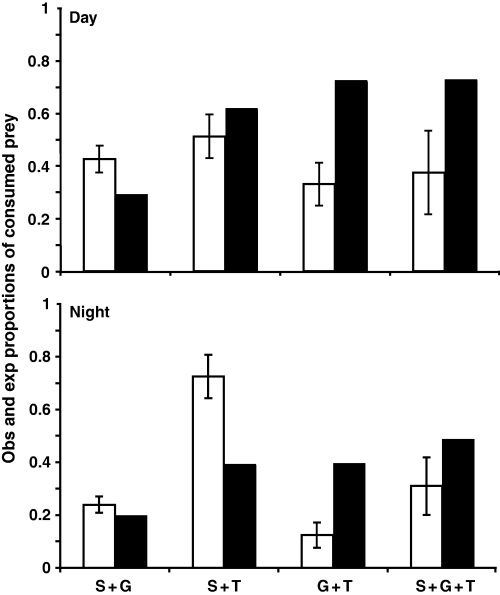
Mean proportions of observed (open bars) and expected (filled bars) consumption of mayflies (± 1 SE) for the two- and three-predator treatments during the day- (top) and night- (lower) trials. Expected proportions of consumed prey were calculated using the multiplicative risk model (Sih et al., 1998; eqns 1 & 2). S + G, stonefly + galaxiid; S + T, stonefly + trout; G + T, galaxiid + trout; S + G + T, stonefly + galaxiid + trout.
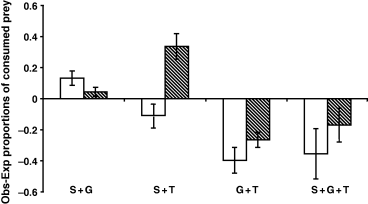
Mean deviations from expected proportions (observed − expected ± 1 SE) of consumed prey for the two- and three-predator treatments for day (open bars) and night (striped bars) trials. Expected proportions were calculated using the multiplicative risk model (Sih et al., 1998; eqns 1 & 2). The deviations were compared to zero in a one sample t-test (i.e. no difference is the null hypothesis). Significant deviations were found for the G + T treatment by both day (t3 = −3.406, P = 0.042) and night (t3 = −4.653, P = 0.019) and the S + T treatment in the night trial (t3 = 4.471, P = 0.021). All other treatments showed no significant deviations from that expected under a multiplicative risk model. S + G, stonefly + galaxiid; S + T, stonefly + trout; G + T, galaxiid + trout; S + G + T, stonefly + galaxiid + trout.
| Treatment | One-sample t-test | |||
|---|---|---|---|---|
| t-value | d.f. | P-value | Mean difference | |
| Day | ||||
| S + G | 2.949 | 3 | 0.060 | 0.1223 |
| S + T | −1.253 | 3 | 0.299 | −0.1287 |
| G + T* | −3.406 | 3 | 0.042 | −0.5398 |
| S + G + T | −2.159 | 3 | 0.120 | −0.5309 |
| Night | ||||
| S + G | 1.366 | 3 | 0.265 | −0.0411 |
| S + T* | 4.471 | 3 | 0.021 | 0.2848 |
| G + T* | −4.653 | 3 | 0.019 | −0.3138 |
| S + G + T | −1.541 | 3 | 0.221 | −0.2150 |
- *Significant deviations.
Discussion
We showed that patterns of consumption changed significantly when predators with different foraging modes were present at the same time. For example, galaxiids + trout consumed less prey than the multiplicative risk model predicted during both day and night trials. Hence there is evidence of interference between the two predatory fish. The differences between day and night trials for the stonefly + trout treatment, with a significant positive deviation from expected at night, imply that different diurnal behaviour of one or both predators led to facilitation of prey consumption when both trout and stoneflies were present at night. Overall, the interaction between predator treatment and time (Fig. 2) indicates that predator consumption rate depends on both predator species and their individual diurnal foraging activities.
In general we saw correct predictions by the model when predators have temporally separated foraging (S + G treatment and S + T day treatment), or if potential positive (S + T) and negative (G + T) interactions somehow cancel each other out (S + G + T treatment). Thus, there was no enhancement or reduction in prey predation risk in such cases. Deviations from the expected number of consumed prey were found with predators that are active at the same time of day or have either different (S + T night treatment) or similar (G + T treatment) foraging strategies. Facilitation coincided with predators displaying different foraging modes, probably causing conflicting prey behaviour and increasing predation risk, while interference interactions between predators with similar foraging behaviour probably led to reduced risk for prey.
Explaining why particular predator combinations in our experiment resulted in facilitation, interference or no interaction depends on knowledge of the characteristics of the predators and prey. In the treatment with the two benthic feeders (Stenoperla and galaxiids) there was neither a positive nor a negative interaction between the predators. Hence, the multiplicative risk model correctly predicted consumption. However, having two predators with similar foraging behaviour does not necessarily explain the lack of interaction. In other experiments with benthic feeders (Cottidae and stoneflies) there have been both positive and negative interactions (Soluk & Collins, 1988), or no interactive effects (Fairchild & Holomuzki, 2005). The direction and magnitude of the interaction seems to depend on prey species identity, where some prey are forced out of refuge by the stonefly (e.g. Ephemerella sp.; Soluk & Collins, 1988), resulting in facilitation. In the same study, predation on another mayfly species (Baetis sp.) was lower than expected when both predators were present, because of interference. In our study the galaxiids were day-active and this behaviour fits with other studies showing less nocturnal foraging behaviour in galaxiids when they originate from streams lacking trout (e.g. McIntosh & Townsend, 1995), as was the case here. Stenoperla were nocturnal, as generally observed (McIntosh, 2000). The different timing of predator foraging activity probably explains why we did not observe any interaction effect of koaro and Stenoperla on mayflies.
Trout and Stenoperla had opposite patterns of foraging activity and methods, trout being more day-active, water-column predators, as expected. In the daytime treatment with Stenoperla and trout there were merely additive consumption effects, which is not what we expected, as the presence of Stenoperla has increased mayfly activity during the day in other studies (Peckarsky et al., 1997). However, our Stenoperla originated from a trout stream and the Stenoperla in this experiment were strictly nocturnal, which would reduce their effect on prey activity during the day and probably explains the lack of facilitation during day in the S + T treatment. During the night, however, there was a significant positive deviation (t3 = 4.471, P = 0.021) from the expected consumption for the S + T treatment (Fig. 4). Stenoperla activity at night is likely to disturb prey and thereby also make mayflies more available to trout, as has been shown in other studies of trout-stonefly systems (e.g. Soluk & Richardson, 1997). Although trout have a higher prey capture rate during the day, even in the one predator treatment they also had relatively high capture rates at night, as has been shown for drift-feeding salmonids feeding at night in other studies (Jenkins, 1969; Angradi & Griffith, 1990; McIntosh & Townsend, 1995).
Fish with different foraging modes, e.g. drift versus benthic feeding, can have various effects on the prey community (Dahl & Greenberg, 1996; Miyasaka & Nakano, 1999). Here, galaxiids and trout probably interfered and consumed less than the expected proportions derived from the multiplicative risk model both by day and night. Both predators attack prey in open water, although galaxiids are viewed as benthivorous. They cause prey to move up into the water column where they pursue and attack (McIntosh & Townsend, 1995, 1996). There are even field observations from non-trout streams that the koaro has a drift-feeding behaviour similar to that of trout (Hayes, 1996). Hence, the two fish species might interfere with each other during the pursuit of prey, leading to risk reduction for prey as we observed.
The three-predator treatment did not show any significant deviation from the expected, whether by day or night (Fig. 4). This phenomenon might be a result of Stenoperla somehow ameliorating the negative interaction between galaxiids and trout shown in the G + T treatment. Thus, more prey were consumed, especially at night, but not to the extent that there is any indication of facilitation as in the night treatment for Stenoperla and trout.
Predicting the effects of multiple predator interactions is likely to be affected by the experience of predators and prey. Mayflies and galaxiids were from non-trout streams and can have had no previous experience of trout as a predator/competitor. Other studies have shown that species naïve to an exotic predator do not behave in the same way as experienced individuals do, both in terms of foraging and avoidance behaviour (summarised in Townsend, 2003). The result should be that the mayflies behave similarly in all treatments. Hence, the effects observed here were interactions between predators per se.
We present evidence of interactive effects between predators as the results from G + T- and S + T-treatments were non-additive. We also showed that it is necessary to study interactions both during the day and night when prey and predators have different diurnal rhythms, because day- and night-active predators affected both the direction and magnitude of the observed interaction. However, further experiments that record predator and prey behaviour are needed to reveal the mechanisms underlying our estimates of prey mortality. As we have seen here a multiplicative risk model, without correction for any sort of interaction, cannot fully predict the amount of consumed prey when predators with different foraging modes and diurnal rhythms are present at the same time. When making predictions it is also necessary to consider whether prey are subjected to risk enhancement or reduction when faced with multiple predators. Moreover, prey diurnal rhythm will influence model predictions of prey consumption. Combining vertebrate and invertebrate predators that also have a predator-prey relationship, i.e. intra-guild predation, may influence the interactions (Wooster et al., 1997). Although we used small trout, Stenoperla may perceive them as a threat, as large trout feed on Stenoperla and reduce their activity.
We conclude that it is necessary to study multiple species interactions if we are to explain the patterns observed in nature as multiplicative models (e.g. individual foraging rates) in most cases do not correctly predict the effects of multiple interacting species. Correctly predicting the outcome of multiple predator interactions depends on knowledge of the participating predators and prey and making predictions will be especially difficult for systems where predators and prey are novel to each other.
Acknowledgments
We thank Jack van Berkel, Rennie Bishop, Nick Etheridge and Linda Morris, University of Canterbury, for their help with the experimental set-up. We are also grateful to Ceasar Bolivar Daniel, Kelly Gutseit, Johanna Stadmark and Geraldine Thiere, who all helped with the sorting of mayflies. Anders Nilsson helped us improve the multiplicative risk model for three predators. We also thank two anonymous reviewers and Alan Hildrew for comments on earlier versions of this manuscript. The project was supported by Travel Grants from the Faculty of Natural Sciences, Lund University to the PhD-students and by The Swedish Council for Environment, Agricultural Sciences and Spatial Planning to P.N. and C.B.




