A post-transplant complication
Conflict of interest: none declared.
Clinical findings
A 48-year-old Chinese man presented with a 1-month history of an expanding purplish nodule on the left lower abdomen. Two years previously, the patient had received a cadaveric renal transplant for end-stage renal failure secondary to chronic hypertension. He had experienced early episodes of rejection, which were treated with the antibody OKT3 (anti-CD3 antibody). The patient’s current immunosuppressive medication consisted of prednisolone 5 mg/day, mycophenolate mofetil 1 g/day, and ciclosporin 400 mg/day. He had no systemic symptoms such as weight loss, pruritus or pyrexia.
Examination revealed a large ulcerated purplish nodule over the left iliac fossa 80 × 50 mm in size (Fig. 1). There was no associated lymphadenopathy or hepatosplenomegaly.
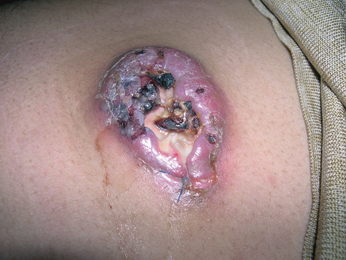
Large ulcerated purplish nodule over the lower abdomen.
A staging full-body computed tomography scan, bone-marrow examination, and myeloma screen were all normal. There was no head, neck or nasal involvement, and a positron emission tomography (PET) scan showed no evidence of disease elsewhere.
Histological findings
An incisional skin biopsy taken from the lesion showed a large nodule with central necrosis, and a bottom-heavy infiltrate composed of abnormal lymphocytes and lymphoplasmacytoid cells, extending into the subcutis. The cells were highly pleomorphic with large nuclei and prominent nucleoli associated with numerous mitoses (Fig. 2). Immunohistochemistry showed the tumour cells to be strongly positive for CD79a, weakly positive for CD138, and negative for CD20 and CD30. There was κ light chain restriction, and in situ staining for Epstein–Barr virus (EBV)-encoded RNA was diffusely positive within the tumour cells.
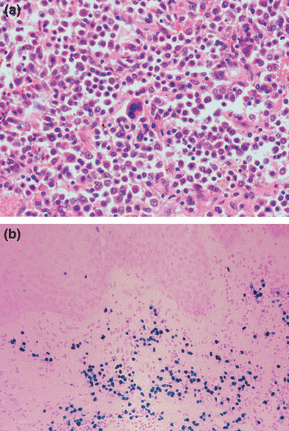
(a) Skin biopsy showing abnormal lymphocytes with prominent nucleoli, and a multinucleated lymphocyte in the centre of the field (haematoxylin and eosin, original magnification × 400). (b) In situ hybridization staining for Epstein–Barr virus-encoded RNA, showing diffuse positivity (bluish–purple) within tumour cells (original magnification × 20).
What is your diagnosis?
Diagnosis
EBV-associated primary cutaneous-post transplant lymphoproliferative disorder, monomorphic B-cell subtype.
Discussion
A diagnosis of primary cutaneous post-transplant lymphoproliferative disorder (PTLD) was made. Other EBV-positive tumours that can affect the skin include metastatic nasopharyngeal carcinoma, metastatic lymphoepithelioma-like carcinoma of other sites, human immunodeficiency syndrome-related lymphomas, and angiocentric immunoproliferative lesions such as extranodal natural killer/T-cell lymphoma.
PTLD is a lymphoid proliferation or lymphoma that develops in a solid-organ or bone-marrow allograft recipient as a consequence of immunosuppression. The risk of lymphoma varies depending on the type of transplant and the immunosuppressive regime. The levels of risk associated with various transplants are: renal < 1%, cardiac and hepatic 1–2%, heart–lung and liver–bowel 5%, and bone-marrow 1%. The risk of developing PTLD is strongly correlated to the intensity of the immunosuppression. OKT3, a monoclonal antibody directed against the pan-T-cell marker CD3, and regimes containing tacrolimus or ciclosporin seem to carry the greatest risk.1
The majority of PTLDs are caused by EBV-induced monoclonal or polyclonal B-cell proliferation in patients with reduced T-cell surveillance as a consequence of rest immunosuppression. Transplant recipients who are EBV seronegative before transplantation have a higher risk of PTLD than seropositive patients. This probably accounts for the higher incidence of PTLDs in the paediatric population. The patient’s pretransplant EBV serology status was unavailable to us, as he had received his transplant in China. He presented with extranodal disease, which is more common than nodal disease. Approximately two-thirds of patients have disease confined to a single site, as observed in this case.2 Involvement of the gastrointestinal tract, central nervous system and the allograft are the most common sites of extranodal involvement. Primary cutaneous disease is very rare.3
PTLDs are a morphologically heterogeneous group of lymphoproliferative disorders, which were classified by Knowles et al.4 into three main groups according to clonality, EBV positivity, and evidence of oncogene/tumour suppressor gene mutations. The three categories are: (i) plasmacytic hyperplasia, (ii) polymorphic B-cell hyperplasia and polymorphic B-cell lymphoma, and (iii) immunoblastic lymphoma or plasmacytoma/multiple myeloma. Post-transplant T-cell lymphomas are recognized but are much less common than B-cell lymphomas. The World Health Organization categorization for PTLDs is based on this original classification, but also includes T-cell neoplasms, Hodgkin’s lymphoma and Hodgkin’s lymphoma-like lymphomas.5
First-line treatment involves a reduction in immunosuppression. If this fails, radiotherapy, local excision, chemotherapy and CD20 monoclonal antibodies, alone or in combination, are then used, depending on the type, stage and location of the lymphoma. Monitoring of EBV viral load in peripheral blood can be used to predict the development of PTLD or relapse after successful treatment.6 Prognosis is difficult to predict, although monoclonal lymphomas with evidence of oncogene or tumour-suppressor gene mutations tend to have a worse prognosis. Overall, the mortality of PTLD in solid-organ transplant recipients is approximately 60%, with a mortality of 80% in bone-marrow recipients.5
There is controversy over the optimal management of individual patients with PTLD. A significant number of patients with polymorphic lesions will regress with immunosuppresion reduction (ISR) treatment. Our patient had a lesion that had rapidly increased in size and histologically resembled diffuse large B-cell lymphoma (DLBCL) with unequivocal evidence of clonality, based on surface immunoglobulin light-chain restriction.
On this basis the patient was treated with ISR and three cycles of CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolane) chemotherapy for DLBCL; rituximab was not administered as the patient’s tumour did not express CD20 antigen. The tumour responded only partially to treatment and was subsequently excised; functional imaging with PET showed no other lesion, making surgery an acceptable form of treatment rather than intensification of chemotherapy. More than 12 months after treatment, the patient remains in complete remission from lymphoma.