Prophylaxis in severe prothrombin deficiency
Severe prothrombin (Factor II, FII) deficiency is an extremely rare autosomal recessive condition with an estimated incidence of 1:2 million and an increased prevalence in populations where consanguineous partnerships are more common. International registry data indicate that patients with a FII of <10 iu/dl have a significant risk of severe spontaneous bleeding including haemarthrosis and intracerebral haemorrhage. To our knowledge there is only one published case report of the use of prophylactic FII infusions in an individual with severe prothrombin deficiency. We describe the case of a boy with severe prothrombin deficiency who was treated with on demand prothrombin complex concentrate up to the age of 6 years, but in response to an increasing number of spontaneous bleeding episodes and increased levels of exercise was started on regular prophylaxis.
Our patient was born by an induced vaginal delivery at term to Caucasian parents at that time residing overseas. He was given intramuscular vitamin K at birth with no adverse effects. At 4 d of age, he bled significantly from the umbilical stump requiring two attempts at suturing to control bleeding. No coagulation screen or factor assays were performed at this time. At 1 month he was investigated for fresh rectal bleeding which required a blood transfusion. At 4 months he was diagnosed with severe prothrombin deficiency when his FII level was found to be between 1–3 iu/dl. His parents were found to have FII levels of 68 and 54 iu/dl and further enquiry revealed that his maternal grandmother and his paternal grandfather were first cousins. Baseline thromboelastometry measurements were insensitive to his prothrombin deficiency but thrombin generation assay of platelet poor plasma was reduced.
His family predominantly lived overseas until he was 6 years old and throughout this time he required on-demand treatment with prothrombin complex concentrate (Prothrombinex, Defix and Octaplex) for trauma-induced bleeding episodes. These tended to be soft-tissue and muscle bleeds although there were at least two haemarthroses when he was 5 years old. The family settled back in the UK when he was 6 years old and about to attend school. At this point he was having a number of soft tissue bleeding episodes with no clear traumatic trigger, so for this reason, and in order that his activities at school need not be circumscribed, he was started on prophylactic prothrombin complex concentrate (Octaplex, Octapharma) 40 iu/kg by Factor IX (FIX) content, administered once a week. He was initially treated at home by one of our haemophilia Clinical Nurse Specialists who then taught his mother to treat him. Aside from occasional gum bleeding associated with erupting adult teeth, which is managed with tranexamic acid mouth wash, he had no spontaneous or trauma-related bleeding episodes for 18 months whilst on prophylaxis. Within the last few weeks he has had one severe spontaneous knee bleed 6 d after treatment and, given that his FII level was 8 iu/dl at 5 d, it is reasonable to conclude that a level below this will not prevent bleeding episodes. We plan to increase the frequency of his prophylaxis to every 5 d to prevent further breakthrough bleeds. He is involved in football, athletics, tennis, cricket and swimming at school. He is now 9 years old and is likely to be able to treat himself within the next 12 months. Table I and Fig 1 show his FII, FIX and Factor X levels and Thrombogram® parameters pre-infusion, and 1 and 4 h post-infusion.
| Clotting parameter (normal range) | Time point | |
|---|---|---|
| Pre (120 h post 40/kg) | 1 h post | |
| PT (12–16 s) | 20·7 | 12·7 |
| FII (50–150 iu/dl) | 8 | 100 |
| FIX (50–150 iu/dl) | 68 | 100 |
| FX (50–150 iu/dl) | 80 | 130 |
| ETP (1585–2482 nmol/l) | 380 | 1387 |
| Peak Height (168–479 nmol/l) | 39 | 269 |
| Time to Peak Height (4·9–10·4 min) | 17 | 9·2 |
- PT, prothrombin time; ETP, endogenous thrombin potential.
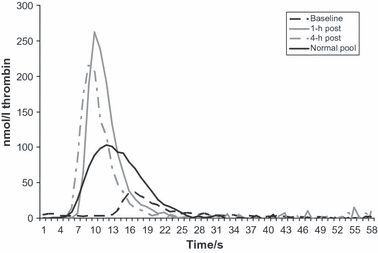
Thrombogram® of baseline, 1- and 4-h post-treatment in platelet poor plasma (PPP) triggered with PPP low reagent (Thrombinoscope Maastricht BV, Netherlands).
Homozygous prothrombin deficiency is the rarest severe bleeding disorder and what scanty evidence exits of the natural history of the condition relies on international and national registries of rare bleeding disorders(Bolton-Maggs et al, 2004; Peyvandi et al, 2006). It is most prevalent in areas of the world where consanguineous marriage is more common and this tends to equate with areas of the world where organisation and funding of inherited bleeding disorder care is rudimentary. It is therefore not surprising that there is only one published case report of the use of prophylactic prothrombin complex concentrate in severe prothrombin deficiency (Lobel et al, 2004). Although our patient required numerous on-demand treatments prior to starting school and had at least two joint bleeds, he did not have recurrent joint damaging bleeding episodes in early life and, as his family were living abroad during this time, prophylaxis would have been logistically impossible even had it been desirable.
Once the family re-settled in the UK and he started full time schooling it became apparent to his parents and the team at his Comprehensive Care Centre that his activities would have to be significantly curtailed in order to prevent recurrent serious bleeding episodes. We felt that this approach would leave him in a significantly worse situation than the other children we manage with severe bleeding disorders who can maintain a fairly normal life-style once on prophylaxis. The risk of spontaneous (or minimal trauma) intracerebral haemorrhage was also a factor in the decision making process. For children with the rarer severe bleeding disorders, where a recombinant treatment product is unlikely ever to be available, the balance between the potential risks of a life-time exposure to pooled plasma products needs to be weighed carefully against the life-time risk of bleed-related morbidity and mortality.




