Parthenotes as a source of embryonic stem cells
The authors declare no conflicts of interest.
Abstract
Abstract. The derivation and study of human embryonic stem cell lines, despite their potential therapeutic usefulness, raise considerable ethical, religious, legal and political concerns because it inevitably leads to the destruction of viable embryos. In an attempt to bridge the division between ethical questions and potential scientific and medical benefits, considerable efforts have been devoted to the search for alternative sources of pluripotent cell lines. In this review we discuss the use of artificial parthenogenesis as a way to create entities, called parthenotes, that may represent an alternative ethical source for pluripotent cell lines. We describe the biological differences between parthenotes and embryos, in order to provide a rationale for the discussion on whether their use can be acceptable as a source of stem cells. We present data derived from animal models on the extent parthenogenetic stem cells are similar to biparental cell lines and discuss these aspects in the context of their extension to the human species. Finally, we present experiments recently carried out in our laboratory that allowed us to generate human parthenotes through artificial activation of human oocytes and to use them as a source for the derivation of parthenogenetic pluripotent cell lines.
INTRODUCTION
Stem cells have the ability to proliferate indefinitely and, in adequate conditions, to differentiate into specialized cell types. They can be obtained from different sources, with different advantages and limitations. Embryonic stem cells are pluripotent and have virtually unlimited self-renewal and differentiation potential. They are derived from the inner cell mass of the blastocyst, as originally shown in the mouse (Evans & Kaufman 1981; Martin 1981) and more recently demonstrated in humans (Thomson et al. 1998). Stem cells obtained in this way possess the highest possible plasticity and may be induced to differentiate into all cell types of the body (Nagy et al. 1993; Brook & Gardner 1997). However, in order to generate these cell lines, it is necessary to deprive the embryo of any further potential to develop into a complete human being. Moving from mice to men, the embryo's potency results in strong ethical considerations. Intentional creation and destruction of nascent human life raises serious ethical, religious, legal and political concerns, and imposes the necessity to identify alternative sources of embryonic stem cells for research and therapeutic purposes.
A number of approaches that allow for the derivation of pluripotent cells but, at the same time, do not involve the generation and destruction of a viable human embryo have been proposed in the last couple of years, in an attempt to bridge the division between ethical questions and potential scientific and medical benefits. To this end, stem cells have been derived from embryos that were ‘blocked’ in their development and, thus, considered clinically dead or developmentally arrested (Zhang et al. 2006). Similarly, cell lines have been obtained from single blastomeres removed from less developed embryos (morulae) using an approach similar to that used for pre-implantation genetic diagnosis for in vitro fertilization (IVF), in which a single cell is extracted from an embryo and tested for genetic disorders (Chung et al. 2006; Klimanskaya et al. 2006). The generation of non-embryonic entities obtained through altered nuclear transfer (ANT) has been proposed as an alternative possibility. Abnormal blastocysts, inherently unable to implant into the uterus but capable of generating customized embryonic stem cells have been created (Condic et al. 2005; Meissner & Jaenisch 2006).
Here, we discuss the use of artificial parthenogenesis as a way to create entities, namely parthenotes, that may represent an alternative ethical source for pluripotent cell lines. We review data from the literature dealing with parthenogenesis in mammalian species. We describe results obtained in our laboratory that have recently allowed us to generate human parthenotes and discuss some experimental evidence showing the possibility to derive pluripotent cell lines from parthenogenetic embryos.
PARTHENOGENESIS IN MAMMALS
Parthenogenesis is the process by which a single egg can develop without the presence of the male counterpart and is a form of reproduction common to a variety of organisms such as fish, ants, flies, honeybees, amphibians, lizards and snakes, that may routinely reproduce in this manner. Mammals are not spontaneously capable of this form of reproduction. However, mammalian oocytes can successfully undergo artificial parthenogenesis in vitro and can be activated by mimicking the calcium wave induced by sperm at normal fertilization, with the use of a calcium ionophore, and stimulated to divide. Mammalian parthenotes can develop to different stages after oocyte activation, depending on the species, but never to term (Table 1). Parthenogenetic activation can be induced at different stages along oocyte meiosis resulting in parthenotes with different chromosome complementation. When parthenogenetic activation is performed in oocytes at the second meiotic metaphase, it results in the extrusion of the second polar body and leads to the formation of a haploid parthenote. This method is rarely used since, in this case, the developmental competence is reduced compared to normal embryos and to diploid parthenotes (Henery & Kaufman 1992).
| Species | Maximum development (days) | Pregnancy length (days) | Reference |
|---|---|---|---|
| Mouse | 10 | 21 | Surani et al. 1986 |
| Rabbit | 10–11 | 31 | Ozil 1990 |
| Pig | 29 | 114 | Kure-bayashi et al. 2000 |
| Sheep | 25 | 150 | Loi et al. 1998 |
| Bovine | 48 | 280 | Fukui et al. 1992 |
| Marmoset monkey | 10–12 | 144 | Marshall et al. 1998 |
Diploid parthenotes can be obtained in two different ways. The most common consists of combining the activation of metaphase-2 oocytes with exposure to an actin polymerization inhibitor, usually cytochalasin B (Balakier & Tarkowski 1976). Alternatively a diploid parthenote can be generated by preventing the extrusion of the first polar body. This protocol leads to the formation of tetraploid oocytes (Kubiak et al. 1991) and the diploid status is then re-established at the end of oocyte maturation with the extrusion of the second polar body. Using one or the other method has important consequences for the genetic make-up of the parthenote in question. Performing oocyte activation before inhibition of the second polar body extrusion determines the formation of highly homozygous parthenotes, since diploid status of the parthenote is obtained after segregation of sister chromatids. In contrast, when the first polar body extrusion is inhibited, parthenotes are genetically identical to each other and have the same heterozygosity as their mother (Kubiak et al. 1991).
The occurrence of a high degree of homozygosity in parthenotes has been evaluated in contrasting ways in the perspective of using these entities as a source of embryonic stem cells.
Homozygosity can be seen as a potential benefit when reduction of immunogenicity of a stem cell derivative is considered. The possibility of generating stem cells that are homozygous for all three sets of HLA (A, B and DR) would exponentially increase the number of phenotypes a graft fully matches. Furthermore, homozygosity has also been suggested to be an advantage to be exploited for selecting cell lines carrying drug response genes, or a disease gene or cancer gene correction, providing a useful research tool for drug testing and development (Lin et al. 2003). At the same time, it must be remembered that homozygosity can represent a severe risk. Loss of heterozygosity could amplify any negative genetic component potentially present in the genotype.
PARTHENOGENESIS AND IMPRINTING
Irrespective of how activation has been performed and what ploidy has been generated, parthenotes are unable to develop to term. In the mouse, the most advanced parthenotes survive to the early limb bud stage, have little extra-embryonic tissue and almost no trophoblast (Kaufman et al. 1977). As shown in Table 1, parthenotes will not develop and will arrest development by day 10 in the mouse, day 11.5 in rabbit, day 21 in sheep and day 29 in pigs. This arrest in development does not seem to be only in that they develop a small trophoblast, since even when supplied with trophoblast cells, parthenotes will stop and die (Newman-Smith & Werb 1995). Studies with chimeras between mouse normal (zygotic) embryos and parthenotes show that parthenotes fail because of some cell-autonomous defects that affect parts of the embryo proper, including skeletal muscle, liver and pancreas (Fundele et al. 1990).
The reason for this arrest in development is believed to be due to genomic imprinting. Genomic imprinting appears to be restricted to eutherian mammals, and has evolved as a result of conflicting concerns of the parental genomes during the growth process. It has been shown that normal mammalian development requires genomic contributions from both the mother and the father. As described by Surani (2001), although oocytes are potentially totipotent in many organisms, this is not so in mammals. This is because the maternal genome is epigenetically modified in the germ line to contain only maternal ‘imprints’, which normally results in repression of certain maternally inherited imprinted genes (Fig. 1). A paternal genome is therefore essential to ‘rescue’ the oocyte, as the maternal genes are imprinted reciprocally to paternal imprints. This explains why both genomes are needed in mammalian development. Maternal and paternal genomes are complementary but not equivalent; therefore, both sets are required for the correct growth process (Smith 2001). This implies that monoparental duplications of regions of a number of chromosomes may be lethal or detrimental to the embryo. At present, more than 50 genes have been identified as imprinted in the human (see Table 2). These display a vast range of functions, ranging from splicing factors, such as Snrpn, to growth factors, such as insulin (Ins1 and Ins2) and Igf-2, to genes that are functional as RNAs, such as H-19 and Xist (reviewed by Bartolomei 1994).
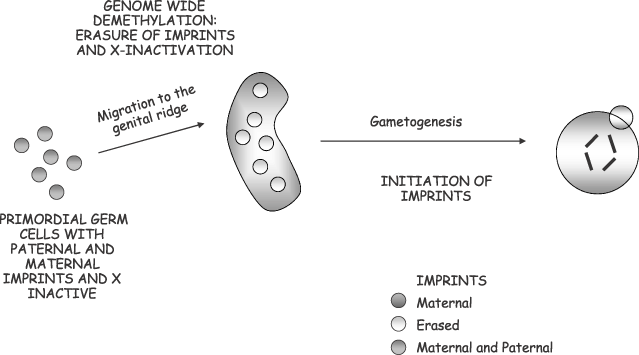
During gametogenesis specific imprints are established in the germ line according to the sex of the parent. The maternal genome is epigenetically modified and contains only the maternal ‘imprints’, resulting in the repression of a number of genes fundamental for development. The paternal genome is essential in order to provide the complementary genes. Both genomes are therefore needed for generating a functional genome.
| Gene | Expressed allele | Protein name or description | RNA description (non-coding RNA only) |
|---|---|---|---|
| ATP10A | Imprinted maternal | ATPase, Class V | |
| CDKN1C | Imprinted maternal | Cyclin-dependent kinase inhibitor | |
| CPA4 | Imprinted maternal | Carboxypeptidase | |
| DIRAS3 | Imprinted paternal | Ras homologue | |
| DLK1 | Imprinted paternal | Delta-like 1 homologue | |
| DLX5 | Imprinted maternal | Homeo box-containing | |
| GNAS | Imprinted maternal | Neuroendocrine secretory protein 55 | |
| GNASXL | Imprinted paternal | Large isoform of GS-a | |
| GRB10 | Imprinted isoform dependent | Growth factor receptor-bound protein | |
| GS-alpha | Imprinted maternal | Stimulatory G-protein, alpha subunit | |
| H19 | Imprinted maternal | RNA of unknown function | |
| HBII-437 | Imprinted paternal | snoRNA | |
| HBII-438 A | Imprinted paternal | snoRNA | |
| HBII-438B | Imprinted paternal | snoRNA | |
| HBII-52 | Imprinted paternal | snoRNA | |
| HYMAI | Imprinted paternal | RNA of unknown function | |
| IGF2 | Imprinted paternal | Insulin-like growth factor 2 | |
| IGF2AS | Imprinted paternal | IGF2 Antisense transcript | |
| IMPO1/ITUP1 | Imprinted paternal | Imprinted transcript variant 1 | |
| INS | Imprinted paternal | Insulin | |
| KCNQ1 | Imprinted maternal | Voltage-gated potassium channel | |
| KCNQ1DN | Imprinted maternal | BWRT protein | |
| KCNQ1OT1 | Imprinted paternal | KCNQ1 Antisense transcript | |
| L3MBTL | Imprinted paternal | Polycomb group | |
| MAGEL2 | Imprinted paternal | MAGE-like protein | |
| MEG3 | Imprinted maternal | RNA of unknown function | |
| MEST | Imprinted paternal | Alpha/Beta hydrolase fold family | |
| MESTIT1 | Imprinted paternal | MEST Antisense transcript | |
| MKRN3 | Imprinted paternal | Makorin, ring finger protein | |
| NDN | Imprinted paternal | Necdin, neuronal growth suppressor | |
| NNAT | Imprinted paternal | Neuroneatin | |
| OSBPL5 | Imprinted maternal | Oxysterol binding protein-like 5 | |
| PEG10 | Imprinted paternal | Retroviral gag pol homologue | |
| PEG3 | Imprinted paternal | Zinc-finger protein | |
| PPP1R9 A | Imprinted maternal | Protein phosphatase inhibitor | |
| SNRPN | Imprinted paternal | Small nuclear ribonucleoprotein | |
| SNURF | Imprinted paternal | SNRP upstream reading frame | |
| TCEB3C | Imprinted maternal | Transcription elongation factor | |
| TP73 | Imprinted maternal | Tumour related protein |
Since both parental genomes are needed for generating a functional genome, it comes as a consequence that parthenogenetic blastocyst-like structures do not possess a functional genome that can be considered distinctive of a human embryo. Their genome is in reality constituted by a double set of an epigenetically imprinted female gamete genome and should be more correctly considered unfertilized eggs that have been activated to initiate cell division. Although they are able to undergo a few cycles of cell division, parthenotes are unlikely to develop beyond the first few divisions, as the centrioles contributed by the human sperm are required for the formation of a functional centrosome (Pickering et al. 1988) and may therefore be considered as a possible alternative source for human pluripotent cells.
HUMAN IN VITRO PARTHENOGENESIS
The above observations demonstrate that epigenetic asymmetry is the main reason why artificial parthenogenesis in mammals leads to the creation of entities that are unable to develop to term. Data from the literature show that human oocytes can be successfully activated but often parthenogenetic development does not proceed beyond the eight-cell stage (Abramczuk & Lopata 1990; Johnson et al. 1990; Winston et al. 1991; Taylor & Braude 1994; Rhoton-Vlasak et al. 1996; Rinaudo et al. 1997; Yamano et al. 2000; Nakagawa et al. 2001a,b; Sengoku et al. 2004). Development of human parthenotes to the blastocyst stage has only comparatively recently been reported (Cibelli et al. 2001; Lin et al. 2003; Rogers et al. 2004). This achievement, together with the establishment of parthenogenetic stem cells in non-human primates (Cibelli et al. 2002), has stimulated new interest in human parthenogenesis because it could eliminate the requirement to produce or disaggregate normal competent human embryos for deriving pluripotent cell lines in vitro (Vrana et al. 2003; Rogers et al. 2004; Kiessling 2005).
Due to the very limited availability of unfertilized human oocytes, data on human parthenogenetic development to the blastocyst stage, at present, are derived only from small groups of oocytes. However, since in Italy no more than three oocytes can be fertilized per IVF cycle (Benagiano & Gianaroli 2004; Ragni et al. 2005), patients undergoing an intracytoplasmic sperm injection procedure from whom more than three good quality oocytes had been retrieved were offered to donate the supernumerary gametes for research. This allowed us to obtain a relatively high number of human oocytes to be used for developing an efficient protocol for parthenogenetic activation and for directly comparing their developmental competence with that of oocytes derived from the same patients whose gametes were undergoing intracytoplasmic sperm injection (Paffoni et al. 2007). Our team fertilized 114 oocytes whereas 104 were artificially activated using a protocol that involved their exposure to the calcium ionophore ionomycin followed by the protein kinase inhibitor 6-dimethylamino purine (6-DMAP). Following this procedure, we could obtain 67.3% of oocytes showing one enlarged pronucleus and one extruded polar body which were therefore considered parthenotes. After 5 days of culture, nine of the parthenotes developed into blastocysts representing 12.9% of the activated oocytes and 8.7% of the total number of oocytes exposed to the activation protocol (Paffoni et al. 2007) (Table 3). Despite higher successful blastocyst rates had been obtained in previous trials (Cibelli et al. 2001; Lin et al. 2003; Rogers et al. 2004), in our experiments, most parameters used for evaluating in vitro development were not significantly different between activated and fertilized oocytes, suggesting that the activation protocol used in our trial reflected the developmental competence of that specific group of oocytes. The most relevant result in the context of using parthenotes as a source for deriving embryonic cell lines, however, was that the combined use of ionomycin and 6-DMAP constantly enabled the development of parthenotes to the blastocyst stage. A possible explanation for this important observation is that, despite a variety of activating agents, such as ethanol, Ca++ ionophores and electroporation induce one prolonged calcium peak that releases oocytes from metaphase arrest, then maturation-promoting factor quickly rises again and further development is arrested. For this reason, the addition of agents like 6-DMAP, which inhibits the reactivation of maturation-promoting factor following kinetics similar to those occurring after fertilization, leads to high in vitro development rates also of human oocytes, confirming the results obtained in several other species including cattle (Lagutina et al. 2004), sheep (Loi et al. 1998), rhesus monkeys (Mitalipov et al. 2001), rabbits (Mitalipov et al. 1999) and pigs (Boquest et al. 2002).
| Time | Oocytes |
|---|---|
| 0 h | 104 |
| 18–20 h activated | 70 |
| Percentage of oocytes | 67.3 |
| 42–44 h cleaved | 64 |
| Percentage of oocytes | 61.5 |
| Percentage of activated | 91.4 |
| 114–116 h blastocyst | 9 |
| Percentage of oocytes | 8.6 |
| Percentage of activated | 12.8 |
DERIVATION OF CELL LINES FROM ANIMAL PARTHENOTES
The first cell lines derived from parthenogenetic embryos were established from mice more that 20 years ago (Kaufman et al. 1983). These pioneering results were followed more recently by the derivation of parthenogenetic cell lines in Macaca fascicularis (Cibelli et al. 2002; Vrana et al. 2003) and in rabbit (Fang et al. 2006; Wang et al. 2006). These were very promising since, in all three species, cell lines exhibited the fundamental properties that characterize normal biparental embryonic stem cells. Cells lines were stable in culture, maintained a normal karyotype, could be differentiated in vitro to form embryoid bodies when cultured in suitable conditions, and could form teratomas when injected in immunodepressed mice. All these data support the hypothesis that it should be possible to derive parthenogenetic stem cells also in the human species and that these cells should, theoretically, be suitable for therapeutic applications.
However, more detailed studies performed on mouse cell lines suggest that cell lineages derived from parthenogenetic cells may be restricted in their differentiation potential. Diploid cells of mouse parthenogenetic lines were able to form chimaeras when injected into normal blastocysts, with participation ranging from 5% to more than 70% but the contribution to skeletal muscle and testis was considerably lower than in other tissue tested (Allen et al. 1994). If mouse embryonic stem cells are aggregated with tetraploid host embryos, tetraploid host cells contribute fully to the development of the extraembryonic membranes while being gradually selected against in the embryo proper. This enables the generation of mice derived entirely from embryonic stem cells (Nagy et al. 1993). When the same procedure was performed with parthenogenetic stem cells, development ceased between days 13 and 15, indicating that parthenogenetic stem cells are unable to form a complete individual (Allen et al. 1994). The restricted developmental potential of parthenogenetic stem cells was further indicated by the analysis of teratomas produced by transfer of aggregates under the kidney capsule, since very little skeletal muscle could be found (Allen et al. 1994). Nevertheless, germline chimaeras could be obtained when parthenogenetic embryonic stem were injected in normal embryos (Allen et al. 1994). However, reduction of totipotency observed in parthenogenetic embryonic stem cells is significantly lower if it is compared to that observed when parthenogenetic embryos are aggregated in chimaeras with normal biparental embryos (Fundele et al. 1989; Fundele et al. 1990). The improvement of parthenogenetic embryonic stem cell developmental potential compared to parthenogenetic embryos may be attributed to the disruption of normal imprinting observed during in vitro culture, thus suggesting that these cells, despite some alterations, may retain the potential to be used for therapeutic purposes.
DERIVATION OF CELL LINES FROM HUMAN PARTHENOTES
Results obtained from the experiments described above have shown that it is possible to derive parthenogenetic embryos from supernumerary human oocytes, and has indicated that it should be possible to use parthenotes as a potential source of pluripotent cells. However, no data are available in the literature reporting the establishment of parthenogenetic stem cell lines from humans. Previous attempts at this have described low attachment of parthenogenetic embryos to feeder cells and arrest of proliferation after few cell divisions (Lin et al. 2003).
We recently have applied a protocol derived from our procedure that has allowed us to obtain pluripotent porcine cell lines (Brevini et al. 2007), with some modifications. Human parthenogenetic blastocyst-like structures have been subjected to enzymatic digestion with pronase in order to remove zonae pellucidae. Digestion was carried out at 38.5 °C on a thermostatically controlled stage while carefully monitoring the process microscopically. Samples were then delicately washed, transferred to fresh drops of medium and were subjected to microsurgery in order to isolate Inner Cell Masses (ICMs). We found that repeated and thorough washing of ICMs from culture oil carry-overs ensured better attachment to the feeder layer and better colony formation.
A further aspect that (in our hands) has appeared to be crucial in order to obtain round, distinct and well-attached colonies was the use of specific feeder cell density. To our understanding, parthenogenetic ICMs show high sensitivity to cell overcrowding on one hand but, at the same time, needed an adequate number of feeder cells to ensure adequate support for their growth. Possibly, this releases correct concentrations of essential factors in the culture microenvironment. Interestingly, although cell-to-cell interactions seemed to play an important role (given that use of medium conditioned by the feeder cells without their presence had been tried) cells did not provide attachment results as satisfactory as when feeder cells were present in the culture vessels. Within 3 days from ICM plating, we could obtain circular colonies with distinct margins of small, round cells that became stable cell lines (Brevini et al. 2006a,b). Currently, these lines have been growing for over 2 years and are being characterized for the expression of pluripotency markers, and are being tested for their ability to differentiate in the various body tissues. Studies addressed to assess genetic stability as well as safety of these cells are also in progress.
It remains to be seen how genetically stable and safe these cells are; aneuploidy in human oocytes, in fact, is relatively high. Several studies on a vast number of human oocytes estimated that chromosome abnormalities are present in 15–20% of them, and indicate both whole chromosome non-disjunction and chromatid separation as main causes its occurrence of (Pellestor et al. 2006). This rate, however, can increase dramatically in parallel with the age of the subject, exceeding 50% in patients over 34 years old (Kuliev et al. 2003; Kuliev et al. 2005). Anomalies are predominantly represented by chromatid errors, which are the major source of aneuploidy in the resulting embryos. Half the detected aneuploidies (49.8%) are of complex nature with involvement of two or more chromosomes, or the same chromosome in both meiotic divisions (Kuliev et al. 2005). In the clinical setting of assisted reproduction this has severe consequences, since in miscarriages approximately have aneuploid cells (Magli et al. 2006). When these data are considered in the perspective of using parthenotes as a possible source of human cell lines, great care will be required to evaluate their karyotype as suggested by some preliminary experimental evidence (Santos et al. 2003).
CONCLUSIONS
We suggest that artificial parthenogenesis represents an alternative source for deriving pluripotent lines of human cells that could also be considered ethically acceptable. At present, reliable protocols are available for the generation of human parthenogenetic embryos from supernumerary IVF oocytes. Some of the conditions that favour good attachment of outgrowths and maintenance in culture of cell lines deriving from these embryos have also been identified.
In our opinion, the uniparental origin and asymmetric imprinting of parthenogenetic cell lines make them a valuable tool for studies addressed to a better understanding of the mechanisms driving early embryo development, as well as genetic imprinting. Whether these factors and other issues related to their parthenogenetic origin may limit their use in research and potential clinical applications remains to be clarified.
ACKNOWLEDGEMENTS
The authors would like to thank Dr S. Antonini for critical discussion and for helping in the preparation of the manuscript.
The experiments described were partially supported by Industrie Farmaceutiche Serono, Roma.