Insight into the molecular pathogenesis of hairy cell leukaemia, hairy cell leukaemia variant and splenic marginal zone lymphoma, provided by the analysis of their IGH rearrangements and somatic hypermutation patterns
Hairy cell leukaemia (HCL) is a rare B-cell malignancy that shares several features with a variant form, HCL-variant, and splenic marginal zone lymphoma (SMZL). Distinction of these three disorders at diagnosis can be difficult, but correct diagnosis is important due to differences in patient management and clinical outcome (Matutes et al, 2003). A fuller understanding of the relationship between these three disorders is needed to help in elucidating their cell of origin and aid in their differential diagnosis.
The presence or absence of somatic hypermutation (SHM) in the immunoglobulin heavy chain (IGH) locus can be used as a tool to classify mature B-cell disorders based on their developmental status in relation to the germinal centre (GC) of the lymphoid follicles (Kuppers et al, 1999). In addition, analysis of IGH variable (IGHV), diversity (IGHD) and joining (IGHJ) gene usage can provide information about immunological features underlying disease pathogenesis.
Whilst a number of studies have investigated IGH rearrangements and SHM in HCL and SMZL, such data are not yet available for HCL-variant. We have investigated the IGH repertoire in a large cohort of HCL-variant and, using a homogenous analytical approach, performed a direct comparison with the IGH repertoire from a representative series of HCL and SMZL.
IGH rearrangements were analysed in 41 cases of HCL-variant, 30 HCL and 113 SMZL (Table SI). Samples were selected based on the availability of DNA material from peripheral blood smears, none of which have been included in previously published IGH rearrangement studies. Diagnoses were based on cell morphology, immunophenotyping and bone marrow and/or spleen histology, according to the World Health Organization (WHO) criteria.
IGHV, IGHD and IGHJ rearrangements were amplified and sequenced from genomic DNA in duplicate, according to the BIOMED-2 protocol (van Dongen et al, 2003) using FR1 and FR2 primers and analysed as previously described (Gonzalez et al, 2005). We identified IGHV, IGHD and IGHJ gene usage and SHM levels by aligning the sequencing data to germline genes using the current ImMunoGeneTics (IMGT) database (http://imgt.cines.fr/) (Tables SII–SIV).
Classically, samples with IGHV genes displaying >2% SHM have been defined as ‘mutated’ to allow for potential polymorphic variations (Schroeder & Dighiero, 1994). Thousands of IGH germline sequences are now available in the IMGT database (http://imgt.cines.fr/), and there are many reports of single nucleotide sequence variations representing ‘bonafide’ somatic mutations, suggesting that this 2% cut-off may have limited biological significance. Applying this current understanding to our data, HCL-variant was found to be more similar to SMZL in terms of IGHV mutation status, with both of these disorders containing a subset of cases that showed no evidence of SHM in their IGHV genes (22% and 15% respectively) (Table I). In contrast, SHM was evident in all cases of HCL (range: 0·5–12%). In addition, the overall distribution of mutation level in HCL was significantly different from that of HCL-variant and SMZL (χ2/Fishers exact: P < 0·001 and P < 0·005 respectively), whilst there was no significant difference between the HCL-variant and SMZL distributions (χ2/Fishers exact: P = 0·4) (Fig 1).
| HCL (n = 30) | HCL-variant (n = 41) | SMZL (n = 113) | |
|---|---|---|---|
| No mutations | 0 (0%) | 9 (22%) | 17 (15%) |
| >0% and <2% | 5 (17%) | 2 (5%) | 19 (17%) |
| >2% | 25 (83%) | 30 (73%) | 77 (68%) |
- HCL, hairy cell leukaemia; SMZL, splenic marginal zone lymphoma.
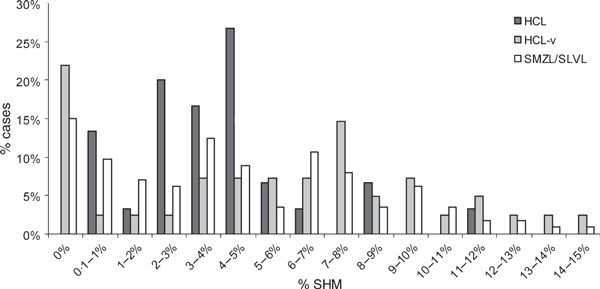
Distribution of HCL, HCL-variant and SMZL cases according to SHM level. Differences between distribution of mutation levels in the three disorders were analysed using chi-square where Fisher’s exact test was applied to sample sizes <10. The overall SHM distribution in HCL was significantly different to that of HCL-variant (P < 0·001) and SMZL (P < 0·005) whilst there was no significant difference between the HCL-variant and SMZL distributions (P = 0·4).
We compared the IGH repertoire seen in our cases to a panel of 206 IGHV, IGHD and IGHJ rearrangements derived from normal peripheral blood CD5−/IgM+ B cells (Brezinschek et al, 1997) (Fig S1). IGHV4-34 was significantly over-represented in both HCL-variant (7/41, 17·1%) and SMZL (14/113, 12·0%) (χ2/Fishers exact: P < 0·05 and P < 0·005 respectively), and was preferentially used in the unmutated subsets of these diseases (HCL-variant: 6/9, 67%; SMZL: 8/17, 47%) compared with the mutated subsets (HCL-variant: 1/32, 3%; SMZL: 6/96, 6%) (hypergeometric distribution: P < 0·001). In SMZL, IGHV1-2 was also significantly over-represented (22/113, 19·5%) (χ2/Fishers exact: P < 0·005) and was preferentially used in cases with <2% mutations (12/36, 33%) compared with cases with >2% mutations (10/77, 13%) (hypergeometric distribution: P < 0·01).
A restricted use of IGHV genes in HCL-variant and SMZL indicates a potential role of antigen selection in their neoplastic transformation. Further supporting this hypothesis, using the IMGT/Junction Analysis tool (http://imgt.cines.fr/) we identified stereotyped complementarity-determining region 3 (HCDR3) sequences (>60% amino acid sequence) with similar length and isoelectric point in the SMZL subsets using IGHV1-2 and IGHV4-34. In contrast, both IGHV1-2 and IGHV4-34 genes were absent in our typical HCL series and based on the distribution of its IGHV subgroup and gene usage there was no evidence of specific antigen involvement in this disease.
Current immunophenotypic and morphological evidence suggest that the precursor cell for SMZL and HCL is not of GC/post-GC origin (Tierens et al, 2003; Forconi et al, 2004). Instead, they may originate from a circulating IgM+ IgD+ CD27+ B-cell subset of SMZ origin, that can acquire SHM independent of the GC reaction as a means of pre-diversification prior to antigen encounter (Weill et al, 2009). Alternatively, a recently discovered memory B-cell population that lacks CD27 expression but has undergone class-switch recombination also makes a plausible candidate for HCL origin. It is also logical to propose that these two non-conventional memory B-cell sub-sets are prime candidates for the normal counterpart of HCL-variant. The bias use of specific IGH genes that have low-level, or completely lack SHM, in subsets of HCL-variant and SMZL suggests that their precursor cells have already been selected by a specific antigen and that these IGH sequences already possess high-affinity for the antigen without the need for mutation. Alternatively, they may code for polyspecific antibodies capable of reacting with a variety of commonly encountered antigens in humans. In contrast, the lack of specific IGHV, IGHD and IGHJ repertoires or stereotypes in HCL, even though all cases present evidence of SHM, suggests that the neoplastic transformation in this entity occurs in a somatically pre-diversified (mutated) cell that has not yet been selected for by antigen. Alternatively, the involvement of multiple antigens/epitopes in HCL pathogenesis may explain the lack of restricted IGHV, IGHD and IGHJ repertoire in this disorder.
We have shown that the hairy-cell lymphoproliferative disorders display different IGHV, IGHD and IGHJ repertoires consistent with them being three distinct entities but that the HCL-variant repertoire shares more similarities with that of SMZL, than of HCL. This supports the WHO re-categorization of HCL-variant as an unclassifiable splenic lymphoma whose biology is distinct from that of typical HCL (Swerdlow et al, 2008).
Acknowledgement
This work was supported by a grant from Cancer Research UK (C17053/A7543) and approved by the Royal Marsden Hospital/Institute of Cancer Research Clinical Research Committee (CRC) and the Ethics Research Committee. We thank Brian Walker and Monica Else for providing editorial assistance during preparation of the manuscript and Nicholas Dickens and Monica Else for advice on statistical analyses. We also owe an enormous amount of gratitude to all of the consultant haematologists who provided patient samples for this study.
Author contributon
E.M and D.G contributed equally to this paper.
Disclosure of conflicts of interest
The authors declare no competing financial interests.




