Salvage therapy of Autoimmune Thrombocytopenic Purpura revealing non-Hodgkin Lymphoma by the thrombopoietin receptor agonist romiplostim
Immune Thrombocytopenic Purpura (ITP) is an autoimmune disease that mainly results from a rapid splenic and hepatic clearance of autoantibody-loaded platelets from the circulation (Koehrer et al, 2010). Secondary causes of ITP must be excluded at diagnosis, including autoimmune conditions, chronic infection (e.g. Hepatitis C virus, human immunodeficiency virus) and lymphoproliferative disorders (LPD), particularly B-cell chronic lymphocytic leukaemia (B-CLL) (Cines et al, 2009). Treatment options for idiopathic ITP are well defined, including steroids, intravenous immunoglobulins (IVIG) in acute ITP, and the anti-CD20 monoclonal antibody rituximab, splenectomy and immune suppressive drugs in chronic ITP (George, 2010). Recently, a breakthrough in chronic ITP treatment has been achieved with the use of the thrombopoietin receptor (TPO-R) agonists, romiplostim (Nplate; Amgen, Thousand Oaks, CA, USA) and eltrombopag (Promacta; GlaxoSmithKline, Research Triangle Park, NC, USA), which produce rapid dose-dependent increases in the platelet count, even in patients with refractory ITP. Moreover, responses to these new agents are generally sustained during therapy and their long-term use appears to be associated with a good safety profile (Nurden et al, 2009). In B-CLL patients, the occurrence of an autoimmune disorder (AID) negatively impact on the prognosis (Zent et al, 2009). Although no therapeutic guidelines are available in this situation, recent reports emphasized the use of rituximab alone (D’Arena et al, 2010) or in combination with chemotherapy (Kaufman et al, 2009; Bowen et al, 2010) or TPO-R agonist (Koehrer et al, 2010; D’Arena & Cascavilla, 2011). However, other causes of thrombocytopenia, including disease progression, hypersplenism and therapy-related thrombocytopenia, must be ruled out in this situation and this generally requires a bone marrow examination (Zent et al, 2009). In addition to ITP in B-CLL, ITP has occasionally been reported in association with Hodgkin lymphomas or non-Hodgkin lymphomas (NHL) of T or B cell origin. However in those single-case reports, treatment was variable and mostly focused on the lymphoma. We thus report here four cases of life-threatening ITP, diagnosed simultaneously to a B-cell NHL, and primarily managed by using the TPO-R agonist, romiplostim.
This retrospective study was approved by our local Review Board. There were four patients, two males and two females, with a median age of 76 (70–83) years (Table I). Three of them (Patients 1, 3 and 4) had no history of haematological malignancy. Patient 2 had been previously diagnosed with B-CLL, but with an initially normal platelet count. The median platelet count at presentation was 3 (3–17) × 109/l. The diagnosis of ITP was based on bone marrow smear in three patients and bone marrow biopsy in one, after the exclusion of other causes of peripheral thrombocytopenia. In all cases, the bone marrow examination showed increased megakaryopoiesis and also showed moderate infiltration (30%) by B-CLL in Patient 2 and refractory cytopenia with multilineage dysplasia (RCMD) in Patient 3. The diagnosis of ITP was thus confirmed in Patient 3 by the positivity of specific anti-platelets antibodies; this test was not performed for the other patients. All patients received 1 mg/kg per d intravenous methylprednisolone at the onset of ITP. Two patients received IVIG 0·5 g/kg per d for 2 d (Patients 1 and 3) while a third (Patient 2) was already receiving monthly IVIG treatment as supportive therapy for immune deficiency in the context of B-CLL. Patients 1, 2 and 3 were treated with romiplostim 5 μg/kg per w s.c. after failure of initial treatment; Patient 4 received romiplostim upfront for acute gastrointestinal bleeding. Patient 3 also received rituximab as a salvage therapy.
| Patients | Age | Sex | Plt | Lymphoma | CTS | IG | R | TF | Plt/R | Treatment | Outcome | FU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | M | 5 | MCL | + | + | − | − | 100 | RCHOP, RDHAP, ASCT | A | 18 |
| 2 | 76 | M | 17 | DLBCL | + | + | + | − | 90 | RCHOP, navelbin, cisplatin | A | 12 |
| 3 | 81 | F | 3 | MZL | + | + | + | + | 36 | – | D | 1 |
| 4 | 83 | F | 3 | MZL | + | − | + | − | 202 | Rituximab | A | 3 |
- Plt, platelet count at diagnosis (×109/l); CTS, corticosteroids; IG, intravenous immunoglobulin; R, rituximab; TF, platelet transfusion; Plt/R, platelet count after romiplostim (×109/l, maximum); FU, follow-up (months); M, male; F, female; MCL, mantle-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; MZL, marginal zone lymphoma; ASCT, autologous stem cell transplantation; RCHOP, rituximab, cyclophosphamide, adriamycin, vincristine, prednisone; RDHAP, rituximab, dexamethasone, cytarabine and cisplatin; A, alive; D, dead.
The mean follow-up was 8·5 (1–18) months. The evolution of the patients’ platelet count upon treatment is detailed in Fig 1. In Patient 1, steroids and IVIG treatment failed and romiplostim was used as salvage therapy, which rapidly normalized the platelet count. This enabled a diagnosis of mantle cell lymphoma (MCL) through a laparoscopic abdominal lymph node biopsy, and treatment by rituximab plus CHOP (cyclophosphamide, adriamycin, vincristine and prednisone), then with RDHAP (rituximab, dexamethasone, cytarabine and cisplatin) and finally, high-dose chemotherapy by BEAM (BCNU, etoposide, cytarabine and melphalan) followed by autologous stem-cell transplantation. This patient is still in complete remission of both MCL and ITP. Patient 2 was diagnosed with DLBCL and was treated by three cycles of R-CHOP and then four cycles of cisplatin and navelbin, due to the concomitant diagnosis of lung epidermoid carcinoma. This patient relapsed from B-CLL, but not from DLBCL, and required further treatment by the anti-CD20 monoclonal antibody Ofatumumab. Patient 3, who had a concomitant diagnosis of RCMD, was diagnosed with marginal-zone lymphoma (MZL) and died from cerebral bleeding due to refractory thrombocytopenia. For Patient 4, romiplostim was initiated immediately after the diagnosis of ITP and resulted in a rapid normalization of the platelet count. An attempt to discontinue romiplostim led to a rapid relapse, which was rapidly corrected after the reintroduction of romiplostim (Fig 1).
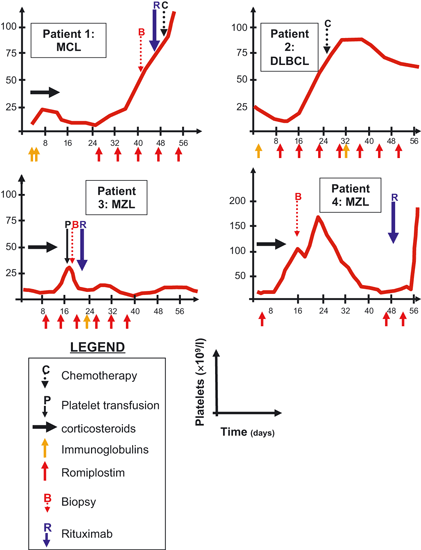
Evolution of the platelet count following treatment in the four study patients. MCL, Mantle Cell Lymphoma; DLBCL, Diffuse Large B-Cell Lymphoma; MZL, Marginal Zone Lymphoma.
Lymphoma-associated ITP is an uncommon condition for which no consensual therapeutic approach is currently available. The TPO-R agonists’ romiplostim and eltrombopag are generally well tolerated and their immediate side effects are moderate. In particular, an increase in marrow reticulin was reported in a few patients, which generally resolved upon treatment discontinuation (Nurden et al, 2009). However in the case of our patients, treatment with romiplostim had multiple goals: (i) to overcome a life-threatening ITP refractory to conventional treatment, (ii) to obtain the histological diagnosis of the lymphoma through a safe and unmodified (e.g. by steroids and/or rituximab) tissue sample biopsy, (iii) to eventually postpone splenectomy and/or to limit the use of steroids in severely immunocompromised patients and (iv) to avoid excessive toxicity of the megakaryocyte lineage after the initiation of chemotherapy. Treatment of the underlying lymphoma was required for two patients, and appeared to be essential for the long-term control of ITP in those cases, as reported (Zent et al, 2009). However, both patients received rituximab, vincristine and cyclophosphamide, which are also used in chronic ITP and may explain the improved platelet count (George, 2010). Importantly, the cost/benefit balance should also be considered when using a TPO-R agonist. However, we considered only a time-limited use of those molecules and the cost may be even less than that of IVIG, although this assertion should be verified in future studies.
Author contributions
Study design: Jerome Tamburini and Didier Bouscary; Data analysis: Chadi Al-Nawakil, Sophie Park, Nicolas Chapuis, Francois Dreyfus, Tali-Anne Szwebel, Laure Gibault, Thierry Molina, Olivier Hermine, Didier Bouscary and Jerome Tamburini; Manuscript writing: Chadi Al-Nawakil, Didier Bouscary and Jerome Tamburini; Patient enrolment: Sophie Park, Olivier Hermine, Didier Bouscary and Jerome Tamburini. All co-authors approved the final version of the manuscript.




